Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of High Refractive Index Hydrogels
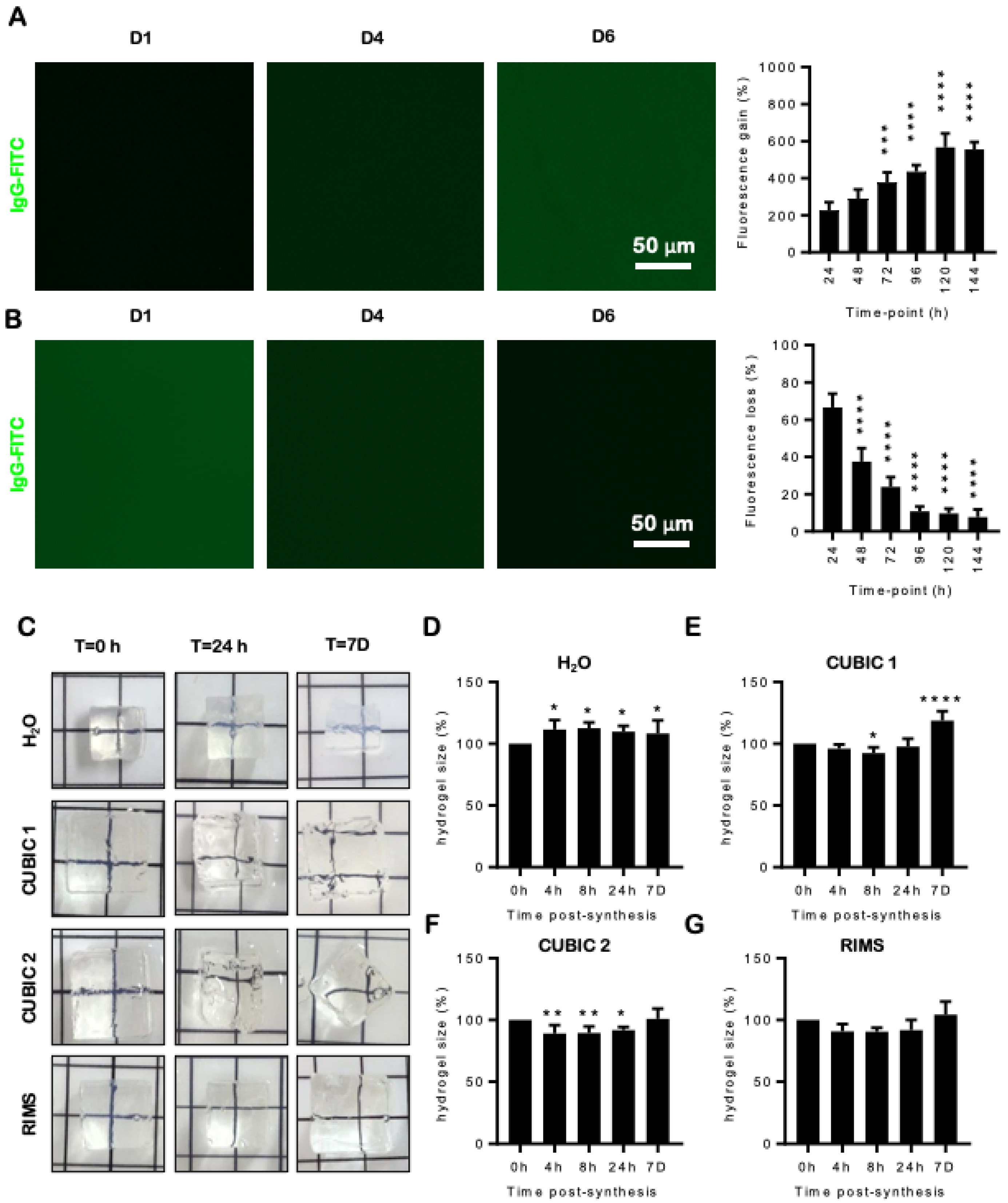
2.2. Penetration of Antibodies and Stability of High Refractive Index Hydrogels
2.3. Hydrogels Change Size and Shape in Different Refractive Index Solutions
2.4. Stable Imaging and 3D Reconstruction of Optically Cleared, Whole-Mount Mouse Organs
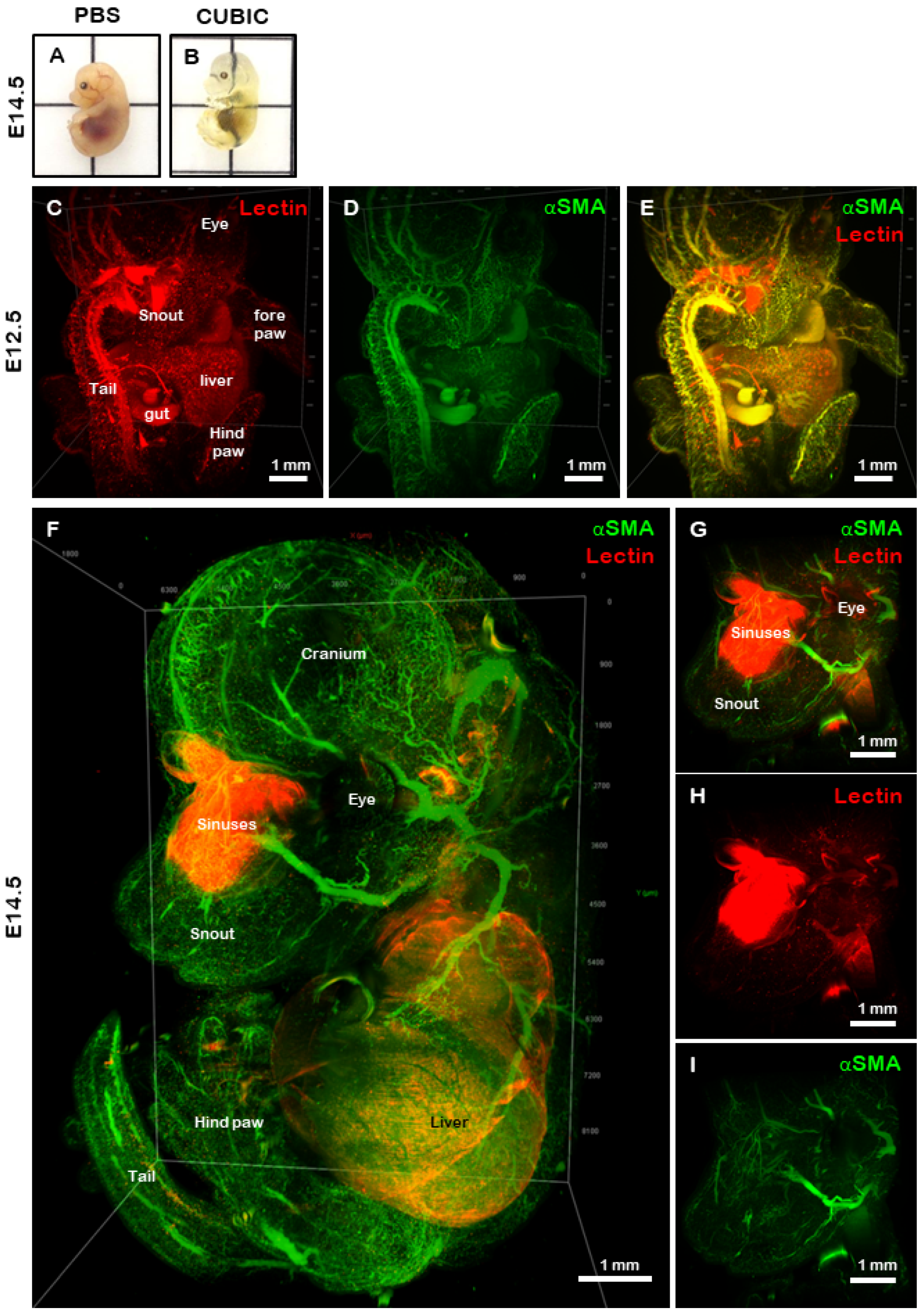
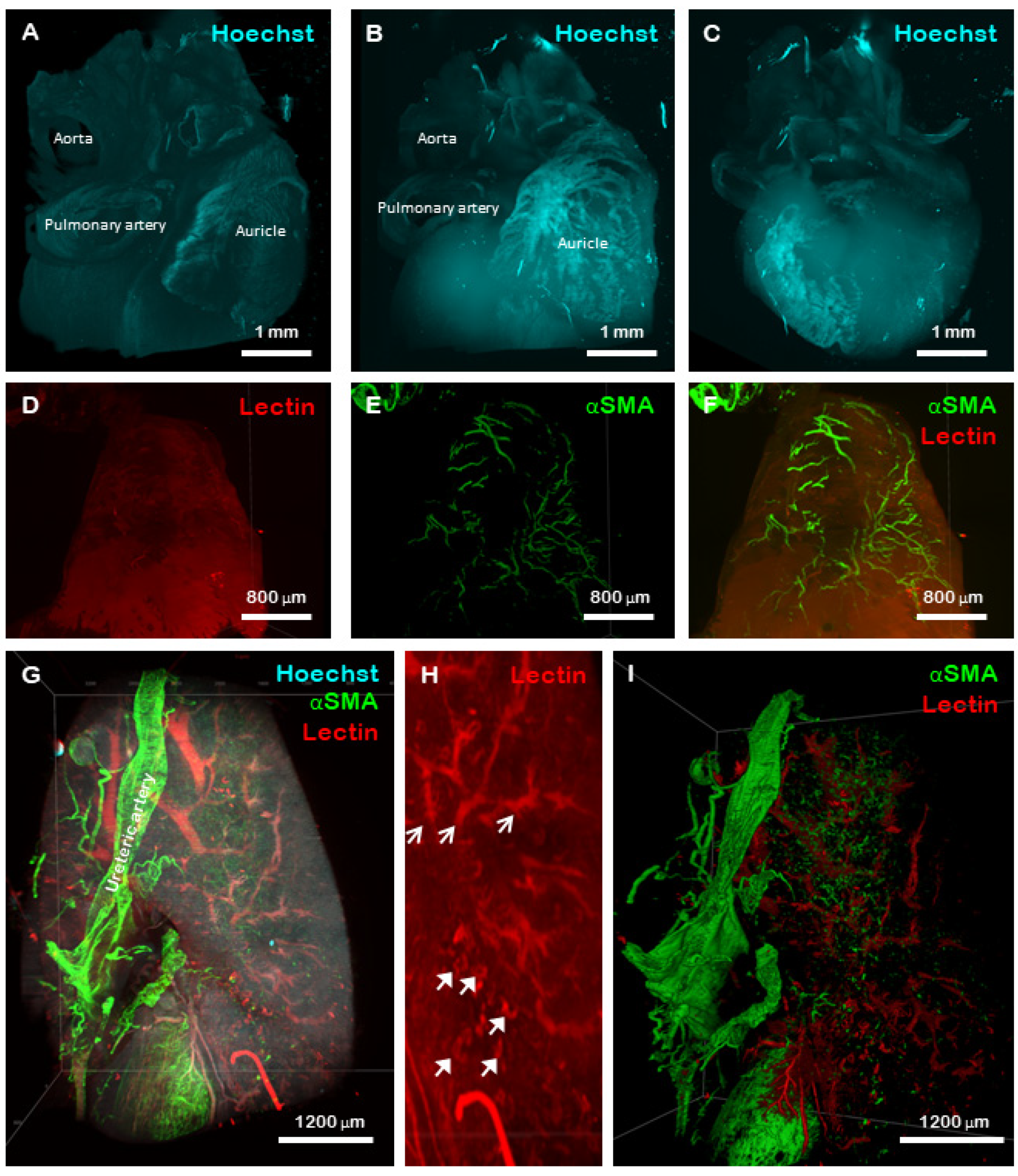
2.5. Discussion
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Synthesis
4.2. Water Content
4.3. Antibody Penetration
4.4. Hydrogel Size Change in Different Refractive Index-Matched Solutions
4.5. Animals
4.6. Tissue Clearing
4.7. Embedding Cleared Tissue in Hydrogel and Immunostaining
4.8. Light Sheet Fluorescence Microscopy (LSFM)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azaripour, A.; Lagerweij, T.; Scharfbillig, C.; Jadczak, A.E.; Willershausen, B.; Van Noorden, C.J. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog. Histochem. Cytochem. 2016, 51, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.; Lobo, E.P.; Delic, N.C.; Myerscough, M.; Lyons, G.; Wakefield, D.; Di Girolamo, N. Keratin-14-Positive Precursor Cells Spawn a Population of Migratory Corneal Epithelia that Maintain Tissue Mass throughout Life. Stem Cell Rep. 2017, 9, 1081–1096. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Deisseroth, K. CLARITY for mapping the nervous system. Nat. Methods 2013, 10, 508–513. [Google Scholar] [CrossRef]
