Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Conductive Hydrogels Applied in Peripheral Nerve Injury
2.1. Conductive Polymers (CPs)-Incorporated CH
2.1.1. PEDOT-Incorporated CH
2.1.2. PANi-Incorporated CHs
2.1.3. PPy-Incorporated CHs
2.2. Carbon-Based Conductive Materials (CBCM)-Incorporated CHs
2.2.1. CNT-Incorporated CH
2.2.2. Graphene-Incorporated CH
3. Challenges and Futures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, C.; Liu, S.Y.; Pi, W.; Zhang, P.X. Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 2021, 16, 1518–1523. [Google Scholar] [CrossRef]
- Hewson, D.W.; Bedforth, N.M.; Hardman, J.G. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia 2018, 73 (Suppl. 1), 51–60. [Google Scholar] [CrossRef] [Green Version]
- Previtali, S.C. Peripheral Nerve Development and the Pathogenesis of Peripheral Neuropathy: The Sorting Point. Neurotherapeutics 2021. [Google Scholar] [CrossRef]
- Nazareth, L.; St John, J.; Murtaza, M.; Ekberg, J. Phagocytosis by Peripheral Glia: Importance for Nervous System Functions and Implications in Injury and Disease. Front. Cell Dev. Biol. 2021, 9, 660259. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, M.; Liu, S.-Y.; Zhang, F.-S.; Wan, T.; Ding, Z.-T.; Zhang, P.-X. Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers 2021, 13, 3957. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Ridgell, S.; Alpaugh, E.S.; Mouhaffel, A.; Kaye, A.J.; Cornett, E.M.; Chami, A.A.; Shah, R.; Dixon, B.M.; Viswanath, O.; et al. Peripheral Nerve Stimulation: A Review of Techniques and Clinical Efficacy. Pain Ther. 2021, 10, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Passero, J.V.; Rawat, A.; Ament, X.H.; Yang, F.; Vidensky, S.; Collins, S.L.; Horton, M.R.; Hoke, A.; Rutter, G.A.; et al. Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice. J. Clin. Investig. 2021, 131, e141964. [Google Scholar] [CrossRef]
- Fissel, J.A.; Farah, M.H. The influence of BACE1 on macrophage recruitment and activity in the injured peripheral nerve. J. Neuroinflamm. 2021, 18, 71. [Google Scholar] [CrossRef]
- Tricaud, N.; Park, H.T. Wallerian demyelination: Chronicle of a cellular cataclysm. Cell. Mol. Life Sci. 2017, 74, 4049–4057. [Google Scholar] [CrossRef] [Green Version]
