Aerogels for Biomedical, Energy and Sensing Applications
Abstract
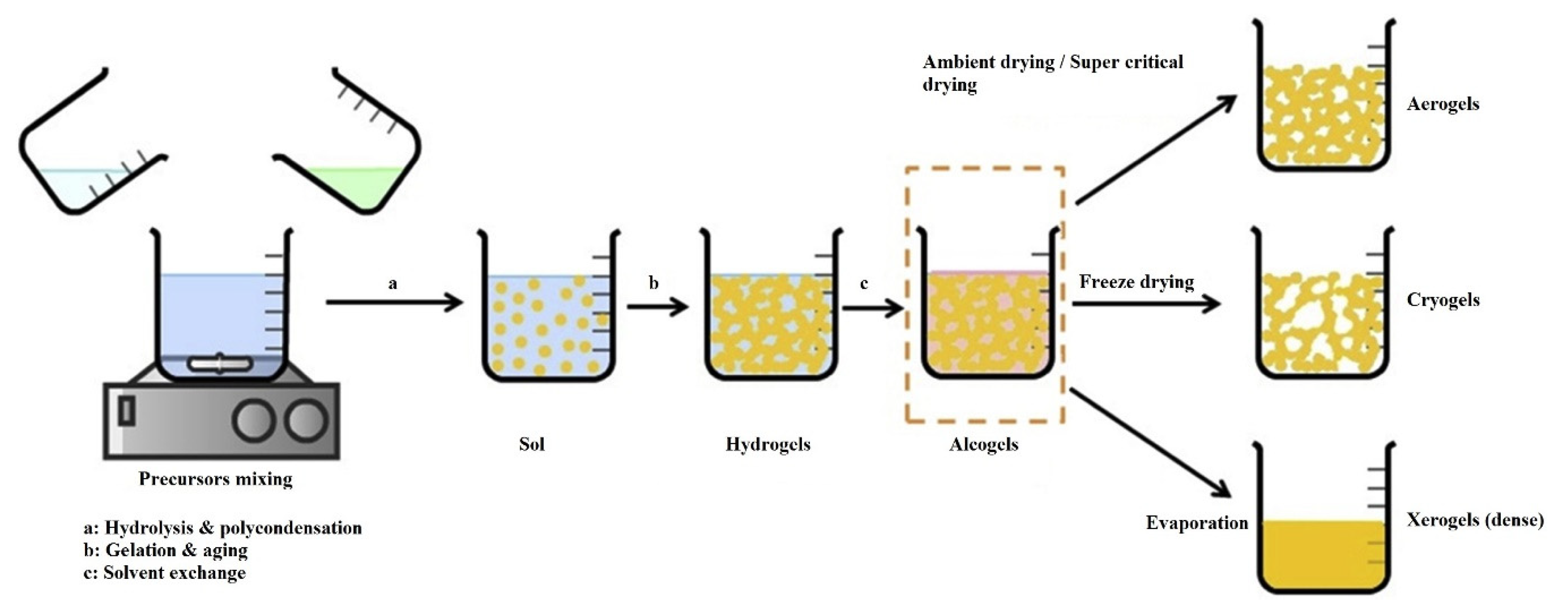
:1. Introduction
2. Properties and Classification of Aerogels
3. Applications of Aerogels
3.1. Aerogels for Biomedical Engineering
3.2. Aerogels for Energy
3.3. Aerogels for Sensors
4. Summary and Future Direction
- ❖
- In this study, a brief history and the applications of aerogels based on their classification and synthesis methods are reviewed and discussed. Aerogels are considered as excellent candidates for biomedical, energy, environment and sensing applications, and especially for high-performance sensors, where high sensitivity is required. Due to their structural characteristics and other properties, including having a highly porous structure, high specific surface area and other specific features provided by the aerogel network, aerogels are suitable for many applications. Therefore, based on the discussion, it is concluded that the combination of low dimensional active building blocks induces fascinating properties in the resulting aerogels, which display satisfactory performances in multiple applications. However, there are many challenges that still need to be addressed.
- ❖
- The economic and bulk production of high-quality aerogels is still a major issue that needs to be solved. Efforts have been made to simplify the synthesis mechanism in order to scale up and to reduce cost. Therefore, freeze drying (lyophilization), modified super critical drying and ambient drying methods were used. However, during the drying conditions, it was not easy to completely maintain the microstructure of the gel, therefore, damage frequently occurred.