- Chung, K.; Wallace, J.; Kim, S.-Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.; et al. Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Susaki, E.; Tainaka, K.; Perrin, D.; Kishino, F.; Tawara, T.; Watanabe, T.M.; Yokoyama, C.; Onoe, H.; Eguchi, M.; Yamaguchi, S.; et al. Whole-Brain Imaging with Single-Cell Resolution Using Chemical Cocktails and Computational Analysis. Cell 2014, 157, 726–739. [Google Scholar] [CrossRef] [Green Version]
- Susaki, E.; Tainaka, K.; Perrin, D.; Yukinaga, H.; Kuno, A.; Ueda, H. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 2015, 10, 1709–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susaki, E.; Ueda, H.R. Whole-body and Whole-Organ Clearing and Imaging Techniques with Single-Cell Resolution: Toward Organism-Level Systems Biology in Mammals. Cell Chem. Biol. 2016, 23, 137–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyagi, Y.; Kawakami, R.; Osanai, H.; Hibi, T.; Nemoto, T. A rapid optical clearing protocol using 2,2′-thiodiethanol for microscopic observation of fixed mouse brain. PLoS ONE 2015, 10, e0116280. [Google Scholar] [CrossRef] [Green Version]
- Ke, M.-T.; Fujimoto, S.; Imai, T. SeeDB: A simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 2013, 16, 1154–1161. [Google Scholar] [CrossRef]
- Hama, H.; Hioki, H.; Namiki, K.; Hoshida, T.; Kurokawa, H.; Ishidate, F.; Kaneko, T.; Akagi, T.; Saito, T.; Saido, T.C.; et al. ScaleS: An optical clearing palette for biological imaging. Nat. Neurosci. 2015, 18, 1518–1529. [Google Scholar] [CrossRef]
- Williams, M.P.I.; Rigon, M.; Straka, T.; Hörner, S.J.; Thiel, M.; Gretz, N.; Hafner, M.; Reischl, M.; Rudolf, R. A Novel Optical Tissue Clearing Protocol for Mouse Skeletal Muscle to Visualize Endplates in Their Tissue Context. Front. Cell. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Jahrling, N.; Saghafi, S.; Weiler, R.; Dodt, H.U. Chemical clearing and dehydration of GFP expressing mouse brains. PLoS ONE 2012, 7, e33916. [Google Scholar] [CrossRef]
