Effect of Alkaline Earth Metal on AZrOx (A = Mg, Sr, Ba) Memory Application
Abstract
:1. Introduction
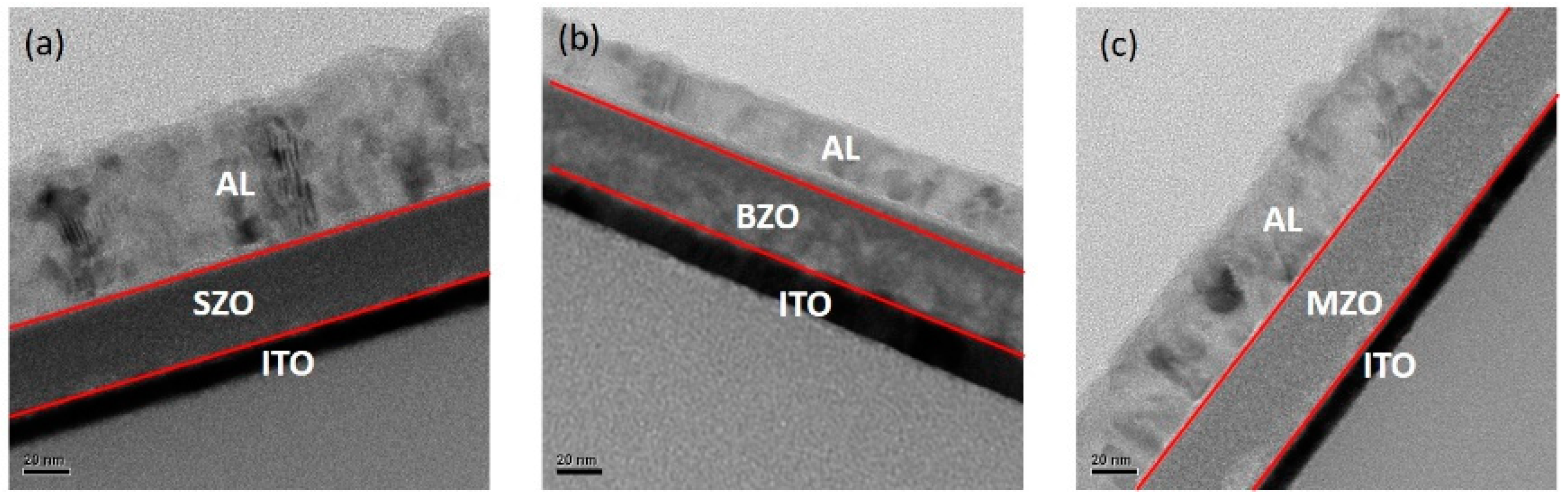
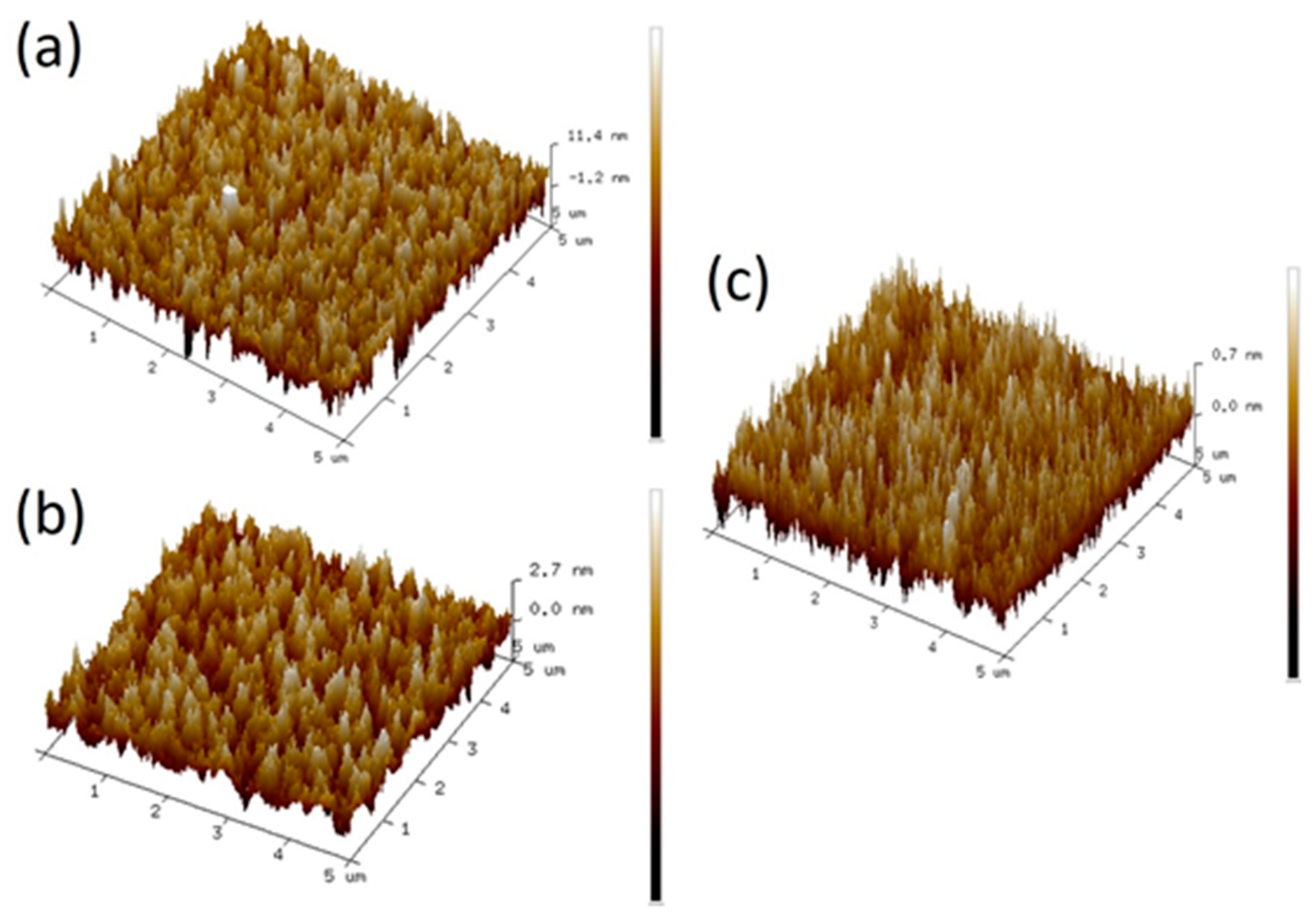
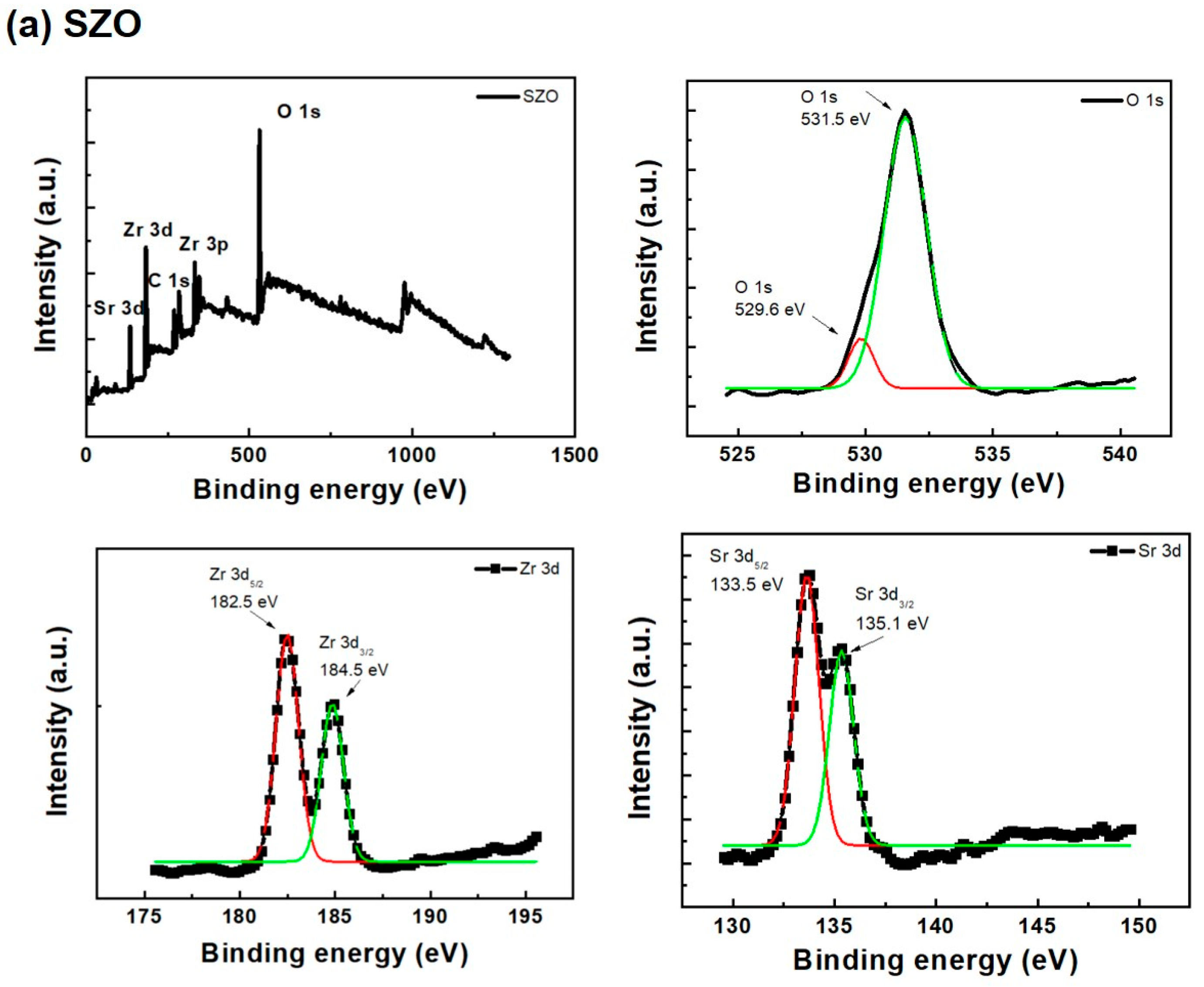
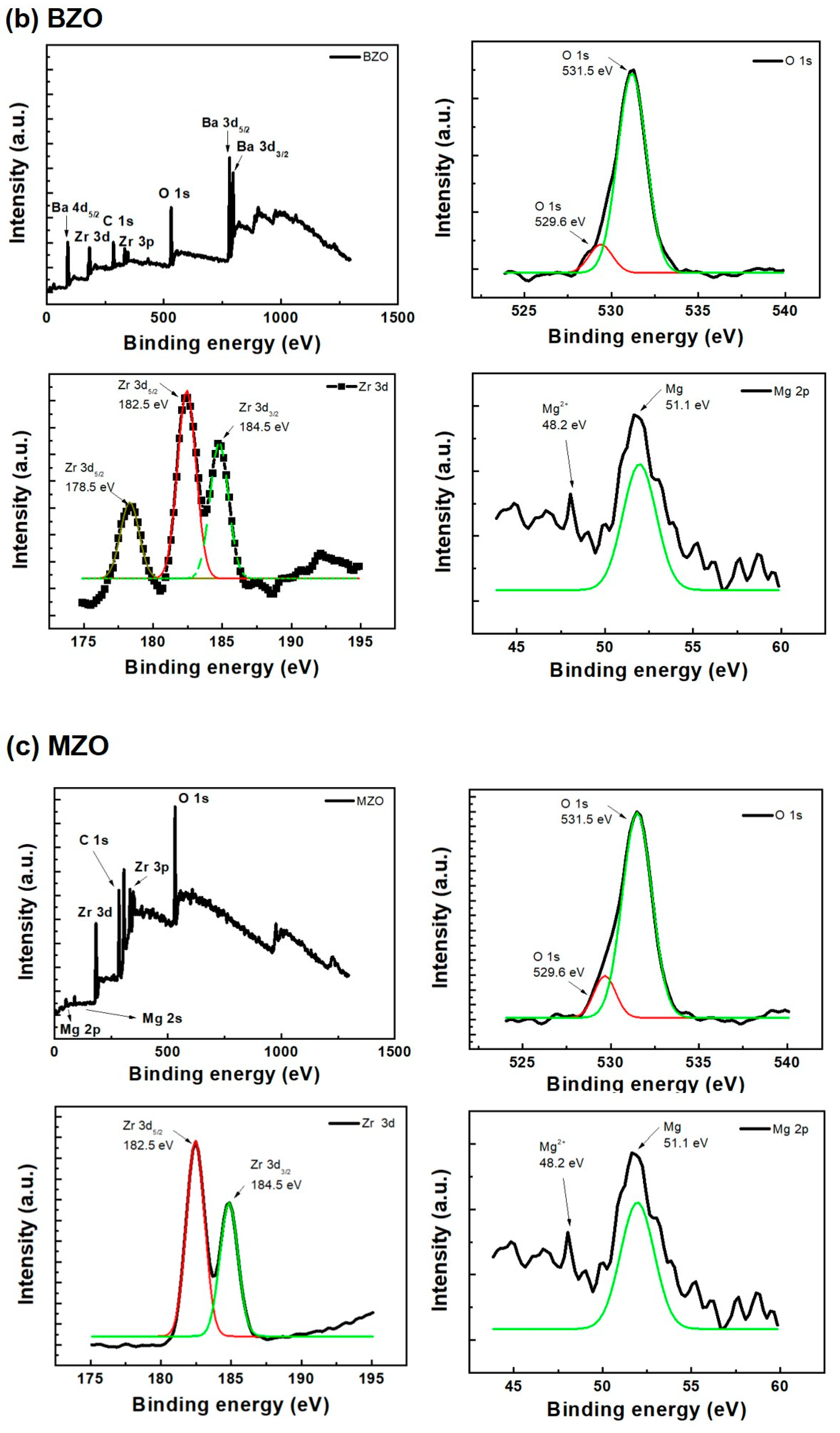
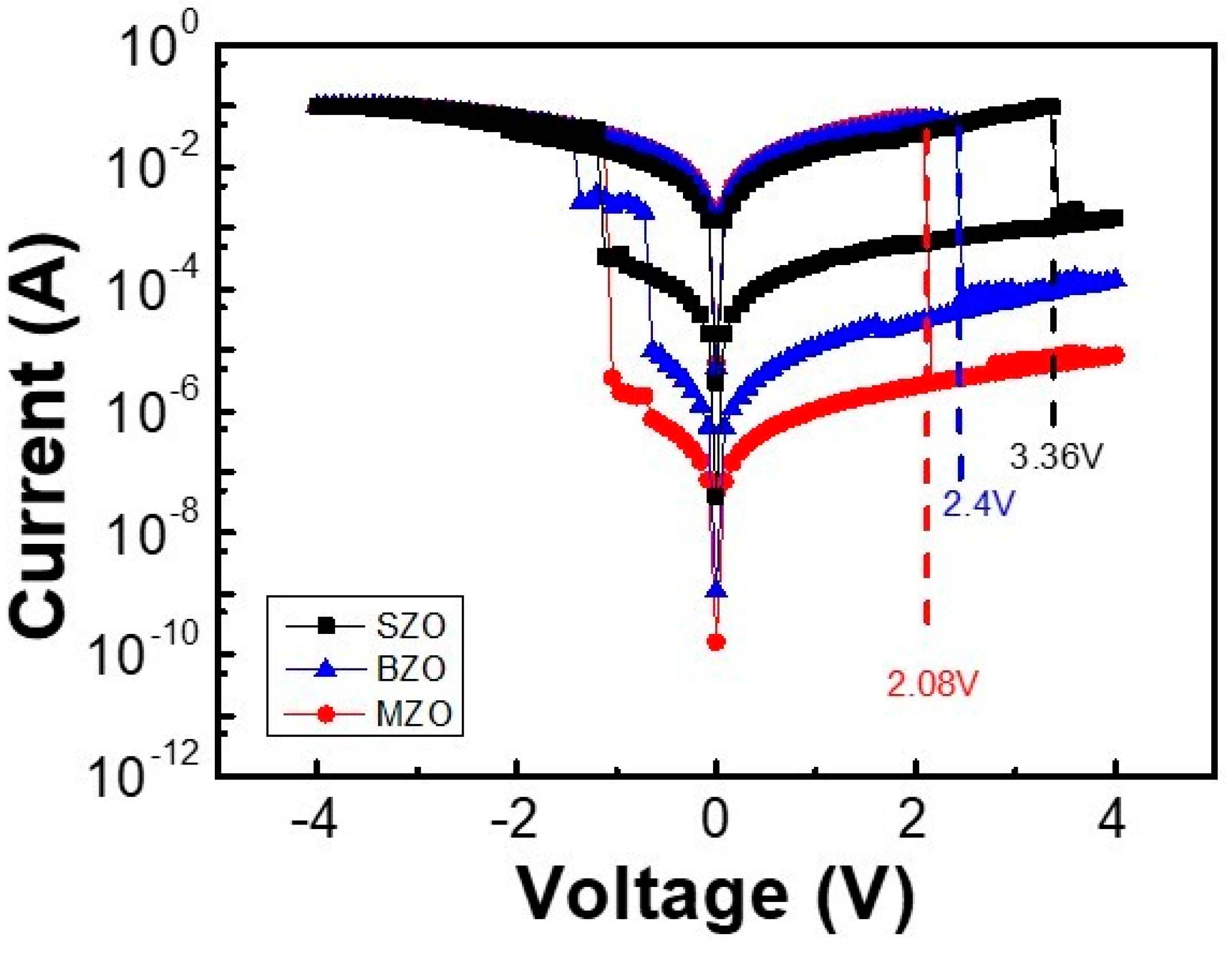
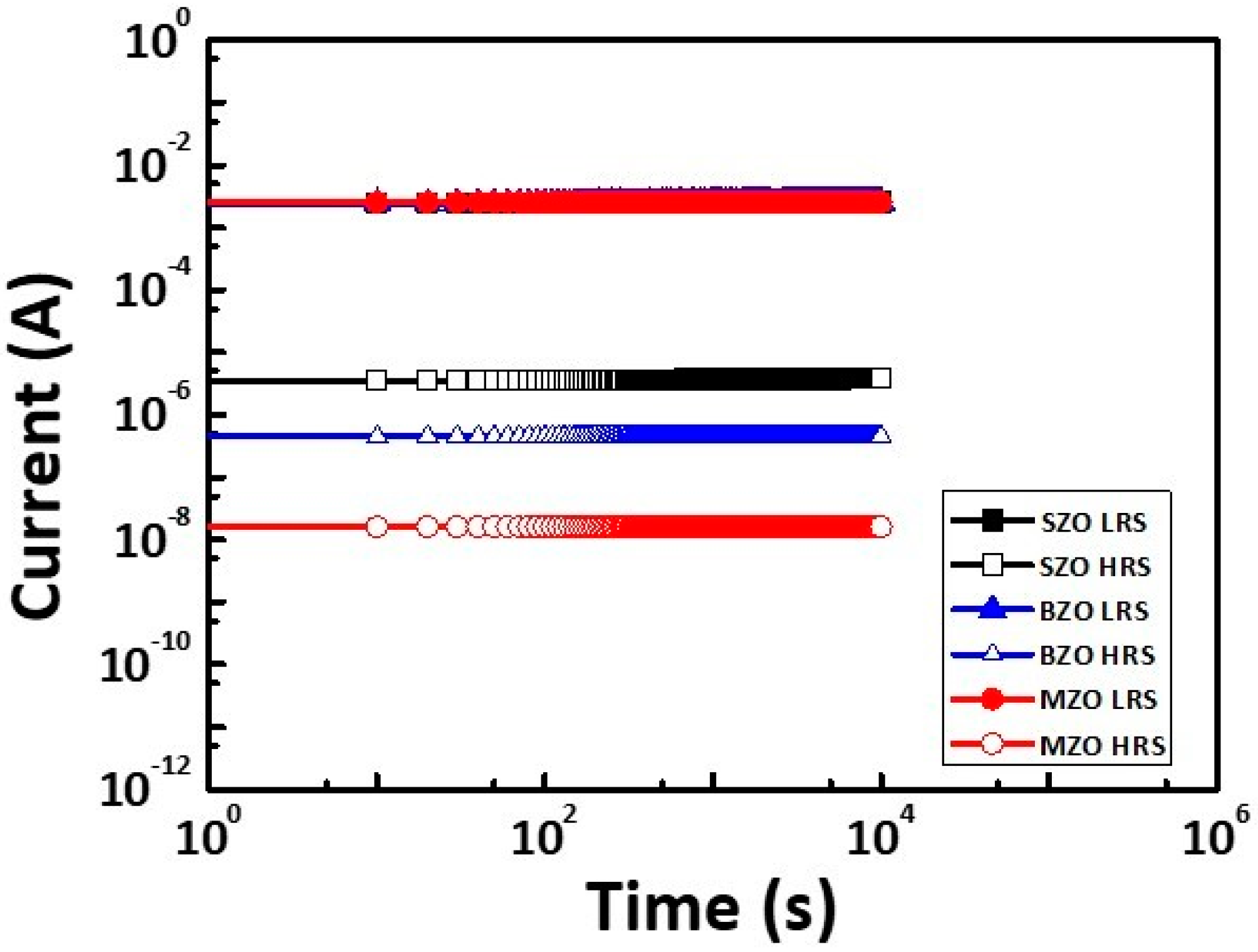
2. Results and Discussion
3. Conclusions
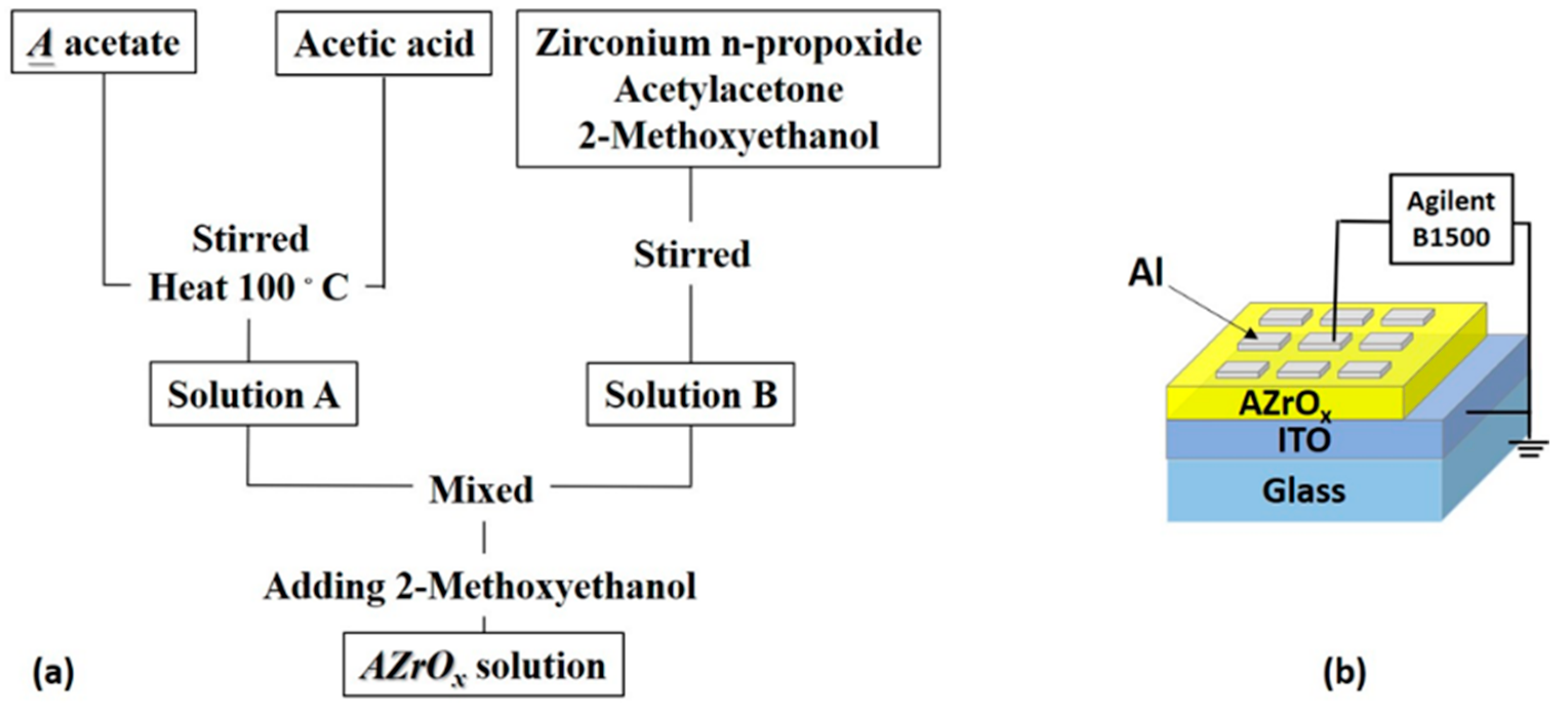
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.Y.; Wang, S.Y.; Tseng, T.Y. Ti-induced recovery phenomenon of resistive switching in ZrO2 thin films. J. Electrochem. Soc. 2010, 157, G166–G169. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lee, D.Y.; Tseng, T.Y.; Lin, C.Y. Effects of Ti top electrode thickness on the resistive switching behaviors of RF-sputtered ZrO2 memory films. Appl. Phys. Lett. 2009, 95, 112904. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, C.Y.; Wu, C.Y.; Hu, C.; Tseng, T.Y. Modified resistive switching behavior of ZrO2 memory films based on the interface layer formed by using Ti top electrode. J. Appl. Phys. 2007, 102, 094101. [Google Scholar] [CrossRef]
- Lee, D.Y.; Tseng, T.Y. Unipolar resistive switching characteristics of a ZrO2 memory device with oxygen ion conductor buffer layer. IEEE Electron. Device Lett. 2012, 33, 803–805. [Google Scholar] [CrossRef]
- Li, Y.; Long, S.; Lv, H.; Liu, Q.; Wang, Y.; Zhang, S.; Lian, W.; Wang, M.; Zhang, K.; Xie, H. Improvement of resistive switching characteristics in ZrO2 film by embedding a thin TiOx layer. Nanotechnology 2011, 22, 254028. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.S.; Tian, M.; Park, S.E. CO2 reforming of methane over modified Ni/ZrO2 catalysts. Appl. Organomet. Chem. 2001, 15, 109–112. [Google Scholar] [CrossRef]
- Azad, A.-M.; Subramaniam, S. Temperature dependence of the dielectric response of BaZrO3 by immittance spectroscopy. Mater. Res. Bull. 2002, 37, 11–21. [Google Scholar] [CrossRef]
- Davies, R.A.; Islam, M.S.; Gale, J.D. Dopant and proton incorporation in perovskite-type zirconates. Solid State Ion. 1999, 126, 323–325. [Google Scholar] [CrossRef]
- Kamitani, A.; Wakana, H.; Adachi, S.; Tanabe, K. Investigation of BaZrO3 and SrZrO3 insulating layers on La–YBCO ground plane for high-Tc devices. Phys. C Supercond. 2004, 412–414, 1414–1418. [Google Scholar] [CrossRef]
- Porter, D.L.; Heuew, A.H. Mechanisms of Toughening Partially Stabilized Zirconia (PSZ). J. Am. Ceram. Soc. 1977, 60, 183–184. [Google Scholar] [CrossRef]
- Lee, K.J.; Chang, Y.C.; Lee, C.J.; Wang, L.W.; Wang, Y.H. Resistive switching properties of alkaline earth oxide-based memory devices. Microelectron. Reliab. 2018, 83, 281–285. [Google Scholar] [CrossRef]
