Impact of Konjac Glucomannan with Different Molecular Weight on Retrogradation Properties of Pea Starch
Abstract
:1. Introduction
2. Results and Discussion
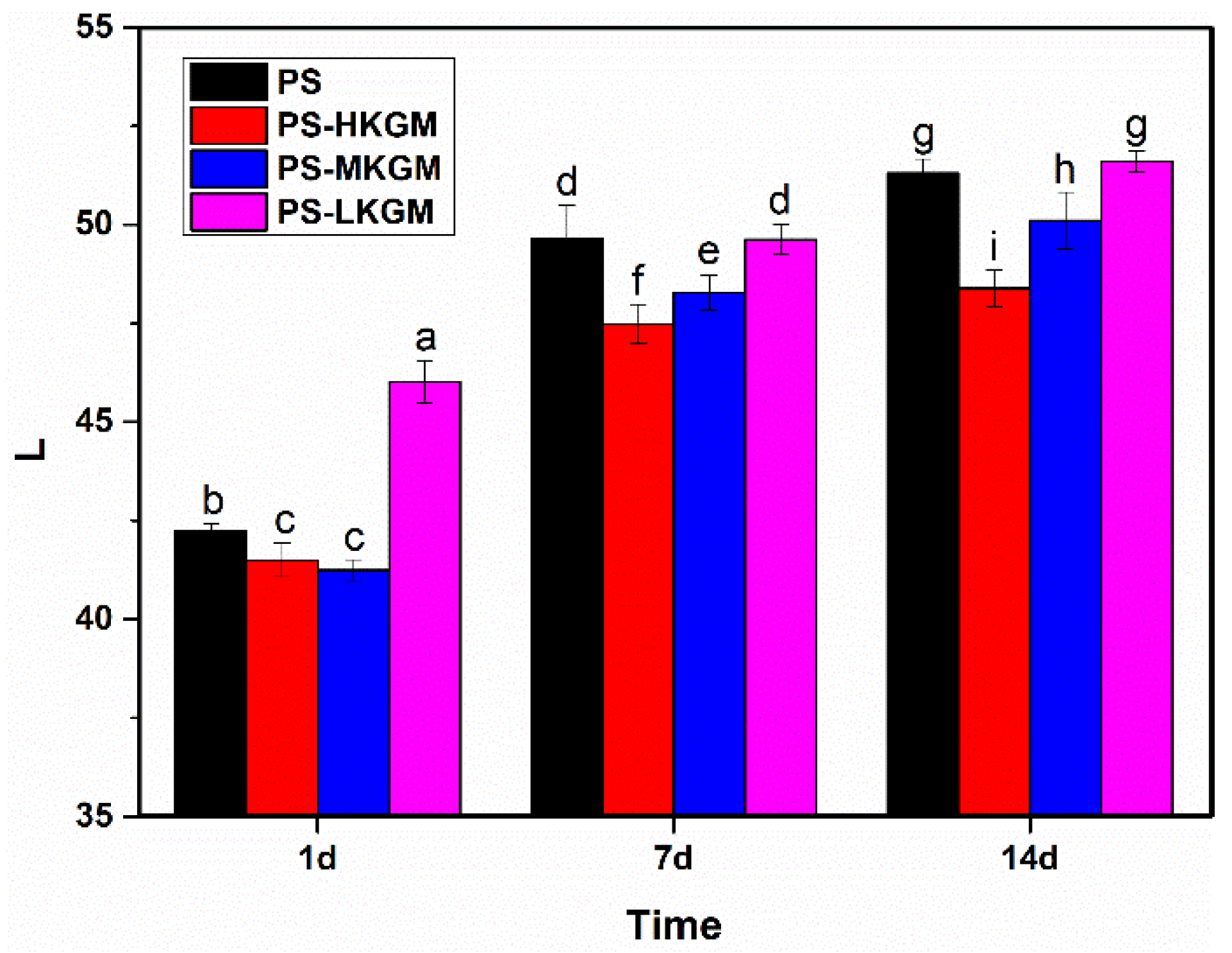
2.1. Lightness (L)
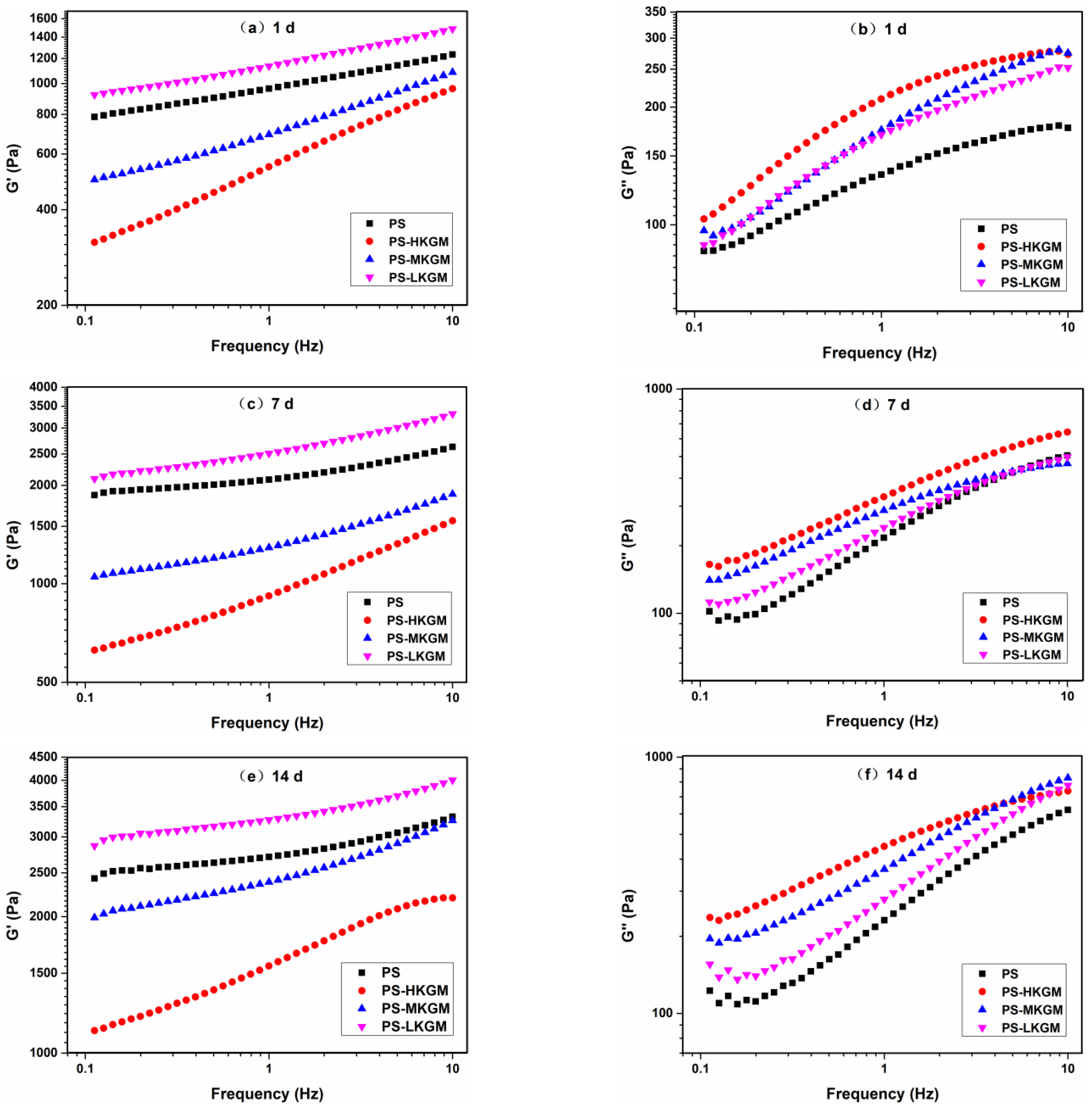
2.2. Dynamic Viscoelastic Properties
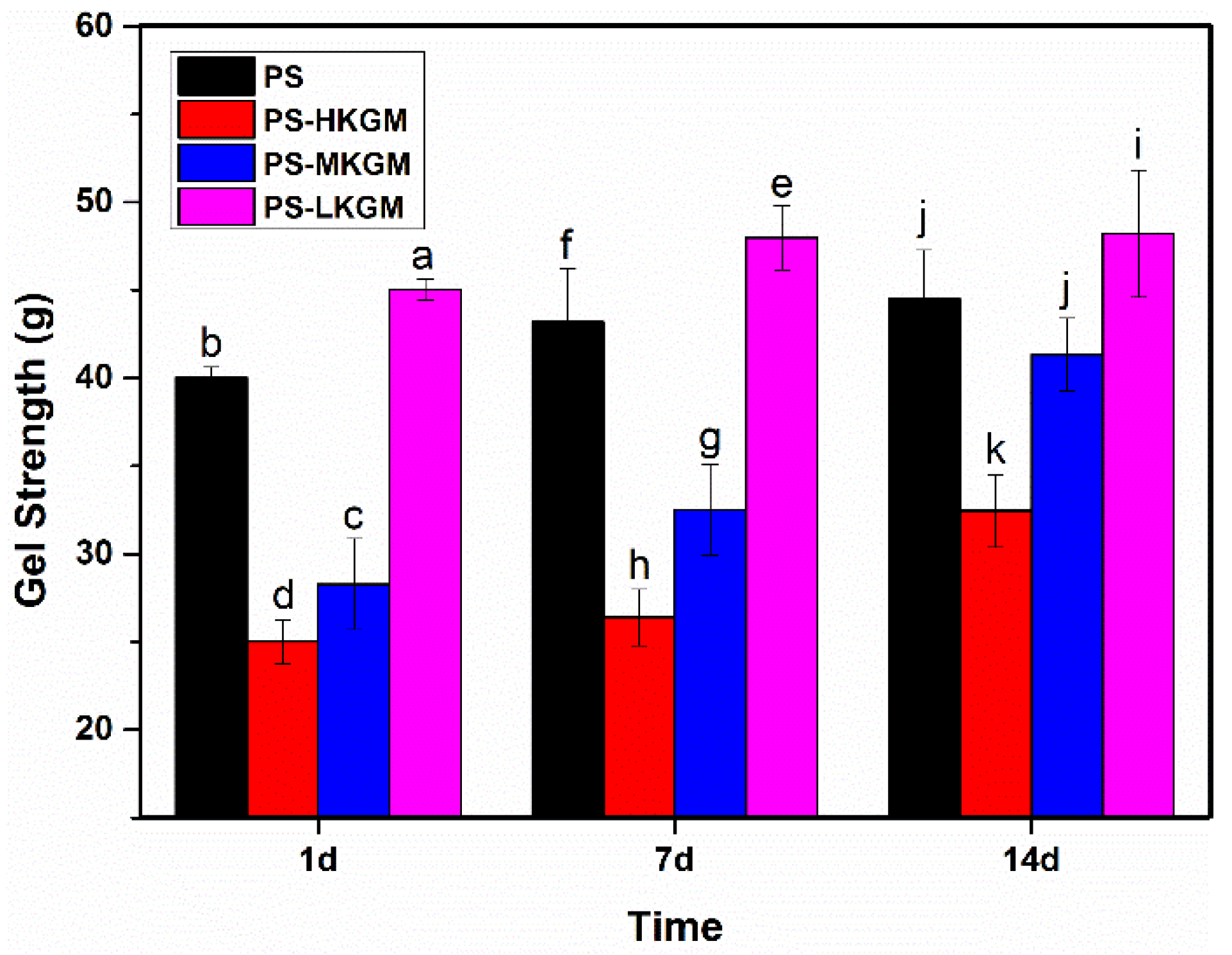
2.3. Gel Strength
2.4. Water Holding Capacity (WHC)
2.5. Moisture Distribution
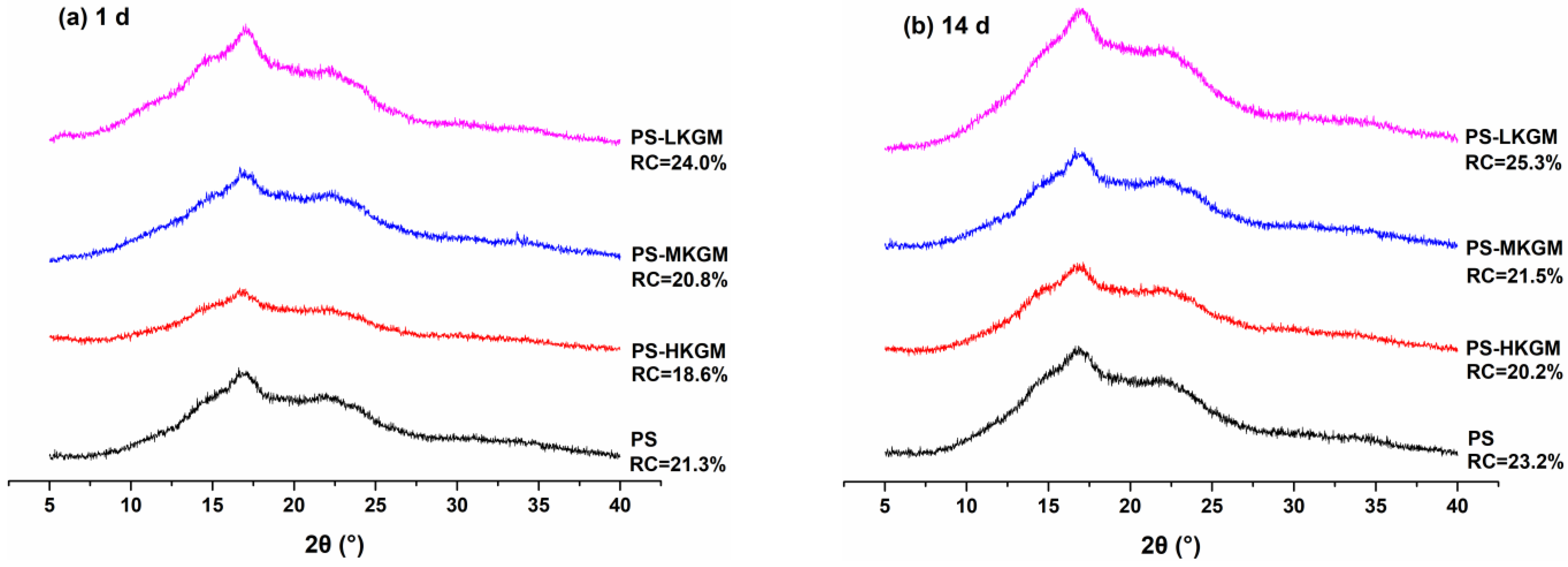
2.6. Crystallinity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PS-KGM Mixtures
4.3. Color Measurement
4.4. Dynamic Rheological Measurement
4.5. Gel Strength
4.6. Water Holding Capacity (WHC)
4.7. Low-Field Nuclear Magnetic Resonance (LF-NMR)
4.8. X-ray Diffractometry (XRD)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Li, F.; Luan, Y.; Li, P.; Dong, X.; Chen, M.; Dai, L.; Sun, Q. Gelatinization, pasting, and rheological properties of pea starch in alcohol solution. Food Hydrocoll. 2021, 112, 106331. [Google Scholar] [CrossRef]
- Matignon, A.; Tecante, A. Starch retrogradation: From starch components to cereal products. Food Hydrocoll. 2017, 68, 43–52. [Google Scholar] [CrossRef]
- Zhen, F.; Chen, J.; Luo, S.J.; Liu, C.M.; Wei, L. Effect of food additives on starch retrogradation: A review. Starch-Starke 2015, 67, 69–78. [Google Scholar]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Cornejo-Ramirez, Y.I.; Martinez-Cruz, O.; Del Toro-Sanchez, C.L.; WongCorral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Wei, X.; Yongzhao, X.; Zhifan, L.; Denglin, L.; Zhao, W.; Yuqing, S.; Ramin Shah, B. Stability, microstructural and rheological properties of complex prebiotic emulsion stabilized by sodium caseinate with inulin and konjac glucomannan. Food Hydrocoll. 