- Quintes, S.; Brinkmann, B.G.; Ebert, M.; Frob, F.; Kungl, T.; Arlt, F.A.; Tarabykin, V.; Huylebroeck, D.; Meijer, D.; Suter, U.; et al. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat. Neurosci. 2016, 19, 1050–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Johnston, A.P. Schwann cells as drivers of tissue repair and regeneration. Curr. Opin. Neurobiol. 2017, 47, 52–57. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Zhou, L.; Li, C.; Zhang, M.; Zhang, F.; Ding, Z.; Wen, Y.; Zhang, P. XT-type DNA hydrogels loaded with VEGF and NGF promote peripheral nerve regeneration via a biphasic release profile. Biomater. Sci. 2021, 9, 8221–8234. [Google Scholar] [CrossRef]
- Li, R.; Li, D.H.; Zhang, H.Y.; Wang, J.; Li, X.K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Ino, D.; Iino, M. Schwann cell mitochondria as key regulators in the development and maintenance of peripheral nerve axons. Cell. Mol. Life Sci. 2017, 74, 827–835. [Google Scholar] [CrossRef]
- Wofford, K.L.; Shultz, R.B.; Burrell, J.C.; Cullen, D.K. Neuroimmune interactions and immunoengineering strategies in peripheral nerve repair. Prog. Neurobiol. 2021, 208, 102172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Baker, J.T.; Rudraiah, S.; Arul, M.R.; Vella, A.T.; Domb, A.J.; Kumbar, S.G. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J. Control. Release 2020, 317, 78–95. [Google Scholar] [CrossRef]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, e1800308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, L.; An, H.; Zhang, P.; Liu, P. Repair of Peripheral Nerve Injury Using Hydrogels Based on Self-Assembled Peptides. Gels 2021, 7, 152. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Zhou, L.P.; Pi, W.; Zhang, P.X. Polymer Scaffolds for Biomedical Applications in Peripheral Nerve Reconstruction. Molecules 2021, 26, 2712. [Google Scholar] [CrossRef]
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Nanotechnology in peripheral nerve repair and reconstruction. Adv. Drug Deliv. Rev. 2019, 148, 308–343. [Google Scholar] [CrossRef]
