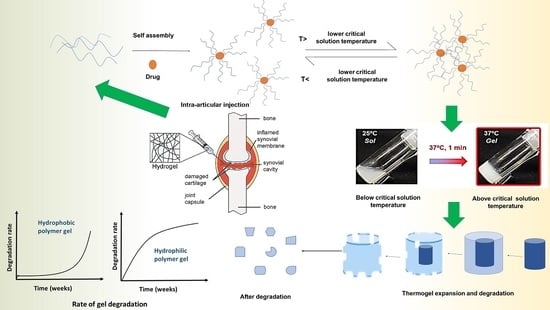
Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery
Abstract
1. Introduction
2. Intra-Articular Administration of Therapeutics
2.1. Types of Intra-Articular Treatments in Joint Diseases such as Osteoarthritis
2.1.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
2.1.2. Hyaluronic Acid
2.1.3. Corticosteroid Injections
2.1.4. Platelet-Rich Plasma (PRP)
2.1.5. Stem Cell Therapy
3. Thermosensitive Gels and Their Comparison to Other Stimuli-Sensitive Gels
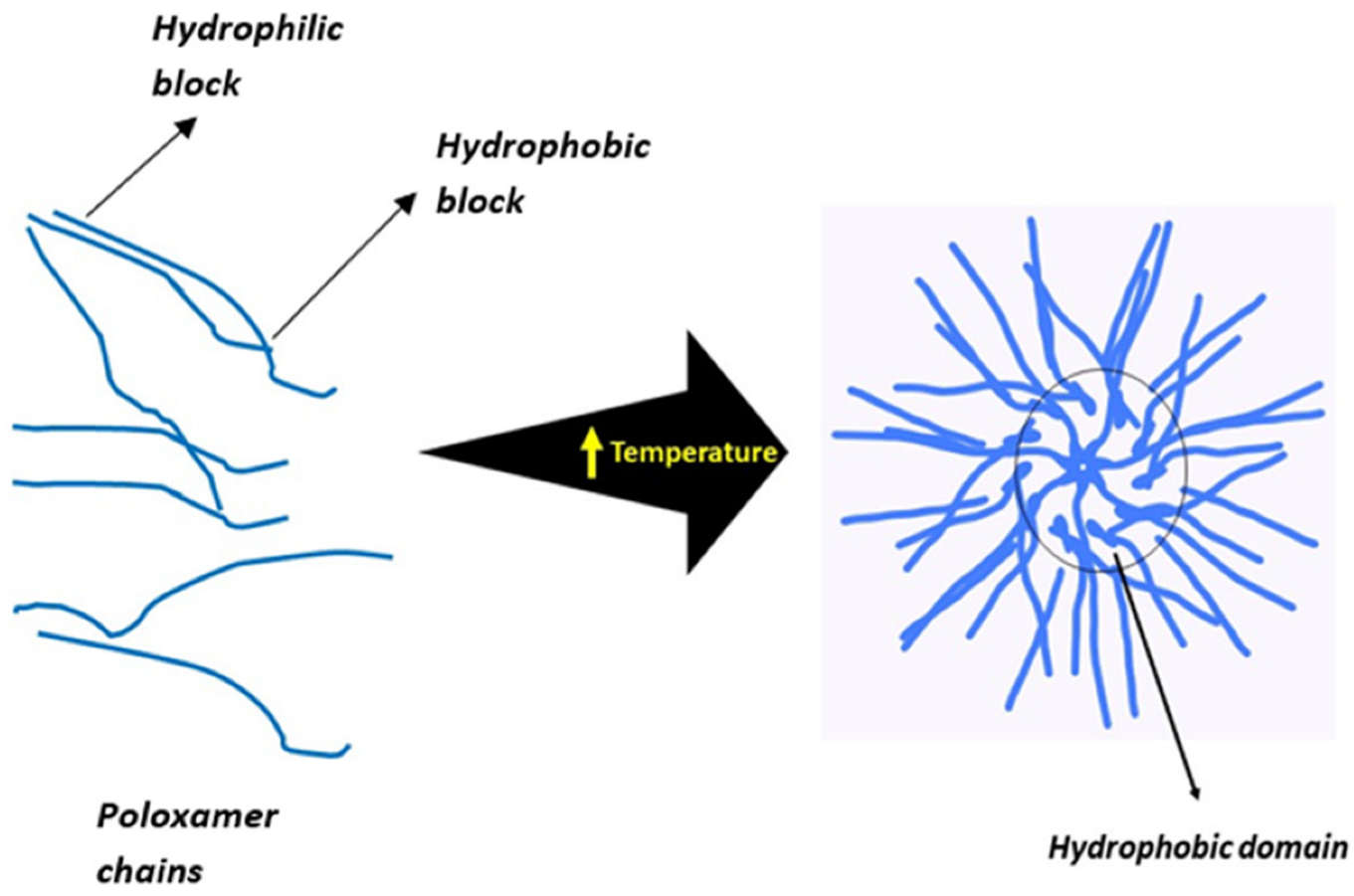
4. Temperature-Sensitive Materials and Gelation Mechanism
4.1. Positive Thermosensitive Hydrogels
4.2. Negative Thermosensitive Hydrogels
5. Thermosensitive In Situ Gels Loaded with Micro and Nanoparticles
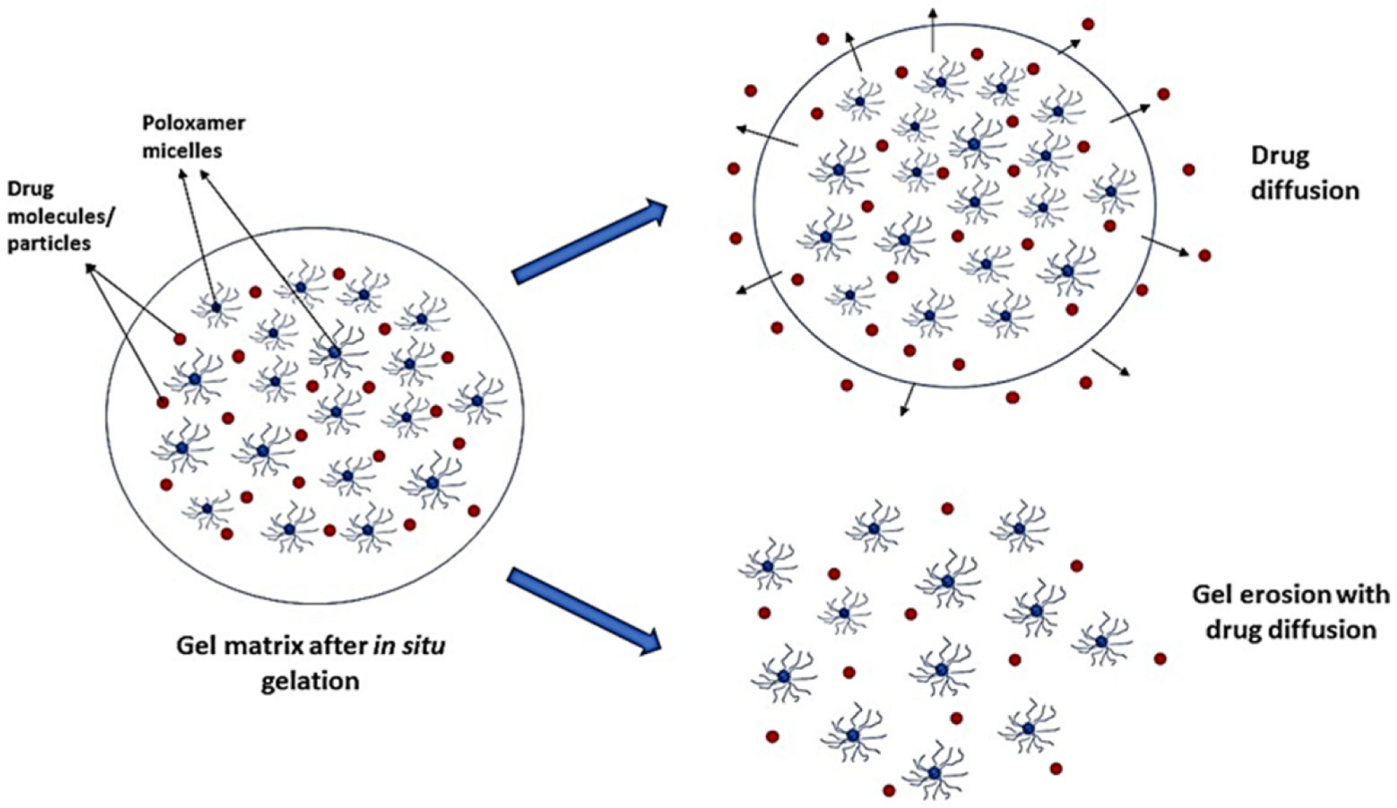
6. Drug Release Mechanism from Thermoreversible In Situ Gels
7. Challenges in Formulation and Evaluation
8. Stability of Thermosensitive Gels In Vitro and In Vivo with Biodegradation
9. Clinical Applications of Intra-Articular Hydrogels
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burt, H.M.; Tsallas, A.; Gilchrist, S.; Liang, L.S. Intra-Articular Drug Delivery Systems: Overcoming the Shortcomings of Joint Disease Therapy. Expert Opin. Drug Deliv. 2009, 6, 17–26. [Google Scholar] [CrossRef]
- Wasserman, A.M. Diagnosis and Management of Rheumatoid Arthritis. Am. Fam. Physician 2011, 84, 1245–1252. [Google Scholar] [CrossRef]
- Smolen, J.S.; Steiner, G. Therapeutic Strategies for Rheumatoid Arthritis. Nat. Rev. Drug Discov. 2003, 2, 473–488. [Google Scholar] [CrossRef]
- Rai, M.F.; Pham, C.T. Intra-Articular Drug Delivery Systems for Joint Diseases. Curr. Opin. Pharmacol. 2018, 40, 67. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, X.; Lin, R.; Sun, A.R.J.; Song, J.; Ye, Z.; Liang, D.; Zhang, M.; Tian, J.; Zhou, X.; et al. Knee Osteoarthritis Therapy: Recent Advances in Intra-Articular Drug Delivery Systems. Drug Des. Devel. Ther. 2022, 16, 1311–1347. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in Intra-Articular Therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Zhang, Z.; Yang, Z.; Huang, G. Intra-Articular Delivery of Tetramethylpyrazine Microspheres with Enhanced Articular Cavity Retention for Treating Osteoarthritis. Asian J. Pharm. Sci. 2018, 13, 229–238. [Google Scholar] [CrossRef]
