Improving the Weak Gel Structure of an Oil-Based Drilling Fluid by Using a Polyamide Wax
Abstract
1. Introduction
2. Results and Discussion
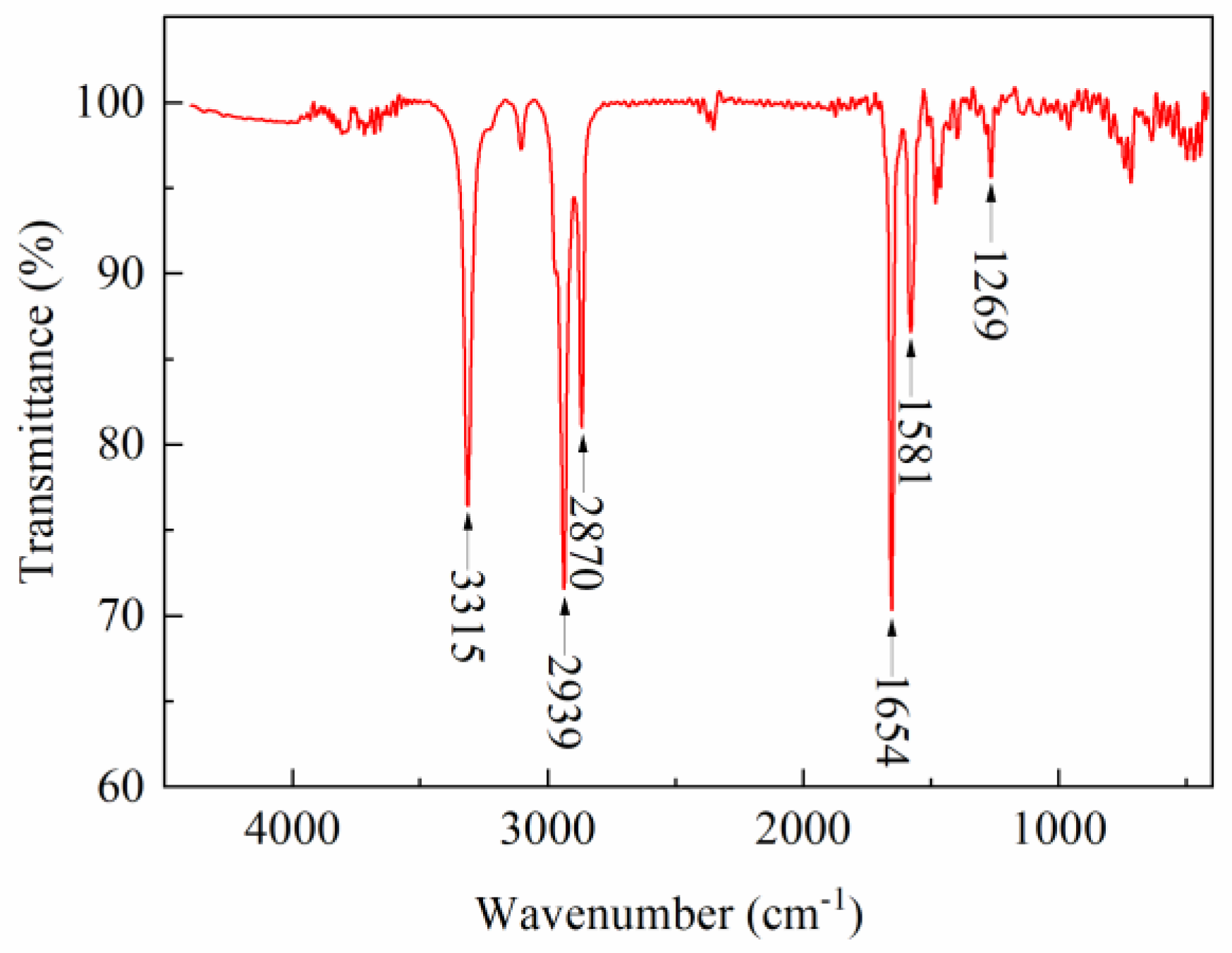
2.1. FTIR
2.2. TGA
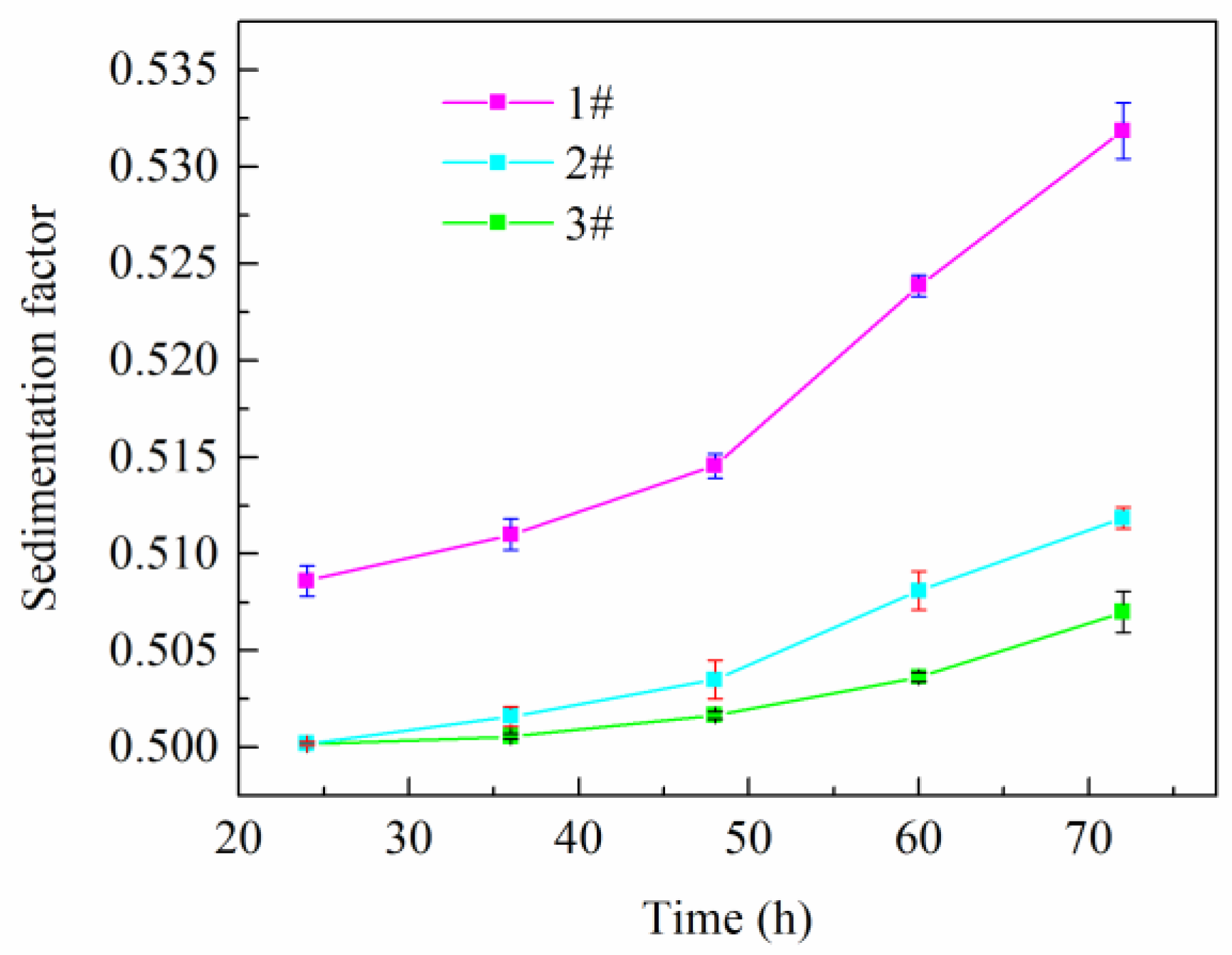
2.3. Emulsion Stability