2020, 105, 105772. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Li, J.; Liang, H.; Wei, X.; Peng, D.; Jiang, Z.; Li, B. Interaction between konjac glucomannan and tannic acid: Effect of molecular weight, pH and temperature. Food Hydrocoll. 2019, 94, 451–458. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Meindrawan, B.; Ofe, O.; Susanto, C.S.; Ayman, A.; Mangindaan, D.; Kasih, T.P. Glucomannan–Beeswax–Chitosan Antimicrobial Edible Coating to Maintain the Storage Quality of Salak Fruit (Salacca zalacca). Macromol. Symp. 2020, 391, 1900164. [Google Scholar] [CrossRef]
- Prabowo, A.S.; Mawarani, L.J. Edible Coating Development of Durian Seeds Starch and Glucomannan with The Addition of Essential Oil As An Antimicrobial to Increase Shelf Life of Tomato and Cauliflower. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012034. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Cao, Y.; Liu, S.; Deng, D.; Zhang, J.; Huang, G. Effects of xanthan and konjac gums on pasting, rheology, microstructure, crystallinity and in vitro digestibility of mung bean resistant starch. Food Chem. 2021, 339, 128001. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, C.; Liu, C.; Wang, P. Effect of Konjac Glucomannan on Gelatinization, Retrogradation, and Gelling Properties of Frozen Wheat Starch. Starch-Starke 2020, 73, 2000025. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Bail, K.L.; Garnier, C.; Llamas, G.; Queveau, D.; Pontoire, B.; Srzednicki, G.; Bail, P.L. Available water in konjac glucomannan–starch mixtures. Influence on the gelatinization, retrogradation and complexation properties of two starches. Food Hydrocoll. 2014, 41, 71–78. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, B.; Yuan, C.; Zou, Y.; Pan, Y. Effects of konjac glucomannan on the rheological, microstructure and digestibility properties of debranched corn starch. Food Hydrocoll. 2019, 100, 105342. [Google Scholar] [CrossRef]