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; García, A.J. Synthesis of Self-Assembled IL-1Ra-Presenting Nanoparticles for the Treatment of Osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599. [Google Scholar] [CrossRef]
- Pawar, V.A.; Manjappa, A.S.; Murumkar, P.R.; Gajaria, T.K.; Devkar, R.V.; Mishra, A.K.; Yadav, M.R. Drug-Fortified Liposomes as Carriers for Sustained Release of NSAIDs: The Concept and Its Validation in the Animal Model for the Treatment of Arthritis. Eur. J. Pharm. Sci. 2018, 125, 11–22. [Google Scholar] [CrossRef]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-Articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium-Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Mansour, H.F. Comparative Studies for Ciprofloxacin Hydrochloride Pre-Formed Gels and Thermally Triggered (in Situ) Gels: In Vitro and in Vivo Appraisal Using a Bacterial Keratitis Model in Rabbits. Pharm. Dev. Technol. 2015, 20, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.A.; Villamagna, I.J.; Borecki, A.; Beier, F.; De Bruyn, J.R.; Hurtig, M.; Gillies, E.R. Thermoresponsive and Covalently Cross-Linkable Hydrogels for Intra-Articular Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 3498–3507. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 246. [Google Scholar] [CrossRef]
- Anita, C.; Munira, M.; Mural, Q.; Shaily, L. Topical Nanocarriers for Management of Rheumatoid Arthritis: A Review. Biomed. Pharmacother. 2021, 141, 111880. [Google Scholar] [CrossRef]
- Dyondi, D.; Lakhawat, R.; Banerjee, R. Biodegradable Nanoparticles for Intra-Articular Therapy. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2009, 3, 33–41. [Google Scholar]
- Oliveira, I.M.; Fernandes, D.C.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. Hydrogels in the Treatment of Rheumatoid Arthritis: Drug Delivery Systems and Artificial Matrices for Dynamic in Vitro Models. J. Mater. Sci. Mater. Med. 2021, 32, 1–13. [Google Scholar] [CrossRef]