2.3.1. Sedimentation Observation
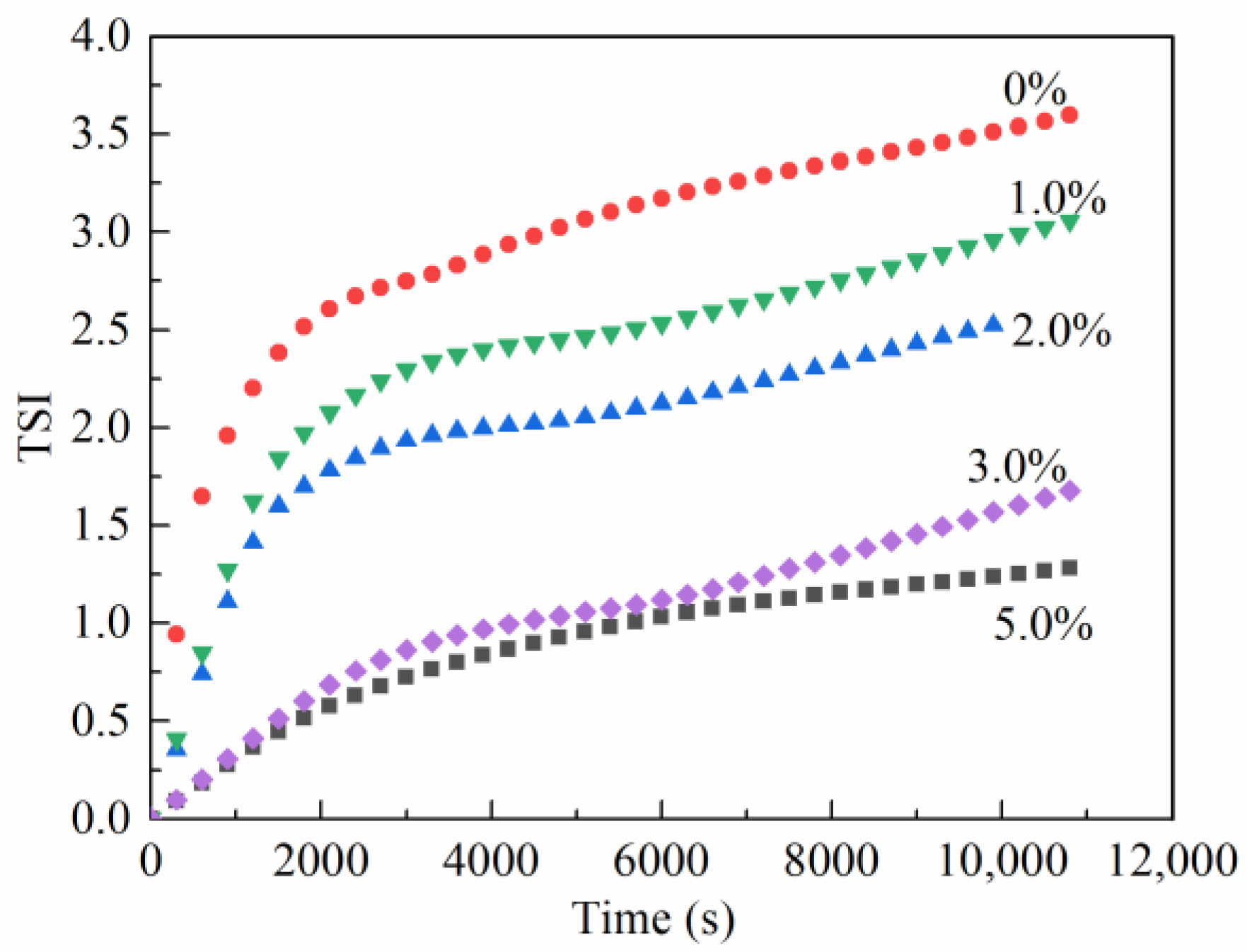
2.3.2. Emulsion Stability Index (TSI)
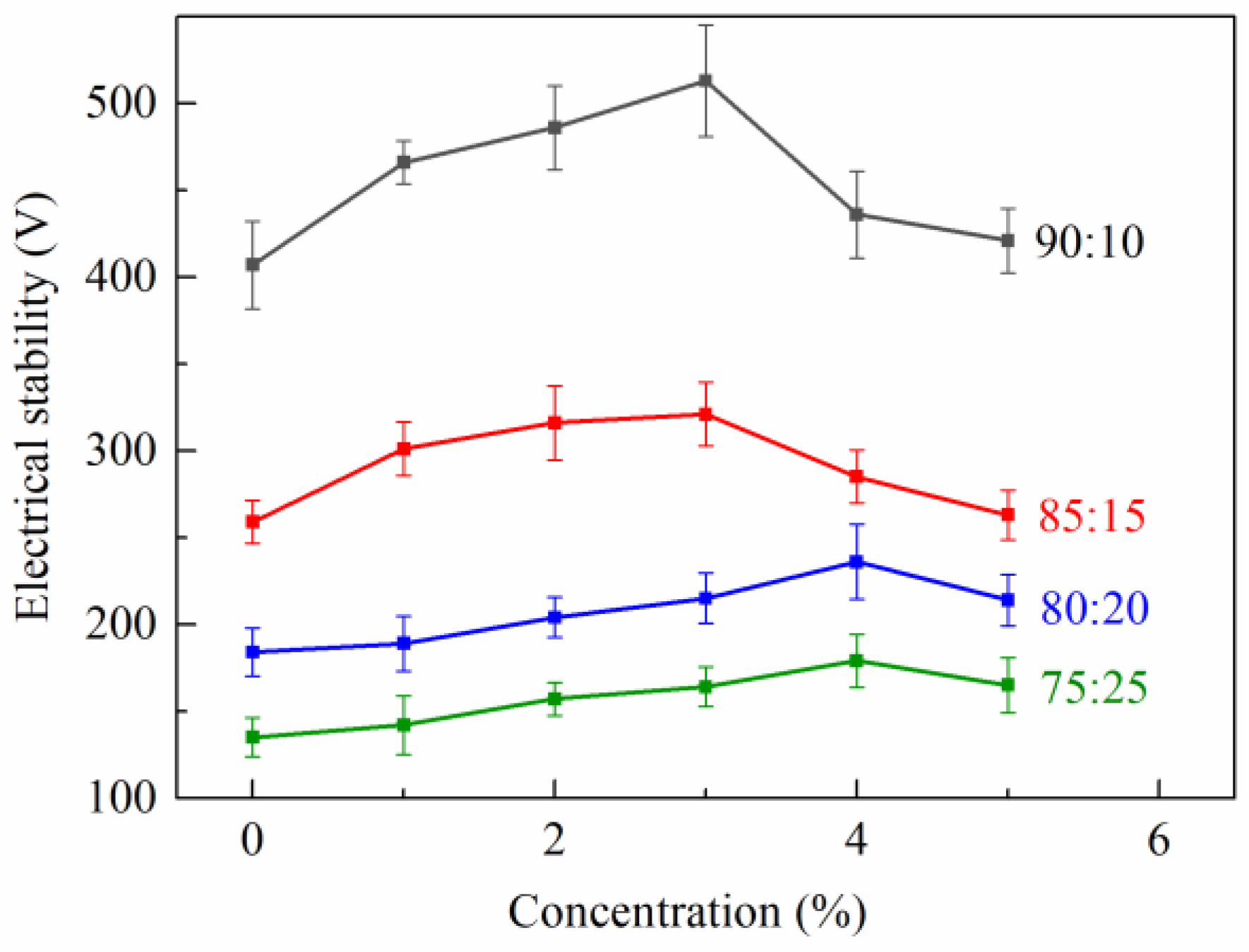
2.3.3. Electrical Stability (ES) Test
2.3.4. Particle Size Analysis of the Emulsion
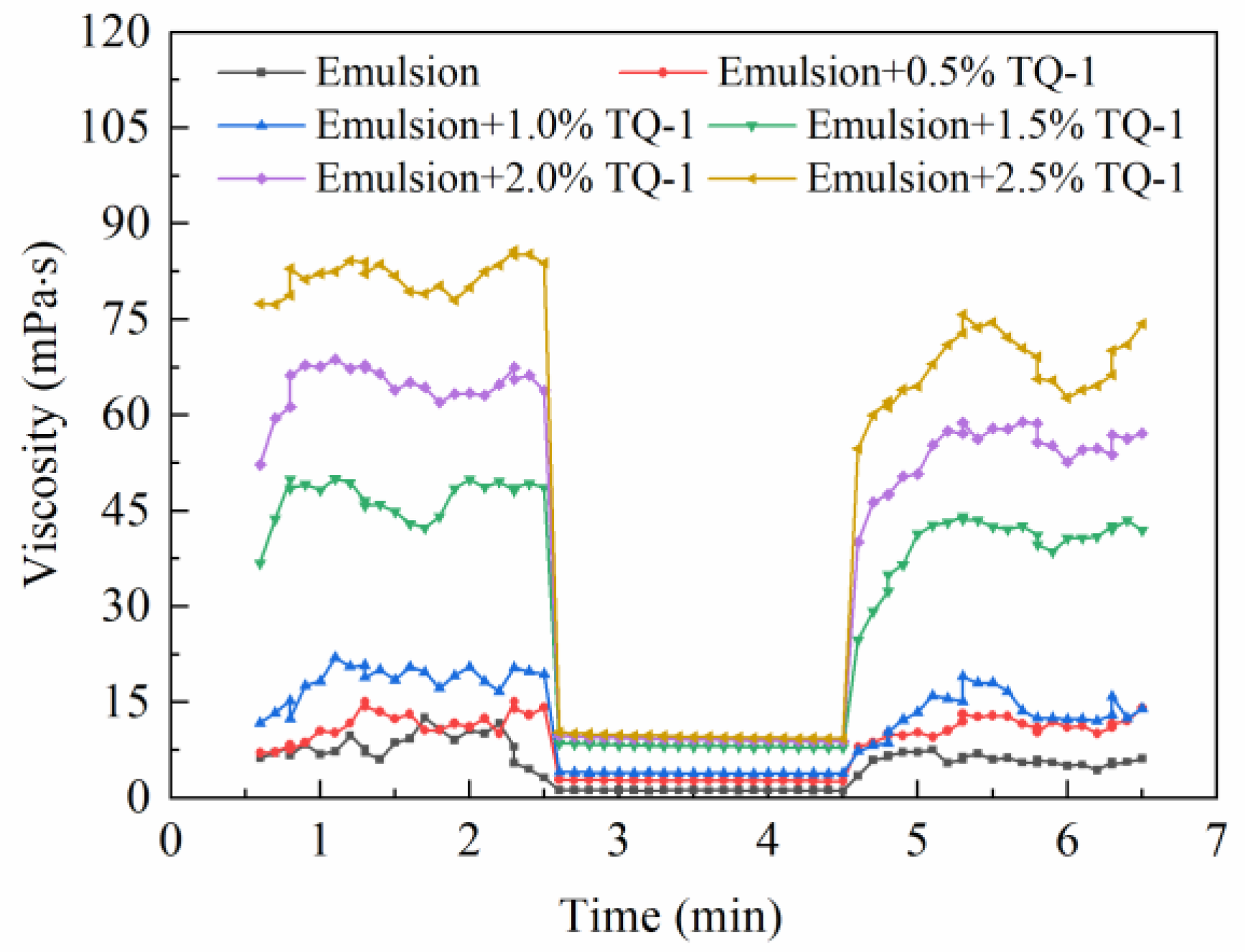
2.4. Rheological Performance