- Xue, W.; Shi, W.; Kong, Y.; Kuss, M.; Duan, B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater. 2021, 6, 4141–4160. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.R.; Jariwala, S.H.; Bilis, Z.; Loverde, J.R.; Pasquina, P.F.; Alvarez, L.M. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials 2018, 186, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Marchesini, A.; Pugliese, P.; De Francesco, F. Nerve repair and regeneration: Biological tubulization limits and future perspectives. J. Cell. Physiol. 2019, 234, 3362–3375. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, D.; Rosenfeld, D.; Anikeeva, P. Emerging Frontier of Peripheral Nerve and Organ Interfaces. Neuron 2020, 108, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Manousiouthakis, E.; Park, J.; Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Towards the translation of electroconductive organic materials for regeneration of neural tissues. Acta Biomater. 2021, in press. [CrossRef]
- Jin, F.; Li, T.; Yuan, T.; Du, L.; Lai, C.; Wu, Q.; Zhao, Y.; Sun, F.; Gu, L.; Wang, T.; et al. Physiologically Self-Regulated, Fully Implantable, Battery-Free System for Peripheral Nerve Restoration. Adv. Mater. 2021, 33, e2104175. [Google Scholar] [CrossRef]
- Yao, X.; Qian, Y.; Fan, C. Electroactive nanomaterials in the peripheral nerve regeneration. J. Mater. Chem. B 2021, 9, 6958–6972. [Google Scholar] [CrossRef]
- Seo, H.; Han, S.I.; Song, K.I.; Seong, D.; Lee, K.; Kim, S.H.; Park, T.; Koo, J.H.; Shin, M.; Baac, H.W.; et al. Durable and Fatigue-Resistant Soft Peripheral Neuroprosthetics for In Vivo Bidirectional Signaling. Adv. Mater. 2021, 33, e2007346. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Barbon, S.; Stocco, E.; Dalzoppo, D.; Contran, M.; De Rose, E.; Parnigotto, P.P.; Macchi, V.; Grandi, C.; De Caro, R. Development of Oxidized Polyvinyl Alcohol-Based Nerve Conduits Coupled with the Ciliary Neurotrophic Factor. Materials 2019, 12, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Ji, H.; Liu, J.; Tu, C.H.; Kappl, M.; Koynov, K.; Vogt, J.; Butt, H.J. Carbon Nanotube-Hydrogel Composites Facilitate Neuronal Differentiation While Maintaining Homeostasis of Network Activity. Adv. Mater. 2021, 33, e2102981. [Google Scholar] [CrossRef] [PubMed]