- Uson, J.; Rodriguez-Garciá, S.C.; Castellanos-Moreira, R.; O’Neill, T.W.; Doherty, M.; Boesen, M.; Pandit, H.; Möller Parera, I.; Vardanyan, V.; Terslev, L.; et al. EULAR Recommendations for Intra-Articular Therapies. Ann. Rheum. Dis. 2021, 80, 1299–1305. [Google Scholar] [CrossRef]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-Engineered Intra-Articular Drug Delivery Systems for Osteoarthritis Therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Poiraudeau, S. Non-Pharmacological Approaches for the Treatment of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 146–154. [Google Scholar] [CrossRef]
- Shi, J.; Fan, K.; Yan, L.; Fan, Z.; Li, F.; Wang, G.; Liu, H.; Liu, P.; Yu, H.; Li, J.J.; et al. Cost Effectiveness of Pharmacological Management for Osteoarthritis: A Systematic Review. Appl. Health Econ. Health Policy 2022, 20, 351. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, R.; Wang, H.; Yang, J.; Xu, J.; Zha, Z. Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 2017, 18, 374–385. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, J.A.D.; Wiegant, K.; van Heerwaarden, R.J.; Spruijt, S.; van Roermund, P.M.; Custers, R.J.H.; Mastbergen, S.C.; Lafeber, F.P.J.G. Knee Joint Distraction Compared with High Tibial Osteotomy: A Randomized Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Sayed Aly, M.N. Intra-Articular Drug Delivery: A Fast Growing Approach. Recent Patents Drug Deliv. Formul. 2008, 2, 231–237. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Öz, U.C.; Bozkir, A. In Situ Hydrogel Formulation for Intra-Articular Application of Diclofenac Sodium-Loaded Polymeric Nanoparticles. Turk. J. Pharm. Sci. 2017, 14, 56. [Google Scholar] [CrossRef]
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-Articular Injections for the Treatment of Osteoarthritis: Focus on the Clinical Use of Hyaluronic Acid. Drugs R D 2011, 11, 13–27. [Google Scholar] [CrossRef]
- Baron, D.; Flin, C.; Porterie, J.; Despaux, J.; Vincent, P. Hyaluronic Acid Single Intra-Articular Injection in Knee Osteoarthritis: A Multicenter Open Prospective Study (ART-ONE 75) with Placebo Post Hoc Comparison. Curr. Ther. Res. Clin. Exp. 2018, 88, 35. [Google Scholar] [CrossRef] [PubMed]
- Suppan, V.K.L.; Tew, M.M.; Wong, B.C.; Chan, H.K.; Chew, Y.W.; Tan, C.S.; Nanta Kumar, V.K.; Shafie, A.A.; Sadashiva Rao, A. One-Year Follow-up of Efficacy and Cost of Repeated Doses versus Single Larger Dose of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis. J. Orthop. Surg. 2020, 28, 2309499019895029. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef]