- ❖
- Surface modification techniques employed for ambient drying inevitably causes a negative effect on the performance of aerogels. The overall properties of aerogels become disturbed during surface modification. The discussed studies demonstrate the superiority of aerogels in comparison with powdered materials in different fields. Therefore, aerogels with no structural variation own superior properties and a large-scale production of low-cost aerogels with superior qualities is vital and should be realized.
- ❖
- Maintain the porosity of the structure, especially the microporosity of the aerogel structure under stress during the fabrication of aerogel products, i.e., biosensors, gas sensors, ion batteries and catalysts. During the fabrication of sensors, aerogels are dispersed in solvents in order to get a uniform dispersion and coated on other substrates to make aerogel-based sensors. During this dispersion process, the microporous structures of aerogels are deteriorated and a decrease in mass and energy transfer is observed in the resultant sensor. However, in a comparison, these sensors still behave better and display a much-increased sensing performance than powder-based sensors. It is still believed that if the intrinsic porosity of the aerogel structures is retained well during the fabrication of sensors, the performance of the aerogel-based sensor can further be improved.
- ❖
- The utilization of thin aerogel films covers the microporous structural damage caused during the synthesis of sensors. Aerogels with excellent mechanical features are the prerequisites for the synthesis of aerogel films. Therefore, the mechanical properties of various types of aerogels, e.g., organic, inorganic and hybrid, should be improved. In this matter, the inclusion of additives (supporting materials) is considered a facile way to increase the overall mechanical properties. Although, when the mechanical properties are enhanced and the structure of aerogel is controlled, the extension of this technique remains a challenge for all kinds of aerogels. Therefore, it is compulsory to develop a common method that works for all types of aerogels to enhance their mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kurundawade, S.R.; Malladi, R.S.; Kulkarni, R.M.; Khan, A.A.P. Natural aerogels for pollutant removal. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–32. [Google Scholar]
- Liu, H.; Du, H.; Zheng, T.; Xu, T.; Liu, K.; Ji, X.; Zhang, X.; Si, C. Recent progress in cellulose based composite foams and aerogels for advanced energy storage devices. Chem. Eng. J. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M. Classification of Textile Polymer Composites: Recent Trends and Challenges. Polymers 2021, 13, 2592. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Wiener, J.; Farooq, A.; Saskova, J.; Noman, M.T. Development of maghemite glass fibre nanocomposite for adsorptive removal of methylene blue. Fibers Polym. 2018, 19, 1735–1746. [Google Scholar] [CrossRef]
- Yang, M.; Choy, K.-l. A nature-derived, flexible and three dimensional (3D) nano-composite for chronic wounds pH monitoring. Mater. Lett. 2021, 288, 129335. [Google Scholar] [CrossRef]
- Ahmad, V.; Ahmad, A.; Khan, S.A.; Ahmad, A.; Abuzinadah, M.F.; Karim, S.; Jamal, Q.M.S. Biomedical applications of aerogel. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–48. [Google Scholar]
- Siddique, J.A.; Ansari, S.P.; Yadav, M. Carbon aerogel composites for gas sensing. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–73. [Google Scholar]
- Qin, L.; Yang, D.; Zhang, M.; Zhao, T.; Luo, Z.; Yu, Z.-Z. Superelastic and ultralight electrospun carbon nanofiber/MXene hybrid aerogels with anisotropic microchannels for pressure sensing and energy storage. J. Colloid Interface Sci. 2021, 589, 264–274. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-based supercapacitors: Material and performance considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Matyáš, J.; Qafoku, N.P.; Kruger, A.A. Silver-functionalized silica aerogels and their application in the removal of iodine from aqueous environments. J. Hazard. Mater. 2019, 379, 119364. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Li, X.; Zhang, Y.; Qu, J.; Yu, Z.-Z. Cellulose/graphene aerogel supported phase change composites with high thermal conductivity and good shape stability for thermal energy storage. Carbon 2016, 98, 50–57. [Google Scholar] [CrossRef]