- Song, R.; Huang, M.; Li, B.; Zhou, B. The Effect of Three Gums on the Retrogradation of Indica Rice Starch. Nutrients 2012, 4, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Yin, L.; Zhang, M.; Zhang, M.; Jia, X. Gelatinization, Retrogradation and Gel Properties of Wheat Starch–Wheat Bran Arabinoxylan Complexes. Gels 2021, 7, 200. [Google Scholar] [CrossRef]
- Cuidan, H.; Xiaohui, Z.; Mengqi, T.; Yulin, Z.; Runqiang, Y.; Zhenxin, G.; Pei, W. Impact of water extractable arabinoxylan with different molecular weight on the gelatinization and retrogradation behavior of wheat starch. Food Chem. 2020, 318, 126477. [Google Scholar] [CrossRef]
- Qiu, S.; Yadav, M.P.; Liu, Y.; Chen, H.; Tatsumi, E.; Yin, L. Effects of corn fiber gum with different molecular weights on the gelatinization behaviors of corn and wheat starch. Food Hydrocoll. 2016, 53, 180–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Yang, N.; Jin, Z.; Xu, X. Comparison of dextran molecular weight on wheat bread quality and their performance in dough rheology and starch retrogradation. LWT 2018, 98, 39–45. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, B.; Pan, Y.; Li, X.M.; Cheng, J.S.; Chen, H.Q. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chem. 2018, 264, 58–63. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Xu, B.; Ren, G.; Li, P.; Li, X.; Han, S.; Liu, J. Effects of inulin with different degree of polymerization on gelatinization and retrogradation of wheat starch. Food Chem. 2017, 229, 35–43. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Fan, X.; Wang, Q.; Wang, P.; Yuan, J.; Yu, Y.; Zhang, Y.; Cui, L. The effect of branched limit dextrin on corn and waxy corn gelatinization and retrogradation. Int. J. Biol. Macromol. 2018, 106, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Yousefi, A.; Ako, K. Steady/dynamic rheological characterization and FTIR study on wheat starch-sage seed gum blends. Food Hydrocoll. 2021, 111, 106380. [Google Scholar] [CrossRef]
- Fang, F. Shear-induced synergistic effects of konjac glucomannan and waxy potato starch on viscosity and gel strength. Food Hydrocoll. 2020, 114, 106540. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Fang, F.; Liu, J.; Zhang, F. The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch. Foods 2021, 10, 422. [Google Scholar] [CrossRef]
- Ma, S.P.; Zhu, P.L.; Wang, M.C. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Anbarani, N.M.; Razavi, S.M.A.; Taghizadeh, M. Impact of sage seed gum and whey protein concentrate on the functional properties and retrogradation behavior of native wheat starch gel. Food Hydrocoll. 2021, 111, 106261. [Google Scholar] [CrossRef]
- Xinbo, Z.; Lijian, W.; Xiping, J.; Yinji, C.; Guanghong, Z. Insight into the mechanism of myofibrillar protein gel influenced by konjac glucomannan: Moisture stability and phase separation behavior. Food Chem. 2021, 339, 127941. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M.; Wang, F.; Wang, N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocoll. 2019, 96, 663–670. [Google Scholar] [CrossRef]
- Sheng, L.; Li, P.; Wu, H.; Liu, Y.; Han, K.; Gouda, M.; Tong, Q.; Ma, M.; Jin, Y. Tapioca starch-pullulan interaction during gelation and retrogradation. LWT 2018, 96, 432–438. [Google Scholar] [CrossRef]
- Ji, N.; Li, F.; Yu, M.; Wang, Y.; Xiong, L.; Sun, Q. Inhibition of Long-Term Retrogradation of Corn, Potato, and Pea Starches by Borax. Starch-Starke 2020, 73, 2000045. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Tong, Q.; Zhang, Z.; Jin, Z. Effect of pullulan on the water distribution, microstructure and textural properties of rice starch gels during cold storage. Food Chem. 2017, 214, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chengxin, H.; Meiying, Z.; Liqiong, Z.; Hua, X.; Qiang, Z. Inhibition from whey protein hydrolysate on the retrogradation of gelatinized rice starch. Food Hydrocoll. 2020, 108, 105840. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Shen, M.; Xiao, Y.; Yu, Q.; Chen, Y.; Xie, J. Dual modifications on the gelatinization, textural, and morphology properties of pea starch by sodium carbonate and Mesona chinensis polysaccharide. Food Hydrocoll. 2020, 102, 105601. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, J.; Lu, H.; Chang, R.; Qiu, L.; Tian, Y. Amylopectin crystal seeds: Characterization and their effect on amylopectin retrogradation. Food Hydrocoll. 2021, 111, 106409. [Google Scholar] [CrossRef]
- Yunyun, W.; Bing, H.; Jinling, Z.; Rui, X.; Yaoqi, T. Effects of starchy seed crystals on the retrogradation of rice starch. Food Chem. 2020, 318, 126487. [Google Scholar] [CrossRef]
- Yu, L.; Yuehuan, X.; Mingyue, S.; Huiliang, W.; Yanming, R.; Jun, Y.; Xiuying, H.; Jianhua, X. Effect of Mesona chinensis polysaccharide on the retrogradation properties of maize and waxy maize starches during storage. Food Hydrocoll. 2020, 101, 105538. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Shang, L.; Zhou, P.; Li, B. An efficient and simple approach for the controlled preparation of partially degraded konjac glucomannan. Food Hydrocoll. 2020, 108, 106017. [Google Scholar] [CrossRef]