- Hwang, Y.H.; An, H.M.; Cho, W.J. Performance improvement of the resistive memory properties of InGaZnO thin films by using microwave irradiation. Jpn. J. Appl. Phys. 2014, 53, 04EJ04. [Google Scholar] [CrossRef]
- Carlos, E.; Branquinho, R.; Martins, R.; Kiazadeh, A.; Fortunato, E. Recent Progress in Solution-Based Metal Oxide Resistive Switching Devices. Adv. Mater. 2021, 33, 2004328. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Zhai, F.; Yi, Z.-Z.; Yin, X.-T.; Dastan, D.; Tajabadi, F.; Jafari, A.; Abbasi, S. Mixed conductivity and the conduction mechanism of the orthorhombic CaZrO3 based materials. Surf. Interfaces 2021, 23, 100905. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, K.J.; Lee, C.J.; Wang, L.W.; Wang, Y.H. Bipolar resistive switching behavior in sol-gel MgTiNiOx memory device. IEEE J. Electron. Devices Soc. 2016, 4, 321–327. [Google Scholar] [CrossRef]
- Lee, K.J.; Chang, Y.C.; Lee, C.J.; Wang, L.W.; Wang, Y.H. Bipolar Resistive Switching Characteristics in Flexible Pt/MZT/Al Memory and Ni/NbO2/Ni Selector Structure. IEEE J. Electron. Devices Soc. 2018, 6, 518–524. [Google Scholar] [CrossRef]
- Chang, Y.C.; Xue, R.Y.; Wang, Y.H. Multilayered barium titanate thin films by sol-gel method for nonvolatile memory application. IEEE Trans. Electron. Devices 2014, 61, 4090–4096. [Google Scholar] [CrossRef]
- Lee, K.J.; Wang, L.W.; Chiang, T.K.; Wang, Y.H. Effects of electrodes on the switching behavior of strontium titanate nickelate resistive random access memory. Materials 2015, 8, 7191–7198. [Google Scholar] [CrossRef] [Green Version]
- Sowinska, M.; Bertaud, T.; Walczyk, D.; Thiess, S.; Calka, P.; Alff, L.; Walczyk, C.; Schroeder, T. In-operando hard X-ray photoelectron spectroscopy study on the