Formulation of Chitosan Microparticles for Enhanced Intranasal Macromolecular Compound Delivery: Factors That Influence Particle Size during Ionic Gelation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Microparticulate Formulations
2.2. Validation of the Fluorescence Spectroscopy Analytical Method
2.3. Preparation and Characterization of Microparticulate Delivery Systems Incorporating FD-4
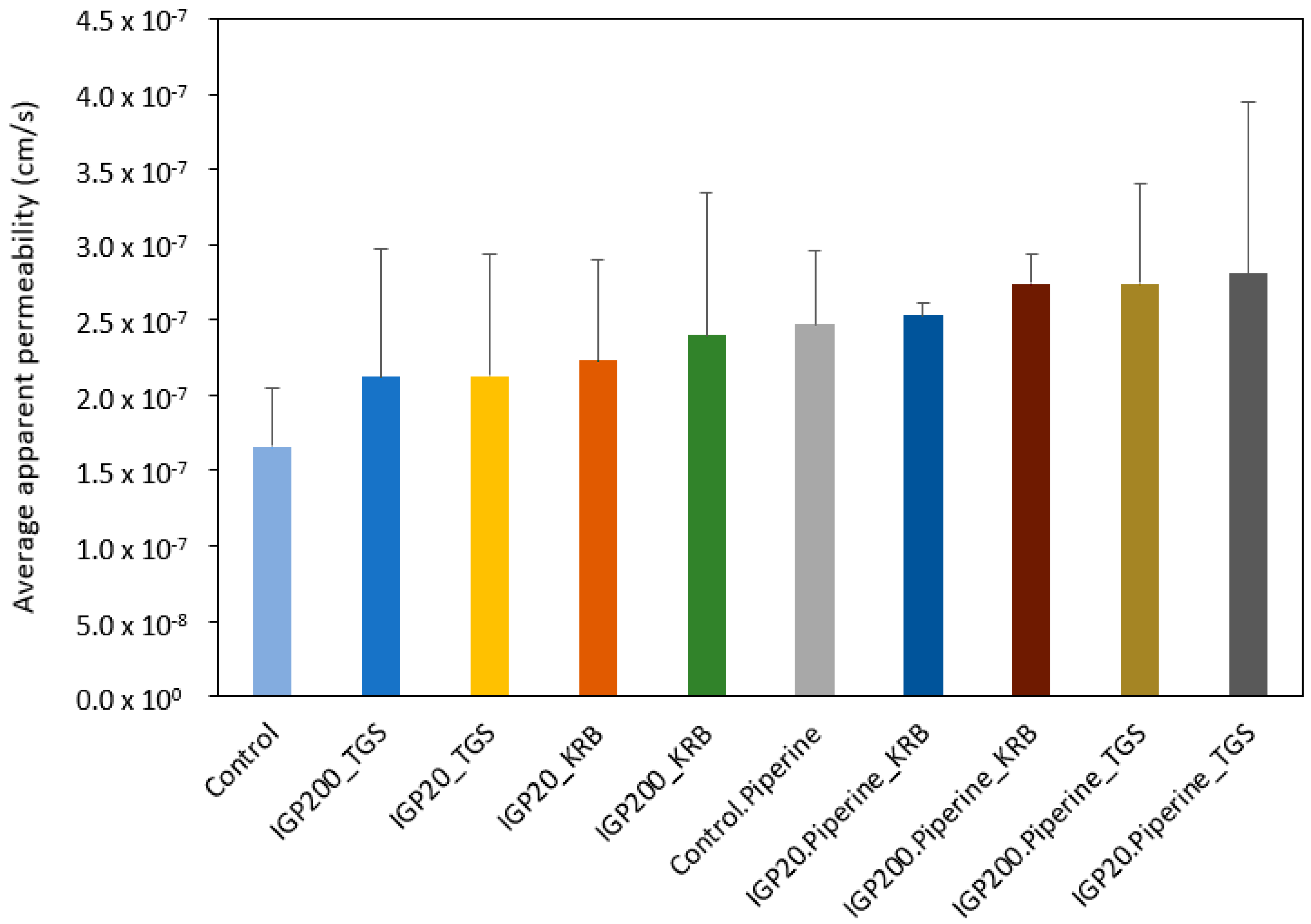
2.4. Ex Vivo Permeation Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Formulation (Phase 1)
4.2.1. Factors That Determine Microparticle Size during Ionic Gelation
4.2.2. Validation of the Fluorescence Spectroscopy Analytical Method
4.2.3. Preparation of the Target Microparticle Delivery Systems Incorporating FD-4
4.3. Ex Vivo Permeation Studies (Phase 2)
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Illum, L. Bioadhesive formulations for nasal peptide delivery. Drugs Pharm. Sci. 1999, 98, 507–539. [Google Scholar]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [PubMed]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Dillon, M.; Taylor, K.A.; Roberts, G.A. Improved method for ir determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Aspden, T.J.; Mason, J.D.; Jones, N.S.; Lowe, J.; Skaugrud, Ø.; Illum, L. Chitosan as a nasal delivery system: The effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Csaba, N.; Köping-Höggård, M.; Alonso, M.J. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int. J. Pharm. 2009, 382, 205–214. [Google Scholar] [CrossRef]
- Hamman, J.H.J.M.d. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-l.; Wu, S.-h.; Tao, Y.; Zang, L.-q.; Su, Z.-q. Preparation and characterization of water-soluble chitosan nanoparticles as protein delivery system. J. Nanomater. 2010, 2010, 898910. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.; Seijo, B.; Ortega-Vinuesa, J.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef]