- Nawrat, P.; Surazyński, A.; Karna, E.; Pałka, J.A. The Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Collagen Metabolism in Cultured Human Skin Fibroblasts. Pharmacol. Res. 2005, 51, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Wang, H.; Chen, J.; Zhang, Z.; Li, F.; Xie, Y.; Huang, Y.; Peng, T.; Cheng, G.; Pan, X.; et al. Self-Assembled Lyotropic Liquid Crystal Gel for Osteoarthritis Treatment via Anti-Inflammation and Cartilage Protection. Biomater. Sci. 2021, 9, 7205–7218. [Google Scholar] [CrossRef]
- Uthman, I.; Raynauld, J.P.; Haraoui, B. Intra-Articular Therapy in Osteoarthritis. Postgrad. Med. J. 2003, 79, 449–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-Articular Treatment of Knee Osteoarthritis: From Anti-Inflammatories to Products of Regenerative Medicine. Phys. Sportsmed. 2016, 44, 101. [Google Scholar] [CrossRef]
- McGarry, J.G.; Daruwalla, Z.J. The Efficacy, Accuracy and Complications of Corticosteroid Injections of the Knee Joint. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1649–1654. [Google Scholar] [CrossRef]
- Hall, M.P.; Band, P.A.; Meislin, R.T.; Jazrawi, L.M.; Cardone, D.A. Platelet-Rich Plasma: Current Concepts and Application in Sports Medicine. J. Am. Acad. Orthop. Surg. 2009, 17, 602–608. [Google Scholar] [CrossRef]
- Demange, M.K.; Sisto, M.; Rodeo, S. Future Trends for Unicompartmental Arthritis of the Knee: Injectables & Stem Cells. Clin. Sports Med. 2014, 33, 161–174. [Google Scholar] [CrossRef]
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review with Quantitative Synthesis. Arthroscopy 2013, 29, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448. [Google Scholar] [PubMed]
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: Adult Stem Cells in Cartilage Regeneration and Tissue Engineering. Nat. Clin. Pract. Rheumatol. 2006, 2, 373–382. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Dashnyam, K.; Lee, J.H.; Mandakhbayar, N.; Jin, G.Z.; Lee, H.H.; Kim, H.W. Intra-Articular Biomaterials-Assisted Delivery to Treat Temporomandibular Joint Disorders. J. Tissue Eng. 2018, 9, 2041731418776514. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-Articular Treatment Options for Knee Osteoarthritis. Nat. Rev. Rheumatol. 2018, 15, 77–90. [Google Scholar] [CrossRef]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Kouchak, M. In Situ Gelling Systems for Drug Delivery. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 20126. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Wang, K.; Wu, X.; Tu, C.; Gao, C. Stimuli-Sensitive Nanotherapies for the Treatment of Osteoarthritis. Macromol. Biosci. 2021, 21, 2100280. [Google Scholar] [CrossRef]