- Ansari, S.P.; Husain, A.; Shariq, M.U.; Ansari, M.O. Conducting polymer-based aerogels for energy and environmental remediation. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–86. [Google Scholar]
- Shi, K.; Huang, X.; Sun, B.; Wu, Z.; He, J.; Jiang, P. Cellulose/BaTiO3 aerogel paper based flexible piezoelectric nanogenerators and the electric coupling with triboelectricity. Nano Energy 2019, 57, 450–458. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Jiang, W.; Xu, Q.; Yu, J.; Liu, L.; Liu, L. Multiscale nanocelluloses hybrid aerogels for thermal insulation: The study on mechanical and thermal properties. Carbohydr. Polym. 2020, 247, 116701. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Louda, P.; Kejzlar, P. Woven textiles coated with zinc oxide nanoparticles and their thermophysiological comfort properties. J. Nat. Fibers 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Zheng, Y.; Wei, H.; Su, Z. Graphene-based hybrid aerogels for energy and environmental applications. Chem. Eng. J. 2021, 420, 129700. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zheng, Y.; Xu, Y.; Zheng, Z.; Chen, X.; Liu, W. Versatile aerogels for sensors. Small 2019, 15, 1902826. [Google Scholar] [CrossRef]
- Rashidi, S.; Esfahani, J.A.; Rashidi, A. A review on the applications of porous materials in solar energy systems. Renew. Sustain. Energy Rev. 2017, 73, 1198–1210. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Amor, N.; Louda, P. Thermophysiological comfort of zinc oxide nanoparticles coated woven fabrics. Sci. Rep. 2020, 10, 21080. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Amor, N.; Yang, T.; Mansoor, T. Thermophysiological comfort of sonochemically synthesized nano TiO2 coated woven fabrics. Sci. Rep. 2020, 10, 17204. [Google Scholar] [CrossRef]
- Rashidi, S.; Esfahani, J.A.; Karimi, N. Porous materials in building energy technologies-A review of the applications, modelling and experiments. Renew. Sustain. Energy Rev. 2018, 91, 229–247. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- Javadi, A.; Zheng, Q.; Payen, F.; Javadi, A.; Altin, Y.; Cai, Z.; Sabo, R.; Gong, S. Polyvinyl alcohol-cellulose nanofibrils-graphene oxide hybrid organic aerogels. ACS Appl. Mater. Interfaces 2013, 5, 5969–5975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Core–shell structuring of pure metallic aerogels towards highly efficient platinum utilization for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2018, 57, 2963–2966. [Google Scholar] [CrossRef] [Green Version]
- Biener, J.; Stadermann, M.; Suss, M.; Worsley, M.A.; Biener, M.M.; Rose, K.A.; Baumann, T.F. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 2011, 4, 656–667. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide aerogels as sorbents for radioactive iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Synergistic hybrid organic–inorganic aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, A.A.P.; Ansari, M.S.; Khan, A.; Kulkarni, R.M.; Bhamare, V.S. Aerogel and its composites: Fabrication and properties. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–17. [Google Scholar]
- Noman, M.T.; Militky, J.; Wiener, J.; Saskova, J.; Ashraf, M.A.; Jamshaid, H.; Azeem, M. Sonochemical synthesis of highly crystalline photocatalyst for industrial applications. Ultrasonics 2018, 83, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Berardi, U. The benefits of using aerogel-enhanced systems in building retrofits. Energy Procedia 2017, 134, 626–635. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-H.; Ding, Y.-D.; Wang, F.; Deng, Z.-P. Thermal insulation material based on SiO2 aerogel. Constr. Build. Mater. 2016, 122, 548–555. [Google Scholar] [CrossRef]
- Hanif, A.; Diao, S.; Lu, Z.; Fan, T.; Li, Z. Green lightweight cementitious composite incorporating aerogels and fly ash cenospheres–Mechanical and thermal insulating properties. Constr. Build. Mater. 2016, 116, 422–430. [Google Scholar] [CrossRef]