- Vauthier, C.; Cabane, B.; Labarre, D. How to concentrate nanoparticles and avoid aggregation? Eur. J. Pharm. Biopharm. 2008, 69, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, A.; Miñarro, M.; García-Montoya, E.; Pérez-Lozano, P.; Carrillo, C.; Sarrate, R.; Sánchez, N.; Ticó, J.; Suñé-Negre, J. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan–tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Magalhães, T.C.; Teixeira, N.M.; Gondim, B.L.C.; Carlo, H.L.; Dos Santos, R.L.; de Oliveira, A.R.; Denadai, Â.M.L. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C 2019, 104, 109885. [Google Scholar] [CrossRef]
- Gerber, W.; Svitina, H.; Steyn, D.; Peterson, B.; Kotzé, A.; Weldon, C.; Hamman, J.H. Comparison of RPMI 2650 cell layers and excised sheep nasal epithelial tissues in terms of nasal drug delivery and immunocytochemistry properties. J. Pharmacol. Toxicol. Methods 2022, 113, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Gerber, W.; Steyn, D.; Kotzé, A.; Svitina, H.; Weldon, C.; Hamman, J. Permeation enhancement effects of leaf materials from different aloe species on in vitro and ex vivo nasal epithelial models. J. Herbmed Pharmacol. 2020, 9, 355–365. [Google Scholar] [CrossRef]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved in vitro model for intranasal mucosal drug delivery: Primary olfactory and respiratory epithelial cells compared with the permanent Nasal cell line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Smith, J.L.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells. Int. Forum Allergy Rhinol. 2020, 10, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-H.; Wu, X.-T.; Guo, W.-C.; Du, Y.-M.; Yu, L.; Tang, J. N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a novel delivery system for Parathyroid Hormone-Related Protein 1–34. Int. J. Pharm. 