Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels
Abstract
:1. Introduction
2. Results and Discussion
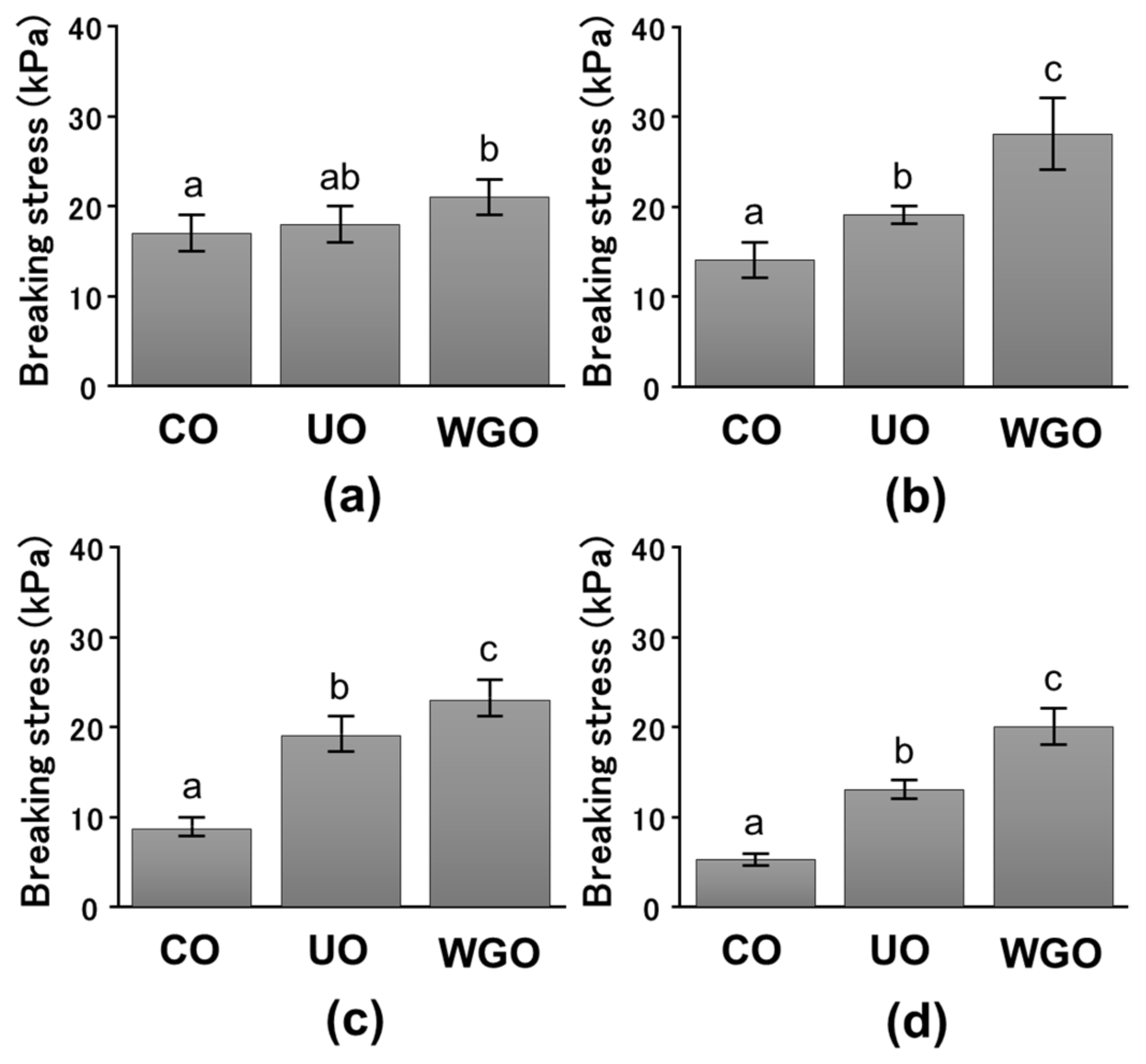
2.1. Effect of WG-Treated Okara on the Mechanical Properties of SPI Gels
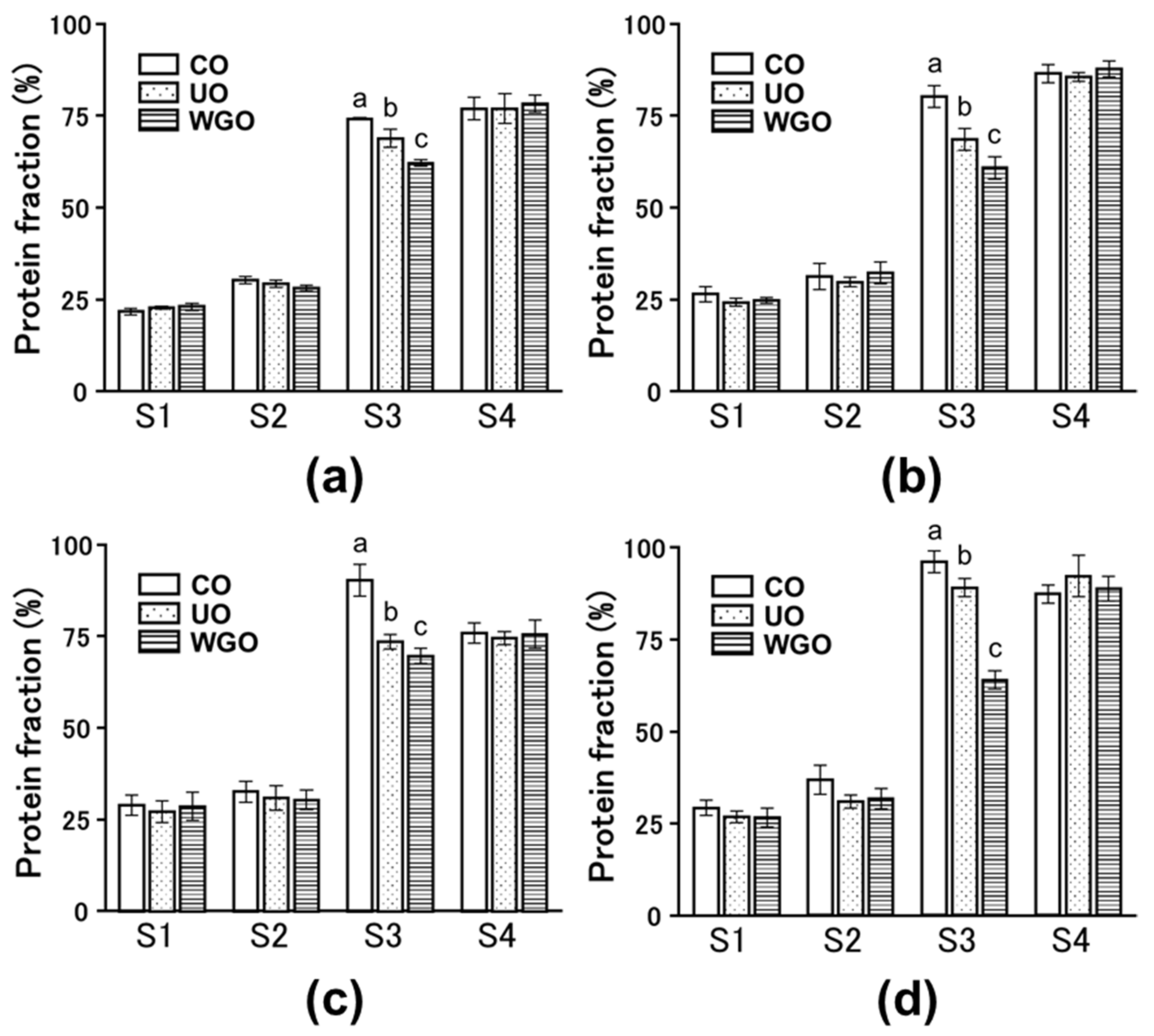
2.2. Effect of WG-Treated Okara on the Intermolecular Forces of SPI Gels
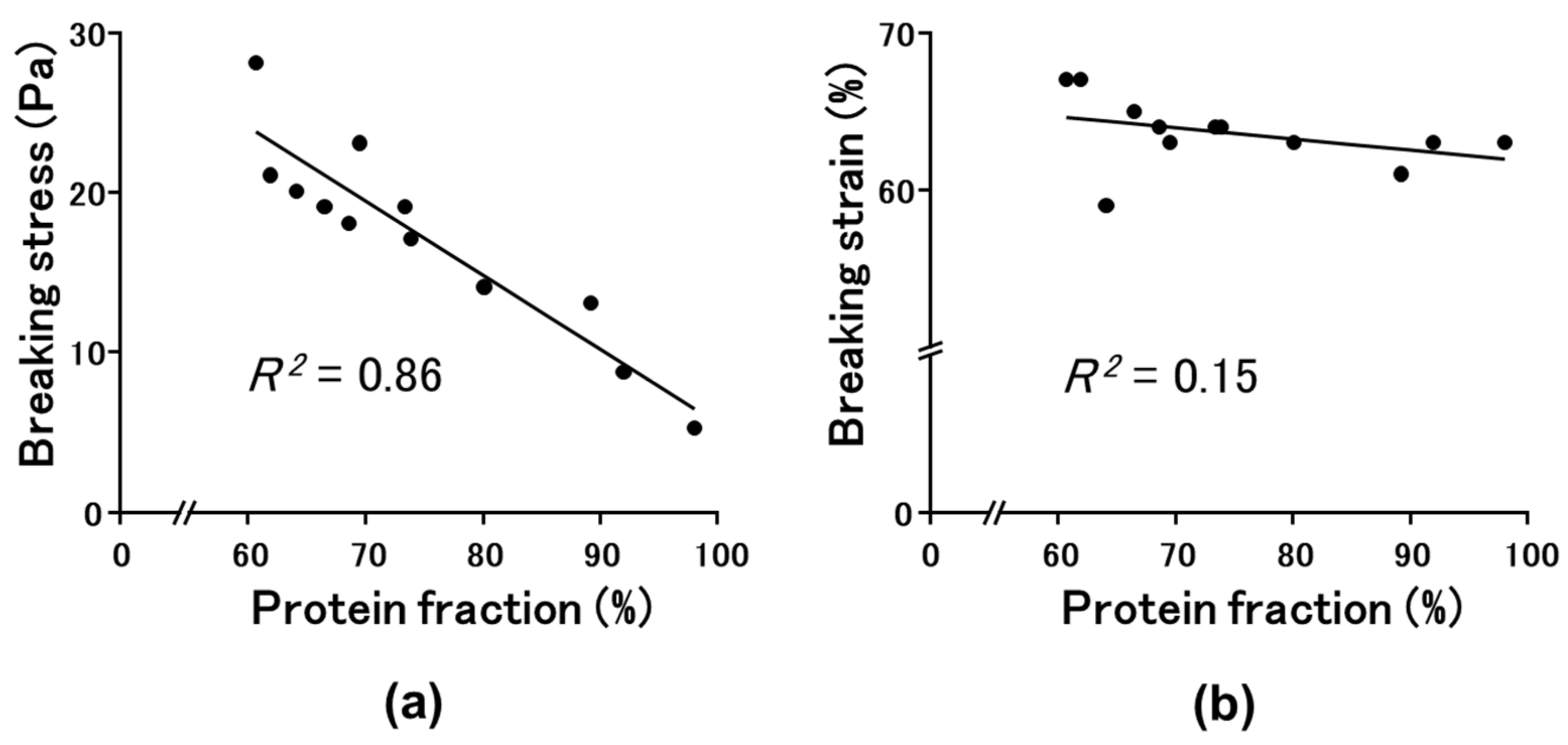
2.3. Relationship between Mechanical Properties and Intermolecular Forces in Okara–SPI Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Low-Protein Okara and WG-Treated Okara
4.3. Preparation of Okara–SPI Gels
4.4. Compression Measurements
4.5. Protein Solubility
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Toole, D.K. Characteristics and Use of Okara, the Soybean Residue from Soy Milk Production A Review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. Soy as a food ingredient. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 149–186. [Google Scholar]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613. [Google Scholar] [CrossRef]
- Nagano, T.; Arai, Y.; Yano, H.; Aoki, T.; Kurihara, S.; Hirano, R.; Nishinari, K. Improved physicochemical and functional properties of okara, a soybean residue, by nanocellulose technologies for food development—A review. Food Hydrocoll. 2020, 109, 105964. [Google Scholar] [CrossRef]
- Pérez-López, E.; Mateos-Aparicio, I.; Rupérez, P. High hydrostatic pressure aided by food-grade enzymes as a novel approach for Okara valorization. Innov. Food Sci. Emerg. Technol. 2017, 42, 197–203. [Google Scholar] [CrossRef]