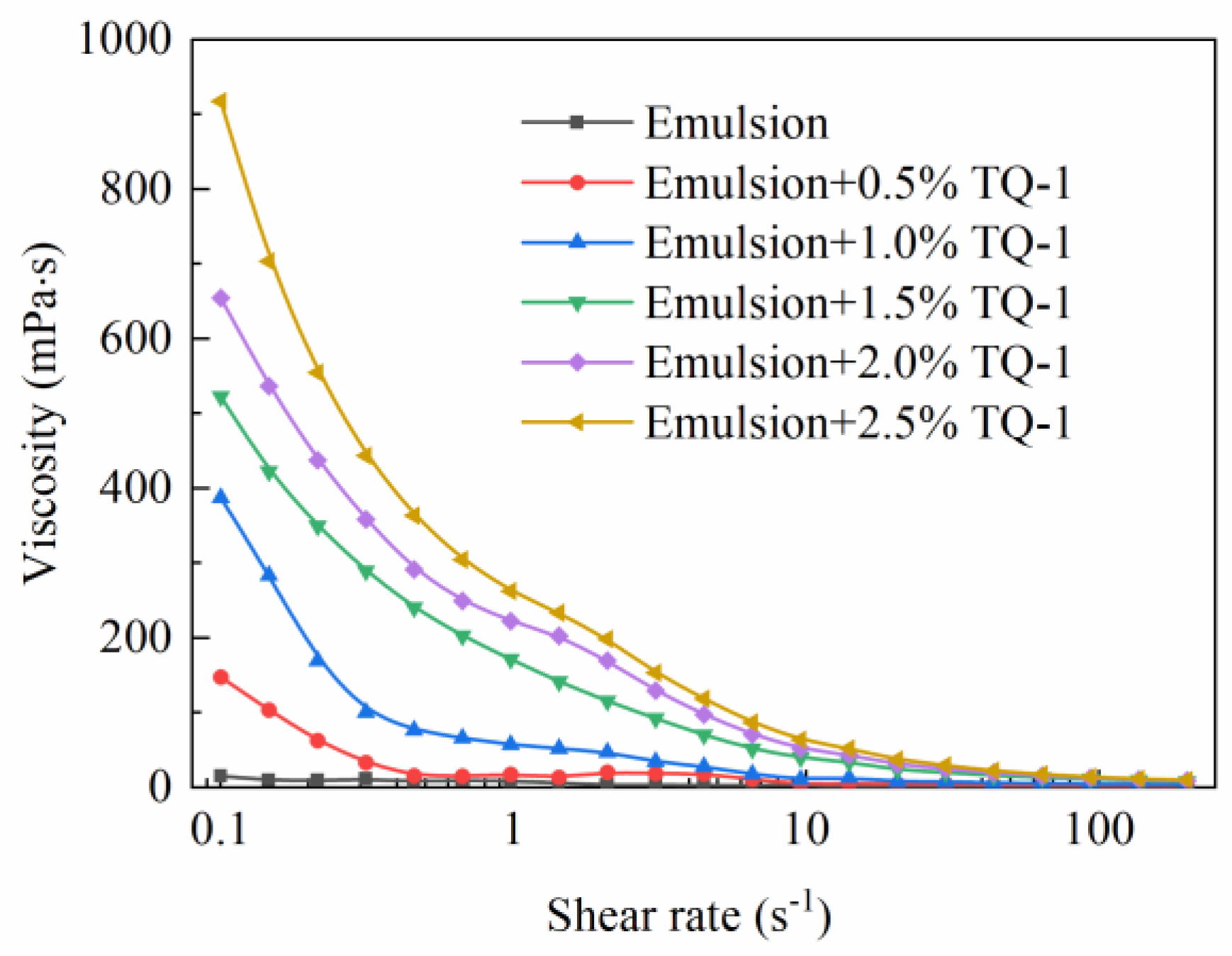
2.4.1. The Effect of TQ-1 on the Viscosity of the Emulsion
2.4.2. The Effect of TQ-1 on the Thixotropy of the Emulsion
2.5. The Effect of TQ-1 on the Performance of OBDFs
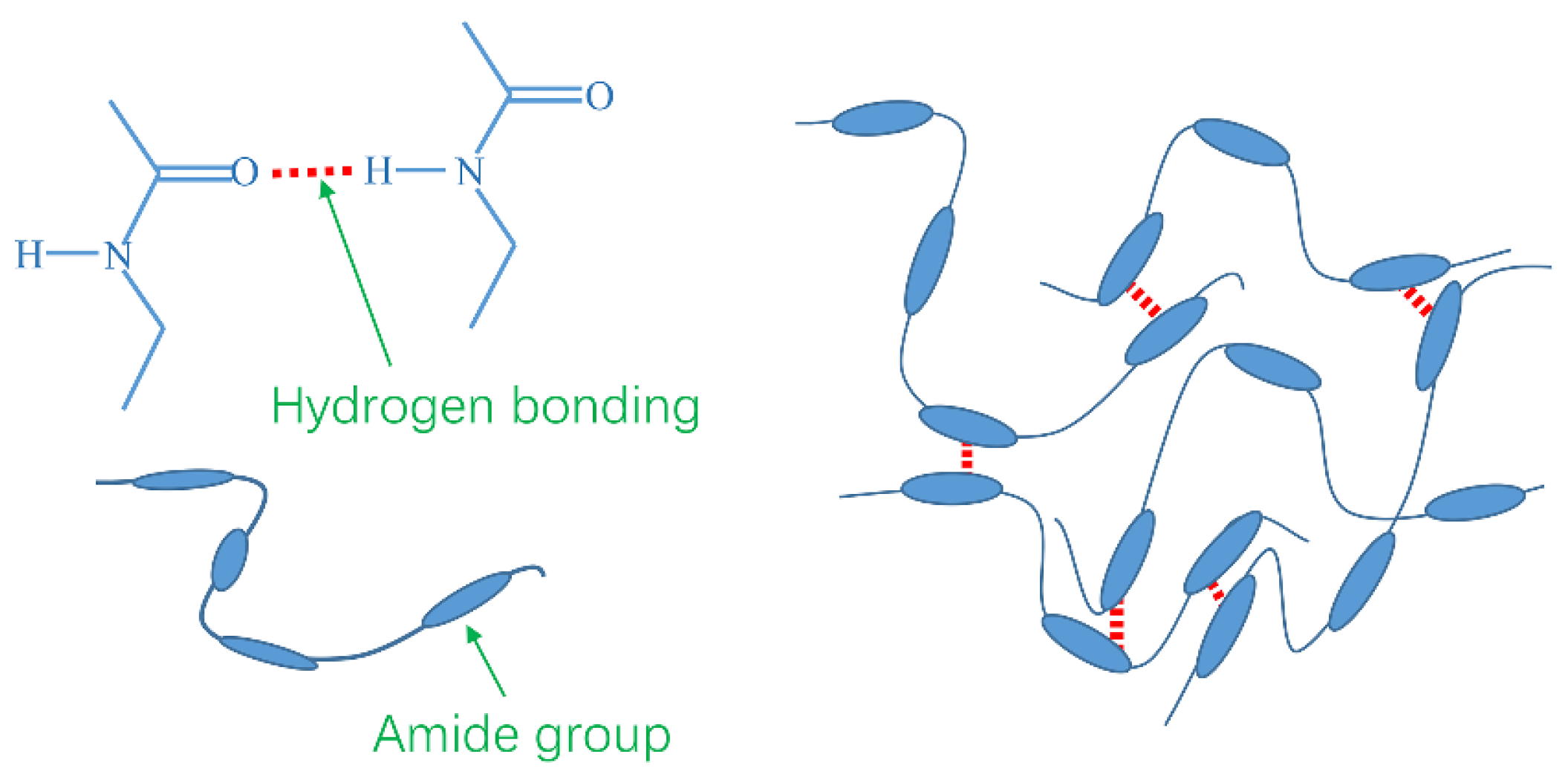
2.6. Mechanism Analysis
3. Conclusions
- (1)
- A polyamide wax TQ-1 for OBDFs was synthesized by dimeric acid and 1,6-hexanediamine, with an initial decomposition temperature of 195 °C and good thermal stability.
- (2)
- After adding TQ-1, both the sedimentation and emulsification stability of the emulsion are greatly improved. For emulsions with different oil–water ratios, TQ-1 can enhance the electrical stability of emulsion to different degrees.
- (3)
- TQ-1 could effectively improve the rheological properties and enhance the shear thinning performance of the emulsion.
- (4)
- TQ-1 increases the demulsification voltage, yield point, and gel strength and reduces settlement of OBDFs.
- (5)
- TQ-1 mainly forms hydrogen bonds through polar amide groups, thus forming a spatial network structure to enhance the weak gel structure of OBDFs.