impact of current compliance and switching cycles on oxygen and carbon defects in resistive switching Ti/HfO2/TiN cells. J. Appl. Phys. 2014, 115, 204509. [Google Scholar] [CrossRef] [Green Version]
- Miao, B.; Mahapatra, R.; Wright, N.; Horsfall, A. The role of carbon contamination in voltage linearity and leakage current in high-k metal-insulator-metal capacitors. J. Appl. Phys. 2008, 104, 054510. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lee, C.-J.; Wang, L.-W.; Wang, Y.-H. Highly Uniform Resistive Switching Properties of Solution-Processed Silver-Embedded Gelatin Thin Film. Small 2018, 14, 1703888. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database; NIST Standard Reference Databas Version 4.1; NIST: Gaithersburg, MA, USA, 2012. [Google Scholar]
- Chang, W.Y.; Ho, Y.T.; Hsu, T.C.; Chen, F.; Tsai, M.J.; Wu, T.B. Influence of crystalline constituent on resistive switching properties of TiO2 memory films. Electrochem. Solid-State Lett. 2009, 12, H135–H137. [Google Scholar] [CrossRef]
- Huang, H.W.; Kang, C.F.; Lai, F.; He, J.H.; Lin, S.J.; Chueh, Y.L. Stability scheme of ZnO-thin film resistive switching memory: Influence of defects by controllable oxygen pressure ratio. Nanoscale Res. Lett. 2013, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastan, D.; Banpurkar, A. Solution processable sol–gel derived titania gate dielectric for organic field effect transistors. J. Mater. Sci. Mater. Electron. 2017, 28, 3851–3859. [Google Scholar] [CrossRef]
- Lee, K.-J.; Chang, Y.-C.; Lee, C.-J.; Wang, L.-W.; Wang, Y.-H. 1T1R Nonvolatile Memory with Al/TiO2/Au and Sol-Gel-Processed Insulator for Barium Zirconate Nickelate Gate in Pentacene Thin Film Transistor. Materials 2017, 10, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.S.; Lee, K.-J.; Wang, Y.H. Magnesium Zirconate Titanate Thin Films Used as an NO2 Sensing Layer for Gas Sensor Applications Developed Using a Sol–Gel Method. Sensors 2021, 21, 2825. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-J.; Wang, Y.-H. Effect of Alkaline Earth Metal on AZrOx (A = Mg, Sr, Ba) Memory Application. Gels 2022, 8, 20. https://doi.org/10.3390/gels8010020
Lee K-J, Wang Y-H. Effect of Alkaline Earth Metal on AZrOx (A = Mg, Sr, Ba) Memory Application. Gels. 2022; 8(1):20. https://doi.org/10.3390/gels8010020
Chicago/Turabian StyleLee, Ke-Jing, and Yeong-Her Wang. 2022. "Effect of Alkaline Earth Metal on AZrOx (A = Mg, Sr, Ba) Memory Application" Gels 8, no. 1: 20. https://doi.org/10.3390/gels8010020
APA StyleLee, K.-J., & Wang, Y.-H. (2022). Effect of Alkaline Earth Metal on AZrOx (A = Mg, Sr, Ba) Memory Application. Gels, 8(1), 20. https://doi.org/10.3390/gels8010020