2010, 393, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Silverio, G.B.; Sakanaka, L.S.; Alvim, I.D.; Shirai, M.A.; Grosso, C.R.F. Production and characterization of alginate microparticles obtained by ionic gelation and electrostatic adsorption of concentrated soy protein. Ciência Rural 2018, 48, e20180637. [Google Scholar] [CrossRef] [Green Version]
- de Moura, S.C.; Berling, C.L.; Germer, S.P.; Alvim, I.D.; Hubinger, M.D. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef]
- Prata, A.; Grosso, C. Production of microparticles with gelatin and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Alvim, I.D.; Cortez, M.A.S. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. J. Food Sci. Technol. 2019, 56, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Consoli, L.; Grimaldi, R.; Sartori, T.; Menegalli, F.C.; Hubinger, M.D. Gallic acid microparticles produced by spray chilling technique: Production and characterization. LWT-Food Sci. Technol. 2016, 65, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of β-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef]
- U.S. Pharmacopeia. Principles of a Sound Drug Formulary System; US Pharmacopeia: Rockville, MD, USA, 2000; Volume 786, pp. 1965–1967. [Google Scholar]
- Santos, E. Formulation of Metformin-Loaded Alginate Microspheres by Ionotropic Gelation-Aerosolization Technique. Sains Malays. 2020, 49, 2513–2525. [Google Scholar]
- Sedyakina, N.; Kuskov, A.; Velonia, K.; Feldman, N.; Lutsenko, S.; Avramenko, G. Modulation of Entrapment Efficiency and In Vitro Release Properties of BSA-Loaded Chitosan Microparticles Cross-Linked with Citric Acid as a Potential Protein–Drug Delivery System. Materials 2020, 13, 1989. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-H.; Kitamura, Y.; Kokawa, M. Liquid-core alginate hydrogel beads loaded with functional compounds of radish by-products by reverse spherification: Optimization by response surface methodology. Int. J. Biol. Macromol. 2017, 96, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; European Coatings: Nurnberg, Germany, 2020. [Google Scholar]