4. Materials and Methods
4.1. Materials
4.2. Preparation of TQ-1
4.3. Characterization
4.3.1. FTIR
4.3.2. TGA
4.4. Emulsion Stability Tests
4.4.1. Preparation of the Emulsion
4.4.2. Emulsion Stability
4.5. Evaluation of Rheological Performance
4.6. Performance of TQ-1 in OBDFs
4.6.1. Preparation of OBDFs
4.6.2. Performance Evaluation of the OBDFs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Abbreviations | Description |
|---|---|
| OBDFs | oil based drilling fluids |
| TQ -1 | polyamide wax |
| FTIR | fourier transform infrared spectroscopy |
| TGA | thermogravimetric analysis |
| TSI | emulsion stability index |
| SF | sedimentation factor |
| HTHP | high-temperature and high-pressure |
| ZnO-NPs | nano-zinc oxide |
| nano-CaCO3 | nano-calcium carbonate |
| PSBR | polystyrene-butadiene rubber |
| YP | yield point |
| AV | apparent viscosity |
| PV | plastic viscosity |
| ES | electrical stability |
| FBRM | focused beam reflectance measurement |
References
- Wenrui, H.; Jingwei, B.; Bin, H. Trend and Progress in Global Oil and Gas Exploration. Pet. Explor. Dev. 2013, 40, 439–443. [Google Scholar]
- Lei, M.; Li, N. The Damage Mechanism of Oil-Based Drilling Fluid for Tight Sandstone Gas Reservoir and Its Optimization. J. Pet. Sci. Eng. 2017, 158, 616–625. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Guan, J.; Sun, Y.; Kuang, X.; Chen, S. A New Type of Whole Oil-Based Drilling Fluid. Pet. Explor. Dev. 2014, 41, 538–544. [Google Scholar] [CrossRef]
- Guo, Q.; Ji, L.; Rajabov, V.; Friedheim, J.; Portella, C.; Wu, R. Shale Gas Drilling Experience and Lessons Learned from Eagle Ford. In Proceedings of the SPE Americas Unconventional Resources Conference, Pittsburgh, PA, USA, 5–7 June 2012; OnePetro: Richardson, TX, USA, 2012. [Google Scholar]
- Elshehabi, T.; Bilgesu, I. Impact of Drilling with Oil Based Mud on Well Control in Horizontal Shale Gas Wells. In Proceedings of the SPE Eastern Regional Meeting, Morgantown, WV, USA, 13–15 October 2015; OnePetro: Richardson, TX, USA, 2015. [Google Scholar]
- Huang, X.; Jiang, G.; He, Y.; An, Y.; Zhang, S. Improvement of Rheological Properties of Invert Drilling Fluids by Enhancing Interactions of Water Droplets Using Hydrogen Bonding Linker. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 467–475. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, G.C.; Wang, K.; Li, X.L. Synthesis of a Dendrimer as Rheological Modifier for Deep-Water Drilling Fluids and Study of Its Interaction with Organo-Clay at Different Temperatures. Key Eng. Mater. 2018, 792, 111–118. [Google Scholar] [CrossRef]
- Du, M.; Liu, P.; Clode, P.L.; Liu, J.; Haq, B.; Leong, Y.-K. Impact of Additives with Opposing Effects on the Rheological Properties of Bentonite Drilling Mud: Flow, Ageing, Microstructure and Preparation Method. J. Pet. Sci. Eng. 2020, 192, 107282. [Google Scholar] [CrossRef]
- Yao, R.; Jiang, G.; Li, W.; Deng, T.; Zhang, H. Effect of Water-Based Drilling Fluid Components on Filter Cake Structure. Powder Technol. 2014, 262, 51–61. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, C. Experimental Investigation of a New Weak-Gel-Type Clay-Free and Water-Based Drilling Fluid with High-Temperature and High-Salt Resistance for Determining Its Optimized Formulation. Energy Sources, Part A Recover. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–18. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, H.; Li, R.; Chen, N. Montmorillonite Modified by Cationic and Nonionic Surfactants as High-Performance Fluid-Loss-Control Additive in Oil-Based Drilling Fluids. J. Dispers. Sci. Technol. 2015, 36, 569–576. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Jaber, M.; Gao, J.; Peng, S. Comparative Study on the Structures and Properties of Organo-Montmorillonite and Organo-Palygorskite in Oil-Based Drilling Fluids. J. Ind. Eng. Chem. 2017, 56, 248–257. [Google Scholar] [CrossRef]
- Finlayson, C.M. Viscosity Increasing Additive for Non-Aqueous Fluid Systems. U.S. Patent 4,208,218, 17 June 1980. [Google Scholar]
- Zhuang, G.; Zhang, Z.; Jaber, M. Organoclays Used as Colloidal and Rheological Additives in Oil-Based Drilling Fluids: An Overview. Appl. Clay Sci. 2019, 177, 63–81. [Google Scholar] [CrossRef]