- De Lima, G.G.; Junior, E.L.S.; Aggio, B.B.; Shee, B.S.; Filho, E.M.M.; Segundo, F.A.S.; Fournet, M.B.; Devine, D.M.; Magalhaes, W.L.E.; de Sa, M.J.C. Nanocellulose for peripheral nerve regeneration in rabbits using citric acid as crosslinker with chitosan and freeze/thawed PVA. Biomed. Mater. 2021, 16, 055011. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2021, 3, e2103691. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Meder, T.; Prest, T.; Skillen, C.; Marchal, L.; Yupanqui, V.T.; Soletti, L.; Gardner, P.; Cheetham, J.; Brown, B.N. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen. Med. 2021, 6, 69. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A. Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational Design of Microfabricated Electroconductive Hydrogels for Biomedical Applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Zhang, Y.; Wang, H.; Xu, Z.; Chen, J.; Bao, R.; Tan, B.; Cui, Y.; Fan, G.; Wang, W.; et al. Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv. Mater. 2018, 30, e1704235. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Daneshvar, E.D.; Egeland, B.M.; Kipke, D.R.; Cederna, P.S.; Urbanchek, M.G. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv. Healthc. Mater. 2012, 1, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, X.; Deng, L.; Ying, D.; Lu, A.; Zhang, L.; Yu, A.; Duan, B. Biocompatible Chitin Hydrogel Incorporated with PEDOT Nanoparticles for Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2021, 13, 16106–16117. [Google Scholar] [CrossRef]
- Xu, D.F.; Fan, L.; Gao, L.F.; Xiong, Y.; Wang, Y.F.; Ye, Q.F.; Yu, A.X.; Dai, H.L.; Yin, Y.X.; Cai, J.; et al. Micro-Nanostructured Polyaniline Assembled in Cellulose Matrix via Interfacial Polymerization for Applications in Nerve Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Dong, M.; Shi, B.; Liu, D.; Liu, J.H.; Zhao, D.; Yu, Z.H.; Shen, X.Q.; Gan, J.M.; Shi, B.L.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Xu, H.X.; Li, X.; Xu, W.J.; Yin, Y.X.; Dai, H.L.; Wang, X.B.; Huang, Z.J.; Xu, P.H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. Rsc. Adv. 2018, 8, 10806–10817. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Xiao, C.; Guan, P.; Zou, Y.; Wen, H.; Liu, C.; Luo, Y.; Tan, G.; Wang, Q.; Li, Y.; et al. Extracellular Matrix-Based Conductive Interpenetrating Network Hydrogels with Enhanced Neurovascular Regeneration Properties for Diabetic Wounds Repair. Adv. Healthc. Mater. 2021, e2101556. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, L.; Tian, Z.M.; Wen, H.Q.; Zhou, L.; Guan, P.F.; Luo, Y.; Chan, C.C.; Tan, G.X.; Ning, C.Y.; et al. Self-curling electroconductive nerve dressing for enhancing peripheral nerve regeneration in diabetic rats. Bioact. Mater. 2021, 6, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Koppes, A.N.; Keating, K.W.; McGregor, A.L.; Koppes, R.A.; Kearns, K.R.; Ziemba, A.M.; McKay, C.A.; Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016, 39, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.M.; Xiao, Q.; Zhao, Y.Y.; Li, J.; Reddy, S.; Shi, X.S.; Su, X.; Chiu, K.; Ramakrishna, S. Engineering an Injectable Electroactive Nanohybrid Hydrogel for Boosting Peripheral Nerve Growth and Myelination in Combination with Electrical Stimulation. Acs Appl. Mater. Interfaces 2020, 12, 53150–53163. [Google Scholar] [CrossRef]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Peng, L.H.; Xu, X.H.; Huang, Y.F.; Zhao, X.L.; Zhao, B.; Cai, S.Y.; Xie, M.J.; Wang, M.Z.; Yuan, T.J.; He, Y.; et al. Self-Adaptive All-In-One Delivery Chip for Rapid Skin Nerves Regeneration by Endogenous Mesenchymal Stem Cells. Adv. Funct. Mater. 2020, 30, 2001751. [Google Scholar] [CrossRef]
- Huang, Q.; Cai, Y.T.; Zhang, X.; Liu, J.C.; Liu, Z.J.; Li, B.; Wong, H.L.; Xu, F.; Sheng, L.Y.; Sun, D.Z.; et al. Aligned Graphene Mesh-Supported Double Network Natural Hydrogel Conduit Loaded with Netrin-1 for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 112–122. [Google Scholar] [CrossRef]
- Luo, H.; Kaneti, Y.V.; Ai, Y.; Wu, Y.; Wei, F.; Fu, J.; Cheng, J.; Jing, C.; Yuliarto, B.; Eguchi, M.; et al. Nanoarchitectured Porous Conducting Polymers: From Controlled Synthesis to Advanced Applications. Adv. Mater. 2021, 33, e2007318. [Google Scholar] [CrossRef]
- Lee, S.; Ozlu, B.; Eom, T.; Martin, D.C.; Shim, B.S. Electrically conducting polymers for bio-interfacing electronics: From neural and cardiac interfaces to bone and artificial tissue biomaterials. Biosens. Bioelectron. 2020, 170, 112620. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talikowska, M.; Fu, X.; Lisak, G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef] [Green Version]
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A Wearable Supercapacitor Based on Conductive PEDOT:PSS-Coated Cloth and a Sweat Electrolyte. Adv. Mater. 2020, 32, e1907254. [Google Scholar] [CrossRef] [PubMed]