- Tadros, T.F. Rheology of Dispersions: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Eirich, F. Rheology V2: Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Murata, H. Rheology-Theory and application to biomaterials. Janeza Trdine 2012, 9, 403–426. [Google Scholar]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Marzok, S.; Ibrahim, M. Development of optimized mucoadhesive thermosensitive pluronic based in situ gel for controlled delivery of Latanoprost: Antiglaucoma efficacy and stability approaches. J. Drug Deliv. Sci. Technol. 2019, 53, 101134. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive In situ gel: Optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Özer, S.; Şenel, B.; Yazan, Y. Preparation and in vitro evaluation of in situ gelling system containing lithium carbonate for parenteral administration. Polym. Bull. 2020, 77, 599–622. [Google Scholar] [CrossRef]
- Wei, G.; Xu, H.; Ding, P.T.; Zheng, J.M. Thermosetting gels with modulated gelation temperature for ophthalmic use: The rheological and gamma scintigraphic studies. J. Control. Release 2002, 83, 65–74. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wong, C.-H.; Chan, H.E.; Lee, W.-Y.; Zuo, Z. Statistical Design of Experiment (DoE) based development and optimization of DB213 in situ thermosensitive gel for intranasal delivery. Int. J. Pharm. 2018, 539, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Rasve, G.; Borade, G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lungare, S.; Bowen, J.; Badhan, R. Development and Evaluation of a Novel Intranasal Spray for the Delivery of Amantadine. J. Pharm. Sci. 2016, 105, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Yang, X.; Trinh, H.M.; Agrahari, V.; Sheng, Y.; Pal, D.; Mitra, A.K. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech 2016, 17, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, D.E.; Dürig, C.; Jähne, E.A.; Smieško, M.; Culot, M.; Gosselet, F.; Cecchelli, R.; Helms, H.C.C.; Brodin, B.; Wimmer, L. In vitro blood–brain barrier permeability predictions for GABAA receptor modulating piperine analogs. Eur. J. Pharm. Biopharm. 2016, 103, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Gerber, W.; Steyn, D.; Kotzé, A.; Svitina, H.; Weldon, C.; Hamman, J. Capsaicin and piperine as functional excipients for improved drug delivery across nasal epithelial models. Planta Med. 2019, 85, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Viegas, D.A.; Rodrigues, M.; Francisco, J.; Falcão, A.; Alves, G.; Santos, A.O. Development and application of an ex vivo fosphenytoin nasal bioconversion/permeability evaluation method. Eur. J. Pharm. Sci. 2016, 89, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- de Pinho Neves, A.L.; Milioli, C.C.; Müller, L.; Riella, H.G.; Kuhnen, N.C.; Stulzer, H.K. Factorial design as tool in chitosan nanoparticles development by ionic gelation technique. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 34–39. [Google Scholar] [CrossRef]
- Yan, J.; Guan, Z.-Y.; Zhu, W.-F.; Zhong, L.-Y.; Qiu, Z.-Q.; Yue, P.-F.; Wu, W.-T.; Liu, J.; Huang, X. Preparation of Puerarin Chitosan Oral Nanoparticles by Ionic Gelation Method and Its Related Kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvern Instruments Ltd. Mastersizer 3000 User Manual; Malvern Instruments Ltd.: Malvern, UK, 2013. [Google Scholar]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- Nakamura, K.; Maitani, Y.; Takayama, K. The enhancing effect of nasal absorption of FITC-dextran 4,400 by β-sitosterol β-D-glucoside in rabbits. J. Control. Release 