- Jinsheng, S.U.N.; HUANG, X.; JIANG, G.; Kaihe, L.Y.U.; Jingping, L.I.U.; Zhiwen, D.A.I. Development of Key Additives for Organoclay-Free Oil-Based Drilling Mud and System Performance Evaluation. Pet. Explor. Dev. 2018, 45, 764–769. [Google Scholar]
- Ma, C.; Li, L.; Yang, Y.P.; Hao, W.W.; Zhang, Q.; Lv, J. Study on the Effect of Polymeric Rheology Modifier on the Rheological Properties of Oil-Based Drilling Fluids. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 292, p. 12106. [Google Scholar]
- Patel, A.D.; Salandanan, C.S. Thermally Stable Polymeric Gellant for Oil-Base Drilling Fluids. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Phoenix, AZ, USA, 9–11 April 1985; OnePetro: Richardson, TX, USA, 1985. [Google Scholar]
- Huang, X.; Sun, J.; Lv, K.; Liu, J.; Shen, H. An Alternative Method to Enhance w/o Emulsion Stability Using Modified Dimer Acid and Its Application in Oil Based Drilling Fluids. RSC Adv. 2018, 8, 26318–26324. [Google Scholar] [CrossRef] [PubMed]
- Mikhienkova, E.I.; Lysakov, S.V.; Neverov, A.L.; Zhigarev, V.A.; Minakov, A.V.; Rudyak, V.Y. Experimental Study on the Influence of Nanoparticles on Oil-Based Drilling Fluid Properties. J. Pet. Sci. Eng. 2022, 208, 109452. [Google Scholar] [CrossRef]
- Noah, A.Z.; El Semary, M.A.; Youssef, A.M.; El-Safty, M.A. Enhancement of Yield Point at High Pressure High Temperature Wells by Using Polymer Nanocomposites Based on ZnO & CaCO3 Nanoparticles. Egypt. J. Pet. 2017, 26, 33–40. [Google Scholar]
- Madkour, T.M.; Fadl, S.; Dardir, M.M.; Mekewi, M.A. High Performance Nature of Biodegradable Polymeric Nanocomposites for Oil-Well Drilling Fluids. Egypt. J. Pet. 2016, 25, 281–291. [Google Scholar] [CrossRef]
- Santos, J.; Calero, N.; Muñoz, J. Optimization of a Green Emulsion Stability by Tuning Homogenization Rate. RSC Adv. 2016, 6, 57563–57568. [Google Scholar] [CrossRef]
- Bukrejewski, P.; Jarczewski, Z.; Matuszewska, A. Evaluation of the Chemical Stability of Diesel Oil with Using Turbiscan Stability Index (TSI). Arch. Motoryz. 2020, 88, 5–18. [Google Scholar]
- Skadsem, H.J.; Leulseged, A.; Cayeux, E. Measurement of Drilling Fluid Rheology and Modeling of Thixotropic Behavior. Appl. Rheol. 2019, 29, 1–11. [Google Scholar] [CrossRef]



| Type | Density (g/cm3) | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV (Pa/(mPa·s)) | G′/G″ (Pa/Pa) | FLHTHP (mL) | ES (V) |
|---|---|---|---|---|---|---|---|---|
| 1# | 2.0 | 52.5 | 52 | 0.5 | 0.01 | 1.5/2.0 | 5.1 | 782 |
| 2# | 2.0 | 59 | 53 | 6.0 | 0.11 | 3.5/5.5 | 4.8 | 1126 |
| 3# | 2.0 | 63 | 54 | 9.0 | 0.16 | 4.0/6.5 | 4.7 | 1347 |
| Order | Additives | Formulation # 1 | Formulation # 2 | Formulation # 3 | Stirring Speed (r/min) | Stirring Time (min) |
|---|---|---|---|---|---|---|
| 1 | White oil | 255 mL | 255 mL | 255 mL | / | / |
| 2 | Main emulsifier | 10.5 g | 10.5 g | 10.5 g | 5000 | 10 |
| 3 | Secondary emulsifier | 4.5 g | 4.5 g | 4.5 g | 5000 | 10 |
| 4 | 20% CaCl2 | 45 mL | 45 mL | 45 mL | 5000 | 20 |
| 5 | CaO | 9 g | 9 g | 9 g | 5000 | 20 |
| 6 | Organoclay | 6 g | 3 g | 3 g | 5000 | 20 |
| 7 | Blown asphalt | 12 g | 12 g | 12 g | 5000 | 20 |
| 8 | TQ-1 | 0 g | 0.9 g | 1.8 g | 5000 | 20 |
| 9 | Barite | 660 g | 660 g | 660 g | 5000 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Meng, X.; Li, M.; Sun, J.; Lv, K.; Gao, C. Improving the Weak Gel Structure of an Oil-Based Drilling Fluid by Using a Polyamide Wax. Gels 2022, 8, 631. https://doi.org/10.3390/gels8100631
Huang X, Meng X, Li M, Sun J, Lv K, Gao C. Improving the Weak Gel Structure of an Oil-Based Drilling Fluid by Using a Polyamide Wax. Gels. 2022; 8(10):631. https://doi.org/10.3390/gels8100631
Chicago/Turabian StyleHuang, Xianbin, Xu Meng, Mao Li, Jinsheng Sun, Kaihe Lv, and Chongyang Gao. 2022. "Improving the Weak Gel Structure of an Oil-Based Drilling Fluid by Using a Polyamide Wax" Gels 8, no. 10: 631. https://doi.org/10.3390/gels8100631
APA StyleHuang, X., Meng, X., Li, M., Sun, J., Lv, K., & Gao, C. (2022). Improving the Weak Gel Structure of an Oil-Based Drilling Fluid by Using a Polyamide Wax. Gels, 8(10), 631. https://doi.org/10.3390/gels8100631