- García-Couce, J.; Schomann, T.; Chung, C.K.; Que, I.; Jorquera-Cordero, C.; Fuentes, G.; Almirall, A.; Chan, A.; Cruz, L.J. Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis. Gels 2022, 8, 488. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Kan, C.-W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Singh, G.; Min, Z.; Shixuan, C.; Xu, K.; Pengcheng, X.; Xueer, W.; Yinghua, C.; Lu, Z.; Lin, Z. Bone Marrow-Derived Mesenchymal Stem Cells Laden Novel Thermo-Sensitive Hydrogel for the Management of Severe Skin Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Bi, B.; Huang, J.; Zhuo, R.; Jiang, X. Thermosensitive and Photocrosslinkable Hydroxypropyl Chitin-Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2018, 192, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Blokzijl, M.M.; Mouser, V.H.M.; Marica, P.; Malda, J.; Hennink, W.E.; Vermonden, T. A Thermo-Responsive and Photo-Polymerizable Chondroitin Sulfate-Based Hydrogel for 3D Printing Applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, J.; Xu, H.; Wang, C.; Yang, Y.; Zhao, Y.; Han, H.; Hou, T.; Yang, G.; Zhang, L.; et al. Construction of a PH-Responsive, Ultralow-Dose Triptolide Nanomedicine for Safe Rheumatoid Arthritis Therapy. Acta Biomater. 2021, 121, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Chiu, I.H.; Hung, M.C.; Vu, Q.L.; Lin, I.L.; Wu, P.C. In Vitro Evaluation of Smart and PH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery. Gels 2022, 8, 406. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; Zhao, J.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.; Zheng, L. Cartilage-Targeting and Dual MMP-13/PH Responsive Theranostic Nanoprobes for Osteoarthritis Imaging and Precision Therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.C. In Situ Synthesis of Magnetic Field-Responsive Hemicellulose Hydrogels for Drug Delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Yeingst, T.J.; Arrizabalaga, J.H.; Hayes, D.J. Ultrasound-Induced Drug Release from Stimuli-Responsive Hydrogels. Gels 2022, 8, 554. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Little, C.; Barai, A.; Burkhardt, D.; Smith, S.; Fosang, A.; Werb, Z.; Shah, M.; Thompson, E. Matrix Metalloproteinase-13 Deficient Mice Are Resistant to Osteoarthritic Cartilage Erosion but not Chondrocyte Hypertrophy or Osteophyte Development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.; Riegel, L.; He, X.; et al. Towards an Arthritis Flare-Responsive Drug Delivery System. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Han, S.; Zeng, X.; Zhu, C.; Pu, Y.; Sun, Y. Multifunctional Thermo-Sensitive Hydrogel for Modulating the Microenvironment in Osteoarthritis by Polarizing Macrophages and Scavenging RONS. J. Nanobiotechnol. 2022, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment Sensitive Hydrogels for Drug Delivery Applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent Advances in Thermo-Sensitive Hydrogels for Drug Delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive Polymers: Insights into Decisive Hydrogel Characteristics, Mechanisms of Gelation, and Promising Biomedical Applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Grau, R. Thermosensitive Poloxamer-Based Injectables as Controlled Drug Release Platforms for Veterinary Use: Development and in-Vitro Evaluation. Int. Res. J. Pharm. Pharmacol. 2020, 9, 1–10. [Google Scholar]
- Bachhav, H.D.; Savkare, A.; Karmarkar, R.; Derle, D. Innovare Academic Sciences Development of Poloxamer Based Thermosensitive In Situ Ocular Gel of Betaxolol Hydrochloride. Int. J. Pharm. Pharm. Sci. 2015, 7, 4–8. [Google Scholar]
- Das, T.; Prakash, V.M.; Kumar, P.; Math, T. Injectable in Situ Gel of Methotrexate for Rheumatoid Arthritis: Development, in Vitro and in Vivo Evaluation. J. Appl. Pharm. Sci. 2019, 9, 40–48. [Google Scholar] [CrossRef]
- Miao, B.; Song, C.; Ma, G. Injectable Thermosensitive Hydrogels for Intra-Articular Delivery of Methotrexate. J. Appl. Polym. Sci. 2011, 122, 2139–2145. [Google Scholar] [CrossRef]
- Khaqan, H.A.; Yar, M.; Imtiaz, U.; Buksh, H.M. Evaluation Hydrogel Drug Delivery Agent for Loading Dexamethosone. Pak. J. Ophthalmol. 2019, 35. Available online: https://www.pjo.org.pk/index.php/pjo/article/view/956 (accessed on 5 October 2022).