- Al Zaidi, I.K.; Demirel, B.; Atis, C.D.; Akkurt, F. Investigation of mechanical and thermal properties of nano SiO2/hydrophobic silica aerogel co-doped concrete with thermal insulation properties. Struct. Concr. 2020, 21, 1123–1133. [Google Scholar] [CrossRef]
- Biesmans, G.; Mertens, A.; Duffours, L.; Woignier, T.; Phalippou, J. Polyurethane based organic aerogels and their transformation into carbon aerogels. J. Non-Cryst. Solids 1998, 225, 64–68. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, R.; Wu, D.; Xu, W.; Ye, Q.; Chen, Z. Preparation and characterization of antibacterial silver-dispersed activated carbon aerogels. Carbon 2004, 42, 3209–3216. [Google Scholar] [CrossRef]
- Krumm, M.; Pueyo, C.L.; Polarz, S. Monolithic zinc oxide aerogels from organometallic sol−gel precursors. Chem. Mater. 2010, 22, 5129–5136. [Google Scholar] [CrossRef]
- Yue, X.; Xiang, J.; Chen, J.; Li, H.; Qiu, Y.; Yu, X. High surface area, high catalytic activity titanium dioxide aerogels prepared by solvothermal crystallization. J. Mater. Sci. Technol. 2020, 47, 223–230. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Ko, E.; Kim, H. Preparation of chitosan aerogel crosslinked in chemical and ionical ways by non-acid condition for wound dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, F.; Wang, W.; Wang, W.; Su, Y.; Jiang, F.; Chen, S.; Riffat, S. Sound absorption characteristics of KGM-based aerogel. Int. J. Low-Carbon Technol. 2020, 15, 450–457. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and applications of ZnO nanostructures (ZONSs): A review. Crit. Rev. Solid State Mater. Sci. 2021, 1–43. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M. Prediction of functional properties of nano TiO2 coated cotton composites by artificial neural network. Sci. Rep. 2021, 11, 12235. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Jamshaid, H.; Ali, A. A novel green stabilization of TiO2 nanoparticles onto cotton. Fibers Polym. 2018, 19, 2268–2277. [Google Scholar] [CrossRef]
- Qian, F.; Troksa, A.; Fears, T.M.; Nielsen, M.H.; Nelson, A.J.; Baumann, T.F.; Kucheyev, S.O.; Han, T.Y.-J.; Bagge-Hansen, M. Gold aerogel monoliths with tunable ultralow densities. Nano Lett. 2019, 20, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Lan, P.C.; Freyman, M.C.; Chen, W.; Kou, T.; Olson, T.Y.; Zhu, C.; Worsley, M.A.; Duoss, E.B.; Spadaccini, C.M. Ultralight conductive silver nanowire aerogels. Nano Lett. 2017, 17, 7171–7176. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Brown, E.; Su, Q.; Li, J.; Wang, J.; Xu, C.; Zhou, C.; Lin, D. 3D printing hierarchical silver nanowire aerogel with highly compressive resilience and tensile elongation through tunable poisson’s ratio. Small 2017, 13, 1701756. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Nie, P.; Cheng, Y.; Lu, X.; Shi, L.; Sun, J. Copper nanowire-based aerogel with tunable pore structure and its application as flexible pressure sensor. ACS Appl. Mater. Interfaces 2017, 9, 14273–14280. [Google Scholar] [CrossRef]
- Schwertfeger, F.; Frank, D.; Schmidt, M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non-Cryst. Solids 1998, 225, 24–29. [Google Scholar] [CrossRef]
- Carlson, G.; Lewis, D.; McKinley, K.; Richardson, J.; Tillotson, T. Aerogel commercialization: Technology, markets and costs. J. Non-Cryst. Solids 1995, 186, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel based thermal insulating cementitious composites: A review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Patil, S.P.; Shendye, P.; Markert, B. Molecular dynamics simulations of silica aerogel nanocomposites reinforced by glass fibers, graphene sheets and carbon nanotubes: A comparison study on mechanical properties. Compos. Part B Eng. 2020, 190, 107884. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L.; Cheng, X.; He, S.; Li, C.; Zhang, H. Flexible silica aerogel composites strengthened with aramid fibers and their thermal behavior. Mater. Des. 2016, 99, 349–355. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Gong, L.; Zhang, H. Aramid fibers reinforced silica aerogel composites with low thermal conductivity and improved mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 84, 316–325. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Noman, M.T.; Wiener, J.; Saskova, J.; Ashraf, M.A.; Vikova, M.; Jamshaid, H.; Kejzlar, P. In-situ development of highly photocatalytic multifunctional nanocomposites by ultrasonic acoustic method. Ultrason. Sonochem. 2018, 40, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Bi-functional renewable biopolymer wrapped CNFs/Ag doped spinel cobalt oxide as a sensitive platform for highly toxic nitroaromatic compound detection and degradation. Chemosphere 2021, 132998. [Google Scholar] [CrossRef]
- Muñoz-Ruíz, A.; Escobar-García, D.M.; Quintana, M.; Pozos-Guillén, A.; Flores, H. Synthesis and characterization of a new collagen-alginate aerogel for tissue engineering. J. Nanomater. 2019, 2019, 2875375. [Google Scholar] [CrossRef] [Green Version]
- Osorio, D.A.; Lee, B.E.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Peces, M.V.; Pérez-Moreno, A.; de-Los-Santos, D.M.; Mesa-Díaz, M.d.M.; Pinaglia-Tobaruela, G.; Vilches-Pérez, J.I.; Fernández-Montesinos, R.; Salido, M.; de la Rosa-Fox, N.; Piñero, M. Chitosan-GPTMS-Silica Hybrid Mesoporous Aerogels for Bone Tissue Engineering. Polymers 2020, 12, 2723. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Subrahmanyam, R.; Gurikov, P.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Cellulose aerogel micro fibers for drug delivery applications. Mater. Sci. Eng. C 2021, 127, 112196. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Miranda, S.; Riemma, S.; Iannone, R. LCA of starch aerogels for biomedical applications. Chem. Eng. Trans. 2016, 49, 319–324. [Google Scholar]
- Saadatnia, Z.; Mosanenzadeh, S.G.; Chin, M.M.; Naguib, H.E.; Popovic, M.R. Flexible, Air Dryable, and Fiber Modified Aerogel-Based Wet Electrode for Electrophysiological Monitoring. IEEE Trans. Biomed. Eng. 2020, 68, 1820–1827. [Google Scholar] [CrossRef]
- Tetik, H.; Zhao, K.; Shah, N.; Lin, D. 3D freeze-printed cellulose-based aerogels: Obtaining truly 3D shapes, and functionalization with cross-linking and conductive additives. J. Manuf. Process. 2021, 68, 445–453. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M. Prediction of Methylene Blue Removal by Nano TiO2 Using Deep Neural Network. Polymers 2021, 13, 3104. [Google Scholar] [CrossRef]
- Noman, M.T.; Petrů, M. Functional properties of sonochemically synthesized zinc oxide nanoparticles and cotton composites. Nanomaterials 2020, 10, 1661. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Ultrasensitive detection of food colorant sunset yellow using nickel nanoparticles promoted lettuce-like spinel Co3O4 anchored GO nanosheets. Food Chem. Toxicol. 2021, 159, 112725. [Google Scholar] [CrossRef]
- Strobach, E.; Bhatia, B.; Yang, S.; Zhao, L.; Wang, E.N. High temperature annealing for structural optimization of silica aerogels in solar thermal applications. J. Non-Cryst. Solids 2017, 462, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, Y.; Wen, Z.-X.; Qiu, Y. An evacuated receiver partially insulated by a solar transparent aerogel for parabolic trough collector. Energy Convers. Manag. 2020, 214, 112911. [Google Scholar] [CrossRef]
- Han, L.; Dong, L.; Zhang, H.; Li, F.; Tian, L.; Li, G.; Jia, Q.; Zhang, S. Thermal insulation TiN aerogels prepared by a combined freeze-casting and carbothermal reduction-nitridation technique. J. Eur. Ceram. Soc. 2021, 41, 5127–5137. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Li, Y.; Cui, S.; Shen, X.; Tan, G. Hydrophobic in-situ SiO2-TiO2 composite aerogel for heavy oil thermal recovery: Synthesis and high temperature performance. Appl. Therm. Eng. 2021, 190, 116745. [Google Scholar] [CrossRef]
- Long, S.; Feng, Y.; He, F.; Zhao, J.; Bai, T.; Lin, H.; Cai, W.; Mao, C.; Chen, Y.; Gan, L. Biomass-derived, multifunctional and wave-layered carbon aerogels toward wearable pressure sensors, supercapacitors and triboelectric nanogenerators. Nano Energy 2021, 85, 105973. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, G.; Zhong, M.; Zhou, X. Dispersed and size-selected WO3 nanoparticles in carbon aerogel for supercapacitor applications. Mater. Des. 2018, 141, 220–229. [Google Scholar] [CrossRef]