- Liu, H.H.; Chien, J.T.; Kuo, M.I. Ultra high pressure homogenized soy flour for tofu making. Food Hydrocoll. 2013, 32, 278–285. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.-u.; Zhang, M. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT Food Sci. Technol. 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Terpiłowski, K.; Mleko, S. Physicochemical properties of cellulose/whey protein fibers as a potential material for active ingredients release. Food Hydrocoll. 2015, 49, 232–239. [Google Scholar] [CrossRef]
- Peng, J.; Calabrese, V.; Ainis, W.N.; Scager, R.; Velikov, K.P.; Venema, P.; van der Linden, E. Mixed gels from whey protein isolate and cellulose microfibrils. Int. J. Biol. Macromol. 2019, 124, 1094–1105. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Wang, Y.; Jin, Y.; Guo, X.; Liu, Y.; Qi, X.; Lei, H.; Xu, H. Heat-induced whey protein isolate gels improved by cellulose nanocrystals: Gelling properties and microstructure. Carbohydr. Polym. 2020, 231, 115749. [Google Scholar] [CrossRef]
- Arai, Y.; Nishinari, K.; Nagano, T. Developing SoybeanProtein Gel-Based Foods from Okara Using the Wet-Type Grinder Method. Foods 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Jin, X.; Qu, R.; Wang, Y.; Li, D.; Wang, L. Effect and mechanism of acid-induced soy protein isolate gels as influenced by cellulose nanocrystals and microcrystalline cellulose. Foods 2022, 11, 461. [Google Scholar] [CrossRef]