- Talaat, W.M.; Haider, Ã.M.; Kawas, A.; Kandil, N.G.; Harding, D.R.K. Chitosan-Based Thermosensitive Hydrogel for Controlled Drug Delivery to the Temporomandibular Joint. J. Craniofacial Surg. 2016, 27, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Tabbakhian, M.; Salmani, Z. Designing of a Thermosensitive Chitosan / Poloxamer In Situ Gel for Ocular Delivery of Ciprofloxacin. Open Drug Deliv. J. 2008, 2, 61–70. [Google Scholar] [CrossRef]
- Jain, D.; Kumar, V.; Mullertz, A.; Shalom, D.B. Newer Trends in in Situ Gelling Systems for Controlled Ocular Drug Delivery. J. Anal. Pharm. Res. 2016, 2, 00022. [Google Scholar] [CrossRef]
- Kim, H.; Song, D.; Ngo, H.V.; Jin, G.; Park, C.; Park, J.B.; Lee, B.J. Modulation of the Clinically Accessible Gelation Time Using Glucono-d-Lactone and Pyridoxal 5′-Phosphate for Long-Acting Alginate in Situ Forming Gel Injectable. Carbohydr. Polym. 2021, 272, 118453. [Google Scholar] [CrossRef]
- Kim, S.R.; Ho, M.J.; Lee, E.; Lee, J.W.; Choi, Y.W.; Kang, M.J. Cationic PLGA/Eudragit RL Nanoparticles for Increasing Retention Time in Synovial Cavity after Intra-Articular Injection in Knee Joint. Int. J. Nanomed. 2015, 10, 5263–5271. [Google Scholar] [CrossRef]
- Elron-Gross, I.; Glucksam, Y.; Biton, I.E.; Margalit, R. A Novel Diclofenac-Carrier for Local Treatment of Osteoarthritis Applying Live-Animal MRI. J. Control. Release 2009, 135, 65–70. [Google Scholar] [CrossRef]
- Sang, X.; Zhao, X.; Yan, L.; Jin, X.; Wang, X.; Wang, J.; Yin, Z.; Zhang, Y.; Meng, Z. Thermosensitive Hydrogel Loaded with Primary Chondrocyte-Derived Exosomes Promotes Cartilage Repair by Regulating Macrophage Polarization in Osteoarthritis. Tissue Eng. Regen. Med. 2022, 19, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Zewail, M.; Nafee, N.; Boraie, N. Intra-Articular Dual Drug Delivery for Synergistic Rheumatoid Arthritis Treatment. J. Pharm. Sci. 2021, 110, 2808–2822. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elnour, M.; Soliman, M.E.; Skouras, A.; Casettari, L.; Geneidi, A.S.; Ishak, R.A.H. Microparticles-in-Thermoresponsive/Bioadhesive Hydrogels as a Novel Integrated Platform for Effective Intra-Articular Delivery of Triamcinolone Acetonide. Mol. Pharm. 2020, 17, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Sandker, M.; Müller, B.; Meyboom, R.; van Midwoud, P.; Bruin, P.; Redout, E.M.; Versluijs-Helder, M.; van der Lest, C.H.A.; Buwalda, S.J.; et al. Release Behavior and Intra-Articular Biocompatibility of Celecoxib-Loaded Acetyl-Capped PCLA-PEG-PCLA Thermogels. Biomaterials 2014, 35, 7919–7928. [Google Scholar] [CrossRef]
- Seo, B.B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.E.; Song, H.R.; Kim, Y.M.; Song, S.C. Injectable Polymeric Nanoparticle Hydrogel System for Long-Term Anti-Inflammatory Effect to Treat Osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef]
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Monteiro, M.; Grandjean, C.; Alonso, M.J. An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-Articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216. [Google Scholar] [CrossRef]
- Yin, N.; Guo, X.; Sun, R.; Liu, H.; Tang, L.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. Intra-Articular Injection of Indomethacin-Methotrexate: In Situ Hydrogel for the Synergistic Treatment of Rheumatoid Arthritis. J. Mater. Chem. B 2020, 8, 993–1007. [Google Scholar] [CrossRef]