- Muniyandi, T.M.; Balamurugan, S.; Naresh, N.; Prakash, I.; Venkatesh, R.; Deshpande, U.; Satyanarayana, N. Li2FeSiO4/C aerogel: A promising nanostructured cathode material for lithium-ion battery applications. J. Alloys Compd. 2021, 887, 161341. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Li, C.; Hu, Y.; Fang, L.; Yang, Q.; Shi, Z.; Xiong, C. Incorporation of Fe3O4 nanoparticles in three-dimensional carbon nanofiber/carbon nanotube aerogels for high-performance anodes of lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127716. [Google Scholar] [CrossRef]
- Jiang, X.; Ban, C.; Li, L.; Li, H.; Hao, J.; Chen, W.; Liu, X. Design of thermoelectric battery based on BN aerogels and Bi2Te3 composites. J. Alloys Compd. 2021, 887, 161280. [Google Scholar] [CrossRef]
- Bhamare, V.S.; Kulkarni, R.M.; Khan, A.A.P. Adsorptive removals of pollutants using aerogels and its composites. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–199. [Google Scholar]
- Eniola, J.O.; Ansari, M.O.; Barakat, M.; Kumar, R. Aerogels in photocatalysis. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–108. [Google Scholar]
- Moheman, A.; Bhawani, S.A.; Tariq, A. Aerogels for waterborne pollutants purification. In Advances in Aerogel Composites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–124. [Google Scholar]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Golder, S.; Narayanan, R.; Hossain, M.; Islam, M.R. Experimental and CFD Investigation on the Application for Aerogel Insulation in Buildings. Energies 2021, 14, 3310. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Wang, J.; Yan, F. The enhanced transfer behavior and tribological properties in deep sea environment of poly (butylene terephthalate) composites reinforced by silica nanoaerogels. Tribol. Int. 2021, 160, 107051. [Google Scholar] [CrossRef]
- Amor, N.; Noman, M.T.; Petru, M.; Mahmood, A.; Ismail, A. Neural network-crow search model for the prediction of functional properties of nano TiO2 coated cotton composites. Sci. Rep. 2021, 11, 13649. [Google Scholar]
- Noman, M.T.; Petru, M. Effect of Sonication and Nano TiO2 on Thermophysiological Comfort Properties of Woven Fabrics. ACS Omega 2020, 5, 11481–11490. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamedsoltani, L. Managing of gas sensing characteristic of a reduced graphene oxide based gas sensor by the change in synthesis condition: A new approach for electronic nose design. Mater. Chem. Phys. 2016, 183, 181–190. [Google Scholar] [CrossRef]
- Thubsuang, U.; Sukanan, D.; Sahasithiwat, S.; Wongkasemjit, S.; Chaisuwan, T. Highly sensitive room temperature organic vapor sensor based on polybenzoxazine-derived carbon aerogel thin film composite. Mater. Sci. Eng. B 2015, 200, 67–77. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Xie, X.; Tao, K.; Liu, C.; Khor, K.A.; Miao, J.; Norford, L.K. 3D superhydrophobic reduced graphene oxide for activated NO2 sensing with enhanced immunity to humidity. J. Mater. Chem. A 2018, 6, 478–488. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, J.; Zou, X.; Pang, X.; Yang, R.; Chen, S.; Fang, Y.; Shao, T.; Luo, X.; Zhang, L. Three-dimensional TiO2/SiO2 composite aerogel films via atomic layer deposition with enhanced H2S gas sensing performance. Ceram. Int. 2018, 44, 1078–1085. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D graphene aerogel-ZnO composites as efficient detection for NO2 at room temperature. Sens. Actuators B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Dong, Y.; Chi, Y.; Chen, G. Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal. Chem. 2013, 85, 8065–8069. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wu, C.-L. Electrical sensing properties of silica aerogel thin films to humidity. Thin Solid Films 2006, 496, 658–664. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ahmadian, F. Thiourea-treated graphene aerogel as a highly selective gas sensor for sensing of trace level of ammonia. Anal. Chim. Acta 2015, 897, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ma, Y.; Song, P.; Leng, J.; Wang, Q. Gas sensor based on rGO/ZnO aerogel for efficient detection of NO2 at room temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 10058–10069. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Santiago, K.S.; Jia, H.-W.; Ahmed, M.M.; Lin, Y.-F.; Yeh, J.-M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Dai, S.; Zhou, X.; Dong, X.; Jiang, Y.; Chen, Y.; Yuan, N.; Ding, J. A highly sensitive piezoresistive sensor based on CNT-rGO aerogel for human motion detection. J. Compos. Mater. 2021, 55, 00219983211020110. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, J.; Chen, S.; Varley, R.J.; Pan, K. 1D/2D nanomaterials synergistic, compressible, and response rapidly 3D graphene aerogel for piezoresistive sensor. Adv. Funct. Mater. 2020, 30, 2003618. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, X.; An, J.; Chen, Z.; Zhang, X. Highly sensitive, flexible, green synthesized graphene/biomass aerogels for pressure sensing application. Compos. Sci. Technol. 2021, 207, 108730. [Google Scholar] [CrossRef]
- Bi, Y.; Hei, Y.; Wang, N.; Liu, J.; Ma, C.-B. Synthesis of a clustered carbon aerogel interconnected by carbon balls from the biomass of taros for construction of a multi-functional electrochemical sensor. Anal. Chim. Acta 2021, 1164, 338514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, H.; Zhang, S.; Lai, X.; Zeng, X. Superhydrophobic MXene@ carboxylated carbon nanotubes/carboxymethyl chitosan aerogel for piezoresistive pressure sensor. Chem. Eng. J. 2021, 425, 130462. [Google Scholar] [CrossRef]







| Aerogel Type | Precursor | Surface Area [m2·g−1] | Density [kg·m−3] | Porosity | Pore Size | Thermal Conductivity [W·m−1·K−1] | Reference |
|---|---|---|---|---|---|---|---|
| Silica aerogels | C4H12O3Si | 600–1000 | 350 | 85–99.9% | 1–100 nm | 0.010–0.020 | [33] |
| Silica aerogels | Na2SiO3 | 600–1000 | 300–350 | - | 20 nm | - | [34] |
| Silica aerogels | C4H12O3Si | 576 | 100 | >90% | 20–100 nm | 0.020 | [35] |
| Silica aerogels | Na2SiO3 | 366 | 40–150 | >90% | 20–100 nm | - | [36] |
| Silica aerogels | Na2SiO3 | 300–400 | 50–80 | 98% | 20–40 nm | 0.016–0.020 | [37] |
| Carbon aerogels | C3H8N2O | 300 | 0.24 | 99% | - | - | [38] |
| Carbon aerogels | C2H3Cl | 1600 | - | 98% | 2 nm | - | [39] |
| Silver aerogels | AgNO3 | 400 | 27 | 98% | 10–100 nm | - | [40] |
| Zinc aerogels | ZnC4H6O4 | 350 | - | 99% | - | - | [41] |
| Titanium aerogels | C16H36O4Ti | 300 | - | 98% | 20 | - | [42] |
| Cellulose aerogels | Wood | - | - | 98% | - | - | [43] |
| Chitosan aerogels | Chitosan powder | 400 | - | 99% | - | - | [44] |
| Biomass aerogels | Konjac glucomannan | - | 47 | 95% | - | - | [45] |
| Aerogel Type | Analyte | Response/Recovery Rate | Sensing Range | Detection Limit | References |
|---|---|---|---|---|---|
| Graphene aerogel | Ammonia | 100 s/500 s | 0.02–85 ppm | 10 ppb | [94] |
| Carbon aerogel | Toluene and n-hexane | 25 s/20 s | - | - | [95] |
| Graphene aerogel | NO2 | 116 s/169 s | 0.1–1 ppm | 50 ppb | [96] |
| TiO2/SiO2 aerogels | H2S | 53 s/74 s | 0.5–50 ppm | 0.5 ppm | [97] |
| ZnO/graphene aerogels | NO2 | 132 s/164 s | 10–200 ppm | 10 ppm | [98] |
| Silica aerogels/ Carbon quantum dots | NO2 | - | 2–10 ppm | 250 ppb | [99] |
| Silica aerogel film | Humidity | 38 s/21 s | 20–90% RH | - | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. https://doi.org/10.3390/gels7040264
Noman MT, Amor N, Ali A, Petrik S, Coufal R, Adach K, Fijalkowski M. Aerogels for Biomedical, Energy and Sensing Applications. Gels. 2021; 7(4):264. https://doi.org/10.3390/gels7040264
Chicago/Turabian StyleNoman, Muhammad Tayyab, Nesrine Amor, Azam Ali, Stanislav Petrik, Radek Coufal, Kinga Adach, and Mateusz Fijalkowski. 2021. "Aerogels for Biomedical, Energy and Sensing Applications" Gels 7, no. 4: 264. https://doi.org/10.3390/gels7040264
APA StyleNoman, M. T., Amor, N., Ali, A., Petrik, S., Coufal, R., Adach, K., & Fijalkowski, M. (2021). Aerogels for Biomedical, Energy and Sensing Applications. Gels, 7(4), 264. https://doi.org/10.3390/gels7040264