- Ertürk, A.; Becker, K.; Jährling, N.; Mauch, C.P.; Hojer, C.D.; Egen, J.; Hellal, F.; Bradke, F.; Sheng, M.; Dodt, H.-U. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 2012, 7, 1983–1995. [Google Scholar] [CrossRef]
- Erturk, A.; Pan, C.; Cai, R.; Quacquarelli, F.P.; Gasemigharagoz, A. Whole organ and organism tissue clearing by uDISCO. Protoc. Exch. 2016. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, S.; Luo, W.; Gao, X.; Men, Y.; Ma, C.; Liu, X.; Yi, Y.; Bugde, A.; Zhou, B.O.; et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 2018, 28, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Power, R.; Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 2017, 14, 360–373. [Google Scholar] [CrossRef]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex-vivo optical clearing methods for biological tissues: Review. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Hillman, E.M.; Voleti, V.; Li, W.; Yu, H. Light-Sheet Microscopy in Neuroscience. Annu. Rev. Neurosci. 2019, 42, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Heath, D.E.; Sharif, A.R.M.; Rayatpisheh, S.; Oh, B.H.L.; Rong, X.; Beuerman, R.; Chan-Park, M.B. High Water Content Hydrogel with Super High Refractive Index. Macromol. Biosci. 2013, 13, 1485–1491. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Nojima, S.; Susaki, E.; Yoshida, K.; Takemoto, H.; Tsujimura, N.; Iijima, S.; Takachi, K.; Nakahara, Y.; Tahara, S.; Ohshima, K.; et al. CUBIC pathology: Three-dimensional imaging for pathological diagnosis. Sci. Rep. 2017, 7, 9269. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.-U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Hahn, C.M.; Saghafi, S.; Jährling, N.; Wanis, M.; Dodt, H.-U. Reduction of Photo Bleaching and Long Term Archiving of Chemically Cleared GFP-Expressing Mouse Brains. PLoS ONE 2014, 9, e114149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giovanna, A.P.; Credi, C.; Franceschini, A.; Müllenbroich, M.C.; Silvestri, L.; Pavone, F.S. Tailored Sample Mounting for Light-Sheet Fluorescence Microscopy of Clarified Specimens by Polydimethylsiloxane Casting. Front. Neuroanat. 2019, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Belle, M.; Godefroy, D.; Couly, G.; Malone, S.; Collier, F.; Giacobini, P.; Chédotal, A. Tridimensional Visualization and Analysis of Early Human Development. Cell 2017, 169, 161–173.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, A.; Fok, S.; Lee, V.; Rye, K.-A.; Di Girolamo, N.; Cochran, B.J. Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging. Gels 2022, 8, 32. https://doi.org/10.3390/gels8010032
Richardson A, Fok S, Lee V, Rye K-A, Di Girolamo N, Cochran BJ. Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging. Gels. 2022; 8(1):32. https://doi.org/10.3390/gels8010032
Chicago/Turabian StyleRichardson, Alexander, Sandra Fok, Victoria Lee, Kerry-Anne Rye, Nick Di Girolamo, and Blake J. Cochran. 2022. "Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging" Gels 8, no. 1: 32. https://doi.org/10.3390/gels8010032
APA StyleRichardson, A., Fok, S., Lee, V., Rye, K.-A., Di Girolamo, N., & Cochran, B. J. (2022). Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging. Gels, 8(1), 32. https://doi.org/10.3390/gels8010032