- Deng, C.; Shao, Y.; Xu, M.; Yao, Y.; Wu, N.; Hu, H.; Tu, Y. Effects of metal ions on the physico-chemical, microstructural and digestion characteristics of alkali-induced egg white gel. Food Hydrocoll. 2020, 107, 105956. [Google Scholar] [CrossRef]
- Pérez-Mateos, M.; Lourenço, H.; Montero, P.; Borderías, A.J. Rheological and biochemical characteristics of high-pressure- and heat-induced gels from blue whiting (Micromesistius poutassou) muscle proteins. J. Agric. Food Chem. 1997, 45, 44–49. [Google Scholar] [CrossRef]
- Utsumi, S.; Kinsella, J.E. Forces involved in soy protein gelation: Effects of various reagents on the formation, hardness and solubility of heat-induced gels made from 7S, 11S, and soy isolate. J. Food Sci. 1985, 50, 1278–1282. [Google Scholar] [CrossRef]
- Nagano, T.; Mori, H.; Nishinari, K. Effect of heating and cooling on the gelation kinetics of 7S globulin from soybeans. J. Agric. Food Chem. 1994, 42, 1415–1419. [Google Scholar] [CrossRef]
- Nagano, T. Contribution of disulfide bonding to viscoelastic properties and microstructures of 11S globulin gels from soybeans: Magnesium chloride-induced gels. Food Sci. Technol. Res. 2013, 19, 51–57. [Google Scholar] [CrossRef]
- Nagano, T.; Akasaka, T.; Nishinari, K. Dynamic viscoelastic properties of glycinin and β-conglycinin gels from soybeans. Biopolymers 1994, 34, 1303–1309. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Liu, Y.; Peng, F.; Wang, X.; Wang, C.; Li, M.; Xu, H. Gel properties and formation mechanism of soy protein isolate gels improved by wheat bran cellulose. Food Chem. 2020, 324, 126876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohydr. Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, W.; Liu, R.; Liu, Y.; Xing, L.; Han, M.; Kang, Z.; Xu, X.; Zhou, G. Improved gel functionality of myofibrillar proteins incorporation with sugarcane dietary fiber. Food Res. Int. 2017, 100, 586–594. [Google Scholar] [CrossRef] [PubMed]



| WG-Treated or Untreated Okara | SPI | |
|---|---|---|
| 3% okara–SPI | 0.48% | 15.52% |
| 5% okara–SPI | 0.80% | 15.20% |
| 10% okara–SPI | 1.60% | 14.40% |
| 15% okara–SPI | 2.40% | 13.60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, Y.; Nishinari, K.; Nagano, T. Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels. Gels 2022, 8, 616. https://doi.org/10.3390/gels8100616
Arai Y, Nishinari K, Nagano T. Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels. Gels. 2022; 8(10):616. https://doi.org/10.3390/gels8100616
Chicago/Turabian StyleArai, Yuya, Katsuyoshi Nishinari, and Takao Nagano. 2022. "Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels" Gels 8, no. 10: 616. https://doi.org/10.3390/gels8100616
APA StyleArai, Y., Nishinari, K., & Nagano, T. (2022). Wet Grinder-Treated Okara Improved Both Mechanical Properties and Intermolecular Forces of Soybean Protein Isolate Gels. Gels, 8(10), 616. https://doi.org/10.3390/gels8100616