- Chiesa, E.; Pisani, S.; Colzani, B.; Dorati, R.; Conti, B.; Modena, T.; Braeckmans, K.; Genta, I. Intra-Articular Formulation of GE11-PLGA Conjugate-Based NPs for Dexamethasone Selective Targeting—In Vitro Evaluation. Int. J. Mol. Sci. 2018, 19, 2304. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Croce, I.; Bassi, A.M.; Vernazza, S.; Caviglioli, G. Chitosan-Clodronate Nanoparticles Loaded in Poloxamer Gel for Intra-Articular Administration. Colloids Surf. B Biointerfaces 2016, 143, 88–96. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Mancuso, A.; Giuliano, E.; Cosco, D.; Paolino, D.; Fresta, M. EtoGel for Intra-Articular Drug Delivery: A New Challenge for Joint Diseases Treatment. J. Funct. Biomater. 2021, 12, 34. [Google Scholar] [CrossRef]
- Shinde, C.; Venkatesh, M.P.; Pramod Kumar, T.; Pai, D.R. Nanostructured Lipid Carrier-Based Smart Gel: A Delivery Platform for Intra-Articular Therapeutics. Autoimmunity 2021, 54, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, P.; Li, B.; Liu, Y.; Wang, M.; Suo, N.; Yang, M.; Zhang, D.; Jin, X. A New Drug Delivery System for Mitomycin C to Improve Intravesical Instillation. Mater. Des. 2016, 110, 849–857. [Google Scholar] [CrossRef]
- Masaro, L.; Zhu, X.X. Physical Models of Diffusion for Polymer Solutions, Gels and Solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between Mesh Size and Equilibrium Degree of Swelling of Polymeric Networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.N.; Metters, A.T.; Bowman, C.N.; Anseth, K.S. Predicting Controlled-Release Behavior of Degradable PLA-b-PEG-b-PLA Hydrogels. Macromolecules 2001, 34, 4630–4635. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In Situ Gelling Systems Based on Pluronic F127/Pluronic F68 Formulations for Ocular Drug Delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-Based in Situ Hydrogels for Controlled Delivery of Hydrophilic Macromolecules after Intramuscular Injection in Rats. Drug. Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Hadgraft, J.; Bye, A.; Brookes, L.G. Drug Release from Pluronic F-127 Gels. Int. J. Pharm. 1986, 32, 223–228. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.K.D.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; De Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 Binary Thermosensitive Hydrogels as Delivery Systems for Infiltrative Local Anesthesia: Physico-Chemical Characterization and Pharmacological Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Wei, G.; Lu, W. Effect of Carrageenan on Poloxamer-Based in Situ Gel for Vaginal Use: Improved in Vitro and in Vivo Sustained-Release Properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Inal, O.; Yapar, E.A. Effect of Mechanical Properties on the Release of Meloxicam from Poloxamer Gel Bases. Indian J. Pharm. Sci. 2013, 75, 700. [Google Scholar]
- Quah, S.P.; Smith, A.J.; Preston, A.N.; Laughlin, S.T.; Bhatia, S.R. Large-Area Alginate/PEO-PPO-PEO Hydrogels with Thermoreversible Rheology at Physiological Temperatures. Polymer 2018, 135, 171–177. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A Poloxamer/Chitosan in Situ Forming Gel with Prolonged Retention Time for Ocular Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef]
- Zeeshan, F.; Sheshala, R.; Wai, N.Z.; SAID, I.D.; Ashraf, K.; Lim, S.M.; Ramasamy, K. Poloxamer and Chitosan-Based In Situ Gels Loaded with Orthosiphon Stamineus Extracts Containing Rosmarinic Acid for the Treatment of Ocular Infections. Turk. J. Pharm. Sci. 2022. [Google Scholar] [CrossRef]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