2002, 79, 147–155. [Google Scholar] [CrossRef]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: Optimization of selected culture conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, Y.K.; Yoon, H.; Kim, J.M.; Kim, C.K. In situ gelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNA. J. Biomed. Mater. Res. 2002, 59, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Nair, H. Formulation and evaluation of thermosensitive biogels for nose to brain delivery of doxepin. BioMed Res. Int. 2014, 2014, 847547. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, K.; Dighe, P.; Kharat, A.; Patil, S. Mathematical models of drug dissolution: A review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar]
- Schmidt, M.C.; Simmen, D.; Hilbe, M.; Boderke, P.; Ditzinger, G.; Sandow, J.; Lang, S.; Rubas, W.; Merkle, H.P. Validation of excised bovine nasal mucosa as in vitro model to study drug transport and metabolic pathways in nasal epithelium. J. Pharm. Sci. 2000, 89, 396–407. [Google Scholar] [CrossRef]
- Wadell, C.; Björk, E.; Camber, O. Nasal drug delivery–evaluation of an in vitro model using porcine nasal mucosa. Eur. J. Pharm. Sci. 1999, 7, 197–206. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Rižner, T.L.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The characterization of the human nasal epithelial cell line RPMI 2650 under different culture conditions and their optimization for an appropriate in vitro nasal model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- StatSoft Inc. Statistica (Data Analysis Software System), Version 7; StatSoft Inc.: Tulsa, OK, USA, 2004. [Google Scholar]
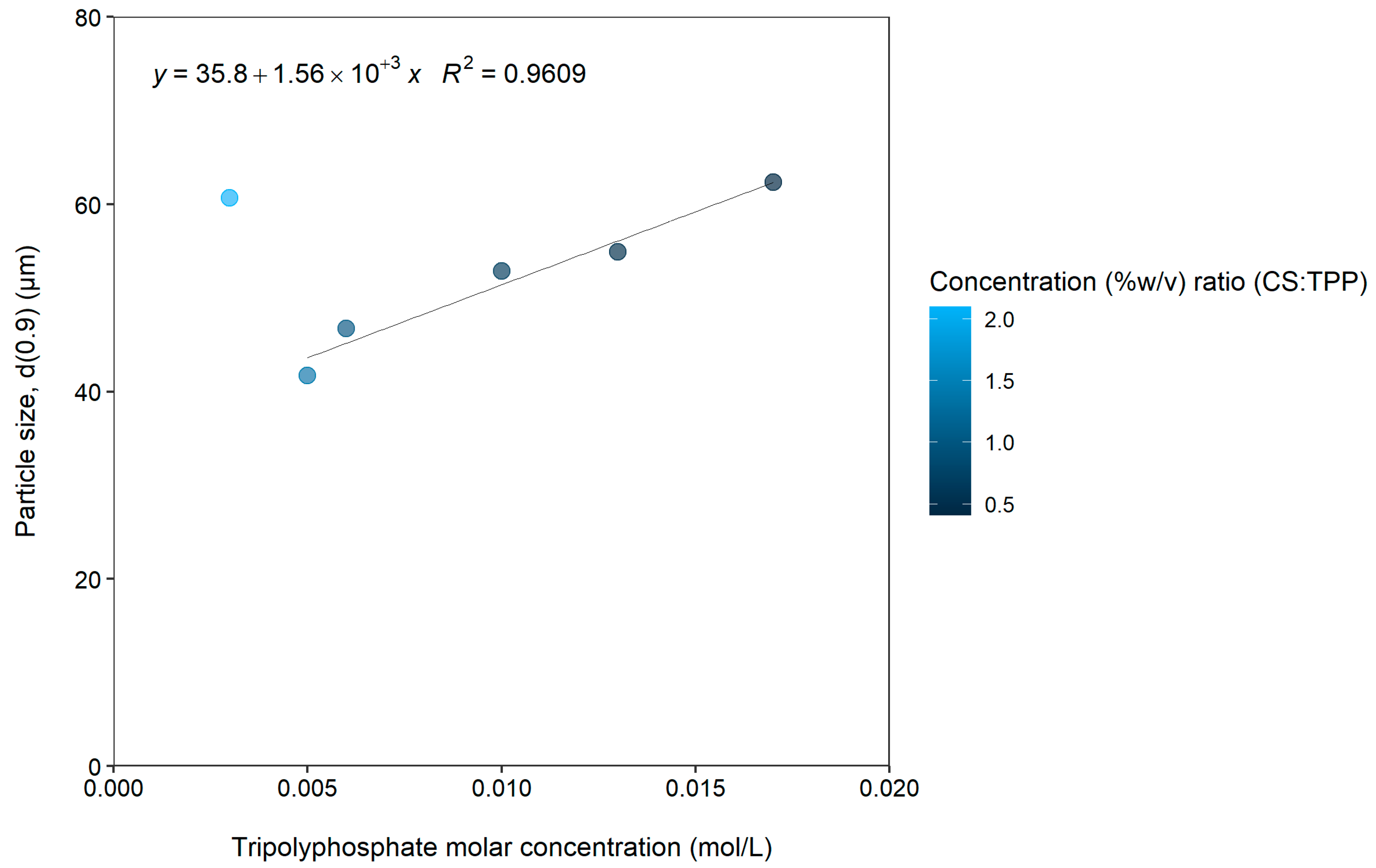
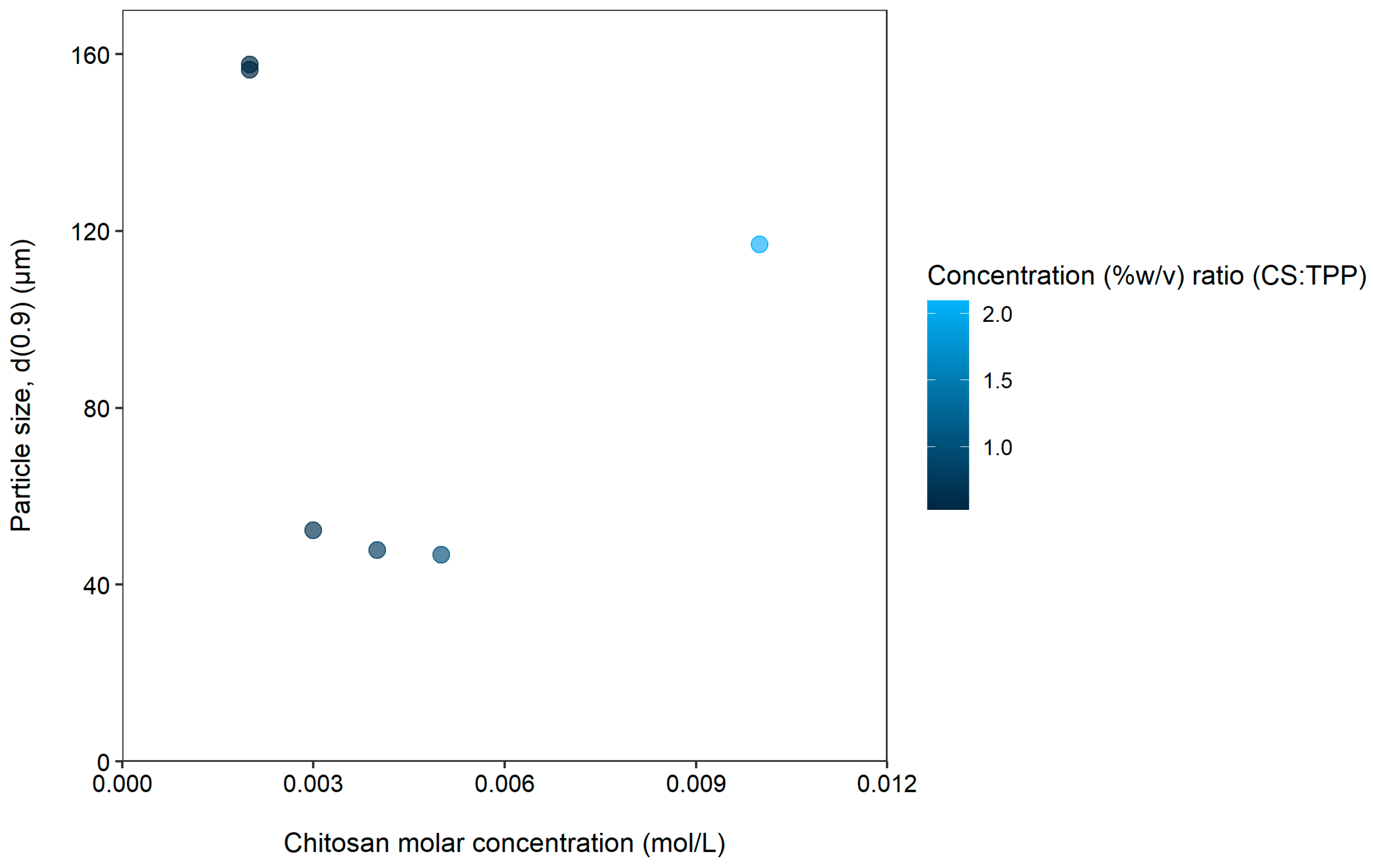



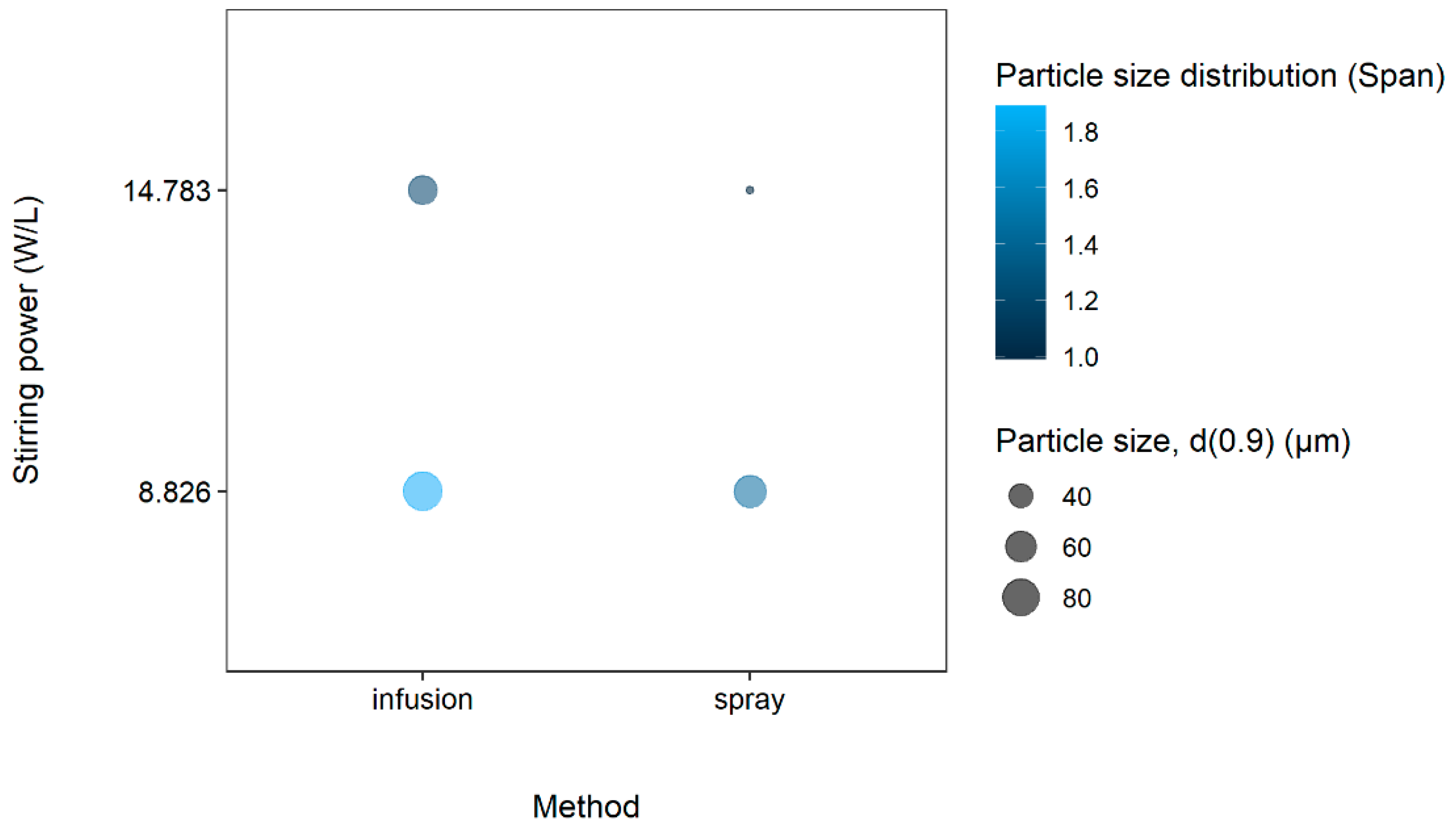
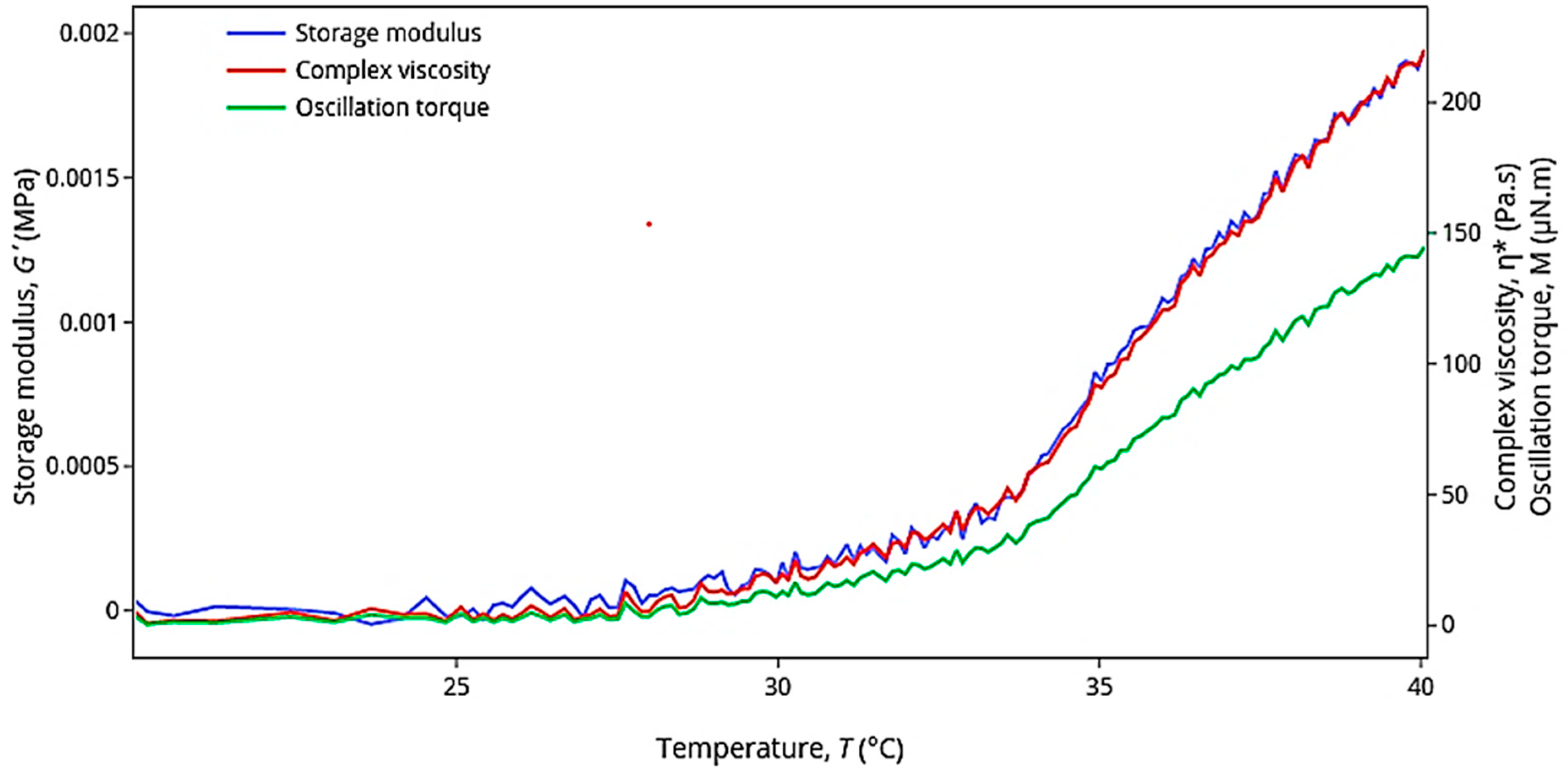