- Horev, Y.D.; Maity, A.; Zheng, Y.; Milyutin, Y.; Khatib, M.; Yuan, M.; Suckeveriene, R.Y.; Tang, N.; Wu, W.; Haick, H. Stretchable and Highly Permeable Nanofibrous Sensors for Detecting Complex Human Body Motion. Adv. Mater. 2021, 33, e2102488. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Bai, T.; Sinclair, A.; Wang, W.; Wu, Q.; Gao, F.; Jia, H.; Jiang, S.; Liu, W. Directed neural stem cell differentiation on polyaniline-coated high strength hydrogels. Mater. Today Chem. 2016, 1–2, 15–22. [Google Scholar] [CrossRef]
- Bao, B.; Rivkin, B.; Akbar, F.; Karnaushenko, D.D.; Bandari, V.K.; Teuerle, L.; Becker, C.; Baunack, S.; Karnaushenko, D.; Schmidt, O.G. Digital Electrochemistry for On-Chip Heterogeneous Material Integration. Adv. Mater. 2021, 33, e2101272. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.Q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef]
- Li, F.; Wang, R.; Song, C.; Zhao, M.; Ren, H.; Wang, S.; Liang, K.; Li, D.; Ma, X.; Zhu, B.; et al. A Skin-Inspired Artificial Mechanoreceptor for Tactile Enhancement and Integration. ACS Nano 2021, 15, 16422–16431. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of Carbon Nanotubes, Graphene, and Their Derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Jordan, J.W.; Townsend, W.J.V.; Johnson, L.R.; Walsh, D.A.; Newton, G.N.; Khlobystov, A.N. Electrochemistry of redox-active molecules confined within narrow carbon nanotubes. Chem. Soc. Rev. 2021, 50, 10895–10916. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, e2001105. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Mo, X.; Wang, H. Reduced Graphene Oxide-Encapsulated Microfiber Patterns Enable Controllable Formation of Neuronal-Like Networks. Adv. Mater. 2020, 32, e2004555. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zheng, T.; Wu, L.; Han, Q.; Lei, Y.; Xue, L.; Zhang, L.; Gu, X.; Yang, Y. Bionic microenvironment-inspired synergistic effect of anisotropic micro-nanocomposite topology and biology cues on peripheral nerve regeneration. Sci. Adv. 2021, 7, eabi5812. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Song, Y.H.; McCrary, M.W.; Schmidt, C.E. Advances in ex vivo models and lab-on-a-chip devices for neural tissue engineering. Biomaterials 2019, 198, 146–166. [Google Scholar] [CrossRef]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef] [PubMed]
- Fledrich, R.; Kungl, T.; Nave, K.A.; Stassart, R.M. Axo-glial interdependence in peripheral nerve development. Development 2019, 146, dev151704. [Google Scholar] [CrossRef] [PubMed]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, P.A.; Goncalves de Pinho, A.R.; Micera, S.; van Wezel, R.J.A.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv. Healthc. Mater. 2018, 7, e1701164. [Google Scholar] [CrossRef] [PubMed]


| Conductive Matrix | In Vitro Studies | In Vivo Studies | Reference |
|---|---|---|---|
| PEDOT + agarose | - | Rat (10 mm peroneal nerve gap) | [47] |
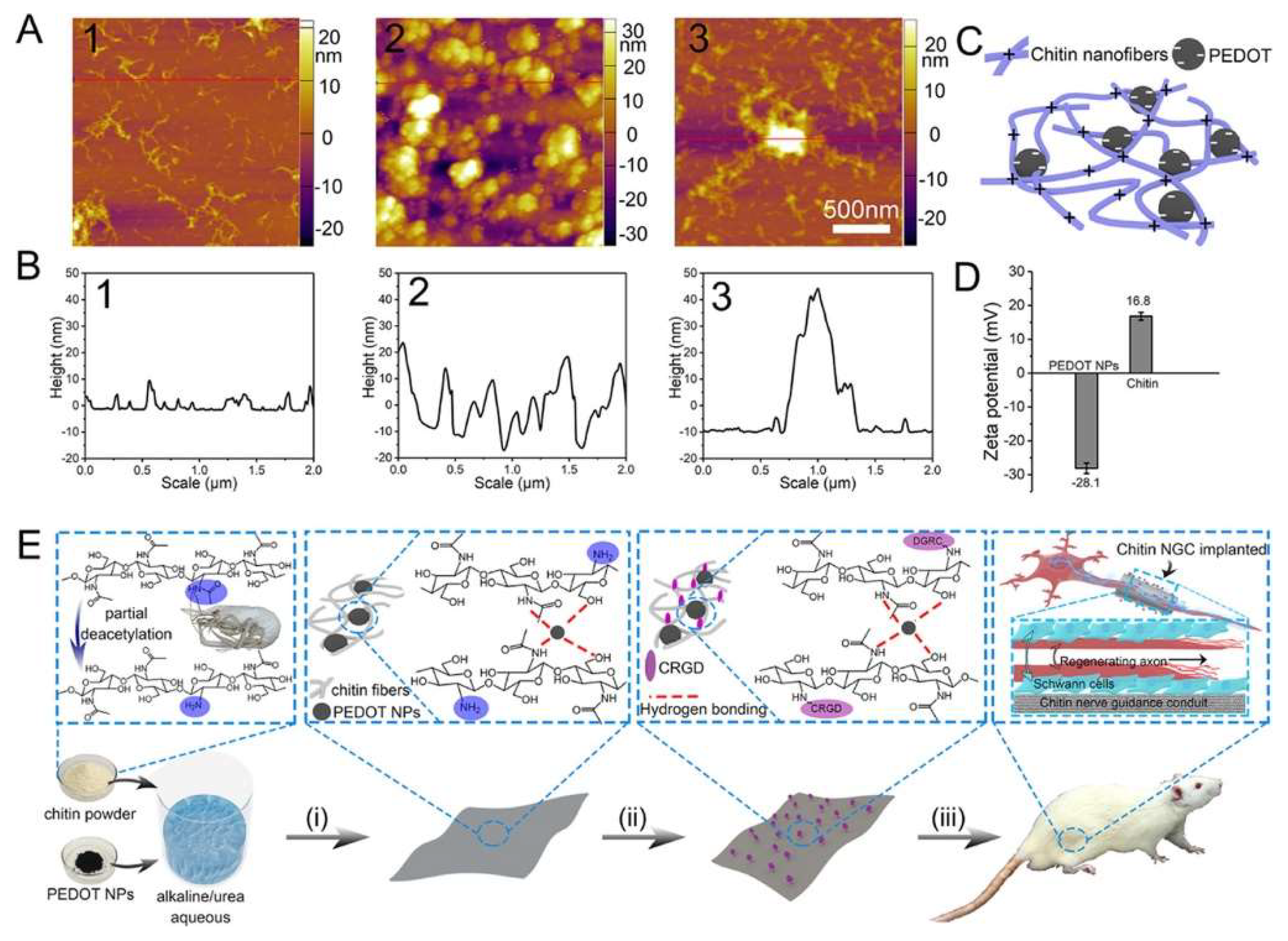
| PEDOT + chitin + CRGD | RSC-96 | Rat (10 mm sciatic nerve gap) | [48] |
| PANi + cellulose | RSC-96 | Rat (5 mm sciatic nerve gap) | [49] |
| PANi + PAM | Toad Sciatic Nerve, NSC, N2a | Rat (10 mm sciatic nerve gap) | [50] |
| PPy + alginate + chitosan | BMMSC, RSC-96, PC-12 | Rat (10 mm sciatic nerve gap) | [51] |
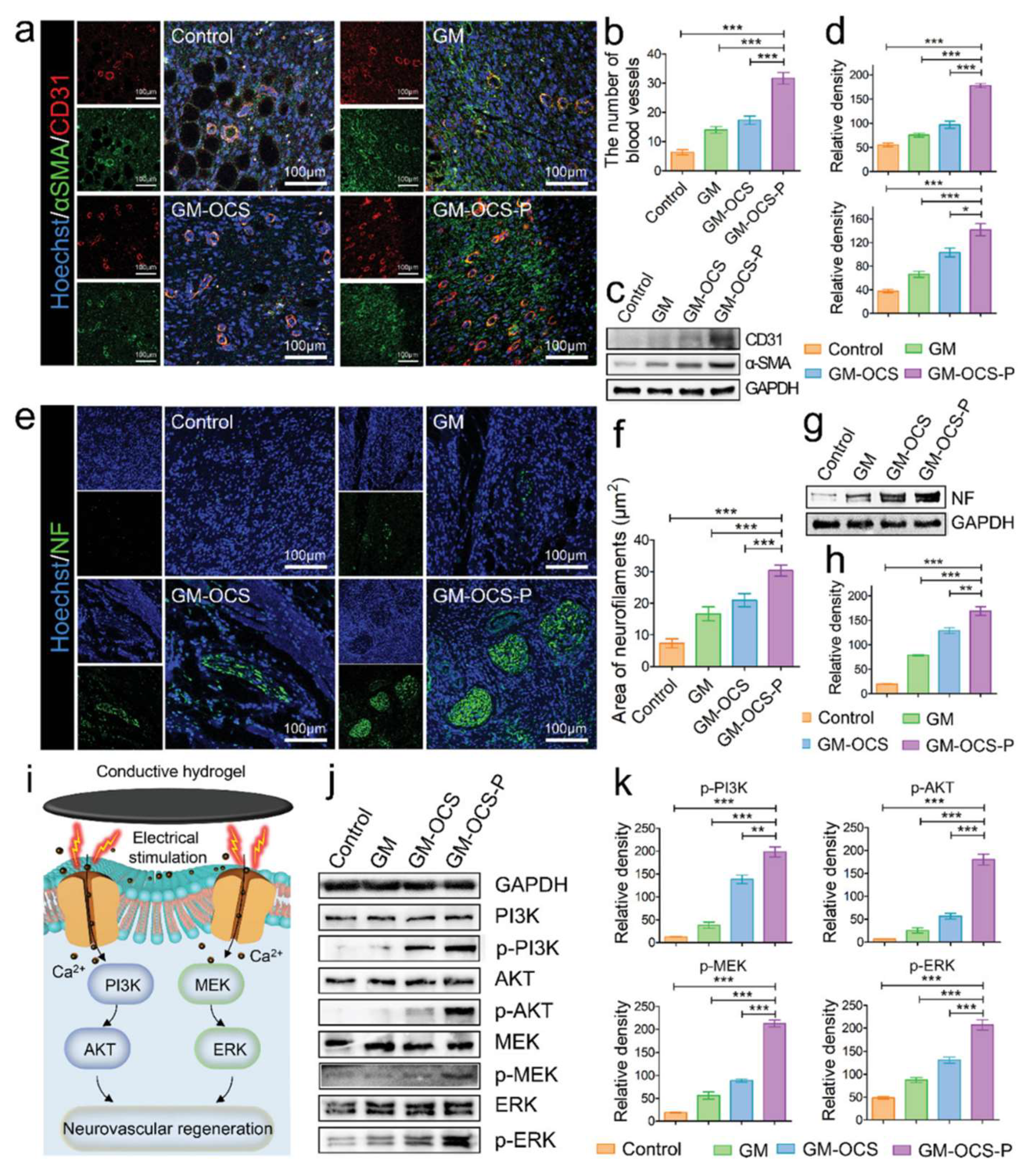
| PPy + GelMA + OCS | Rat DRG, PC-12 | Rat (diabetic skin wound) | [52] |
| PPy + TA | Rat DRG, RSC-96, PC-12 | Rat (diabetic sciatic nerve crush injury) | [53] |
| CNT + Matrigel | Rat DRG | - | [54] |
| CNT + SAP | Rat DRG | - | [55] |
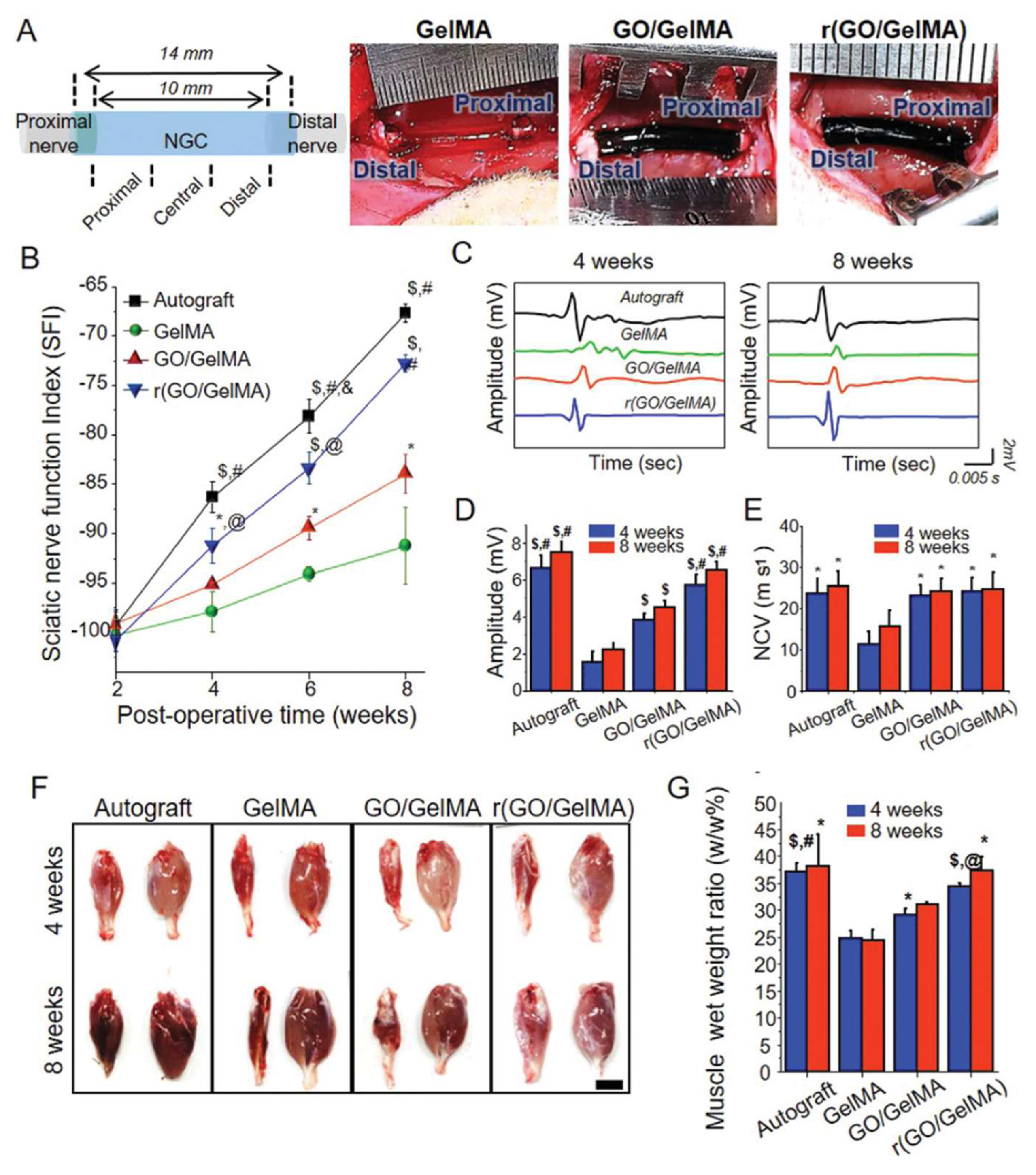
| reduced (GO/GelMA) | PC-12 | Rat (10 mm sciatic nerve gap) | [56] |
| GO + PPy + alginate | BMMSC | Rat (skin nerves removed) | [57] |
| Graphene + GelMA + alginate | RSC-96 | Rat (10 mm sciatic nerve gap) | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, M.; Liu, S.; Li, C.; Ding, Z.; Wan, T.; Zhang, P. Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration. Gels 2022, 8, 41. https://doi.org/10.3390/gels8010041
Zhang F, Zhang M, Liu S, Li C, Ding Z, Wan T, Zhang P. Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration. Gels. 2022; 8(1):41. https://doi.org/10.3390/gels8010041
Chicago/Turabian StyleZhang, Fengshi, Meng Zhang, Songyang Liu, Ci Li, Zhentao Ding, Teng Wan, and Peixun Zhang. 2022. "Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration" Gels 8, no. 1: 41. https://doi.org/10.3390/gels8010041
APA StyleZhang, F., Zhang, M., Liu, S., Li, C., Ding, Z., Wan, T., & Zhang, P. (2022). Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration. Gels, 8(1), 41. https://doi.org/10.3390/gels8010041