- Von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Mendez, M.A. Tapioca starch-galactomannan systems: Comparative studies of rheological and textural properties. Int. J. Biol. Macromol. 2019, 122, 1173–1183. [Google Scholar] [CrossRef]

| T21 (ms) | T22 (ms) | ||||||
|---|---|---|---|---|---|---|---|
| PS-HKGM | PS-MKGM | PS-LKGM | PS | PS-HKGM | PS-MKGM | PS-LKGM | |
| 1 d | 9.22 ± 1.06 a | 10.00 ± 2.83 a | 12.74 ± 2.46 a | 72.39 ± 6.83 a | 253.23 ± 10.60 a | 335.98 ± 15.55 a | 364.25 ± 10.31 a |
| 7 d | 6.14 ± 0.92 ab | 3.51 ± 0.74 bc | 11.75 ± 2.13 a | 59.15 ± 4.39 a | 200.92 ± 5.94 b | 253.23 ± 15.68 b | 297.64 ± 6.83 b |
| 14 d | 1.75 ± 0.16 b | 4.04 ± 0.62 b | 5.34 ± 0.11 b | 43.29 ± 3.82 a | 174.75 ± 10.28 bc | 174.75 ± 13.57 c | 200.92 ± 5.63 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, S.; Ding, L.; Zhang, Y.; He, J.; Li, B. Impact of Konjac Glucomannan with Different Molecular Weight on Retrogradation Properties of Pea Starch. Gels 2022, 8, 651. https://doi.org/10.3390/gels8100651
Wang S, Chen S, Ding L, Zhang Y, He J, Li B. Impact of Konjac Glucomannan with Different Molecular Weight on Retrogradation Properties of Pea Starch. Gels. 2022; 8(10):651. https://doi.org/10.3390/gels8100651
Chicago/Turabian StyleWang, Shishuai, Shuo Chen, Lidong Ding, Ying Zhang, Jiaxin He, and Bin Li. 2022. "Impact of Konjac Glucomannan with Different Molecular Weight on Retrogradation Properties of Pea Starch" Gels 8, no. 10: 651. https://doi.org/10.3390/gels8100651