| Formulation | CS Molar Concentration (mol/L) | TPP Molar Concentration (mol/L) | Concentration (%w/v) Ratio (CS:TPP) * | Span | d(0.9) (µm) |
|---|---|---|---|---|---|
| 1 | 0.005 | 0.017 | 0.409 | 1.28 | 62.385 |
| 2 | 0.013 | 0.524 | 1.24 | 54.913 | |
| 3 | 0.01 | 0.699 | 1.25 | 52.906 | |
| 4 | 0.006 | 1.048 | 1.22 | 46.763 | |
| 5 | 0.005 | 1.395 | 1.3 | 41.707 | |
| 6 | 0.003 | 2.097 | 1.25 | 60.709 | |
| 7 | 0.002 | 0.006 | 0.524 | 1.8 | 157.692 |
| 8 | 0.002 | 0.524 | 3.06 | 156.534 | |
| 9 | 0.003 | 0.699 | 1.24 | 52.286 | |
| 10 | 0.004 | 0.839 | 1.26 | 47.769 | |
| 11 | 0.005 | 1.048 | 1.22 | 46.763 | |
| 12 | 0.01 | 2.097 | 2 | 117.014 |
| Parameter | Small Microparticles | Large Microparticles |
|---|---|---|
| d(0.9) (µm) | 22.194 | 190.218 |
| Span | 0.986 | 2.099 |
| Chitosan molar concentration (mol/L) | 0.005 | 0.02 |
| Tripolyphosphate molar concentration (mol/L) | 0.006 | 0.013 |
| Dispersing technique | Atomizing spray | Infusion droplet |
| Flow rate (mL/min) | 2.5 | 25 |
| Stirring power (W/L) | 17.17 | 8.8 |
| Temperature (°C) | 8 | 40 |
| Fraction | FD-4 Concentration (mol/L) | |
|---|---|---|
| Small Microparticle Delivery System | Large Microparticle Delivery System | |
| Fraction on external surface of microparticles | 0.167 | 0.174 |
| Fraction released from microparticles | 0.299 | 0.281 |
| Fraction bound to the microparticles | 0.005 | 0.005 |
| Measured concentration in all fractions | 0.471 | 0.461 |
| Theoretical concentration | 0.5 | 0.5 |
| Entrapment efficiency (%) | 59.78 | 56.24 |
| Net loss (%) | 5.90 | 7.80 |
| Formulation | Criteria | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Weibull |
|---|---|---|---|---|---|---|
| IGP20.Piperine_KRB | R2-adjusted | −0.5894 | 0.8211 | 0.4431 | 0.9894 | 0.9856 |
| AIC | 85.9625 | 66.2664 | 76.1901 | 41.7018 | 43.6409 | |
| MSC | −2.1729 | 0.0155 | −1.0871 | 2.7449 | 2.5295 | |
| IGP200.Piperine_KRB | R2-adjusted | −0.0545 | 0.7450 | 0.7083 | 0.9762 | 0.9637 |
| AIC | 81.6471 | 68.8267 | 69.2687 | 47.2805 | 52.7882 | |
| MSC | −1.3086 | 0.1159 | 0.0668 | 2.5099 | 1.8980 | |
| IGP20.Piperine_TGS | R2-adjusted | −0.2424 | 0.8091 | 0.6334 | 0.9916 | 0.9879 |
| AIC | 83.9562 | 66.3932 | 72.5389 | 38.2104 | 42.8303 | |
| MSC | −1.6574 | 0.2941 | −0.3888 | 3.4255 | 2.9122 | |
| IGP200.Piperine_TGS | R2-adjusted | 0.3023 | 0.8401 | 0.8641 | 0.9772 | 0.9609 |
| AIC | 78.6442 | 65.3663 | 63.8679 | 47.6297 | 51.9458 | |
| MSC | −0.6865 | 0.7888 | 0.9553 | 2.7595 | 2.2799 |
| Formulation | Chitosan Concentration (mol/L) | Tripolyphosphate Concentration (mol/L) | Concentration (%w/v) Ratio (CS:TPP) * |
|---|---|---|---|
| 1 | 0.005 | 0.017 | 0.409 |
| 2 | 0.013 | 0.524 | |
| 3 | 0.01 | 0.699 | |
| 4 | 0.006 | 1.048 | |
| 5 | 0.005 | 1.395 | |
| 6 | 0.003 | 2.097 | |
| 7 | 0.002 | 0.006 | 0.524 |
| 8 | 0.002 | 0.524 | |
| 9 | 0.003 | 0.699 | |
| 10 | 0.004 | 0.839 | |
| 11 | 0.005 | 1.048 | |
| 12 | 0.01 | 2.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weyers, M.; Peterson, B.; Hamman, J.H.; Steenekamp, J.H. Formulation of Chitosan Microparticles for Enhanced Intranasal Macromolecular Compound Delivery: Factors That Influence Particle Size during Ionic Gelation. Gels 2022, 8, 686. https://doi.org/10.3390/gels8110686
Weyers M, Peterson B, Hamman JH, Steenekamp JH. Formulation of Chitosan Microparticles for Enhanced Intranasal Macromolecular Compound Delivery: Factors That Influence Particle Size during Ionic Gelation. Gels. 2022; 8(11):686. https://doi.org/10.3390/gels8110686
Chicago/Turabian StyleWeyers, Morné, Bianca Peterson, Josias H. Hamman, and Jan H. Steenekamp. 2022. "Formulation of Chitosan Microparticles for Enhanced Intranasal Macromolecular Compound Delivery: Factors That Influence Particle Size during Ionic Gelation" Gels 8, no. 11: 686. https://doi.org/10.3390/gels8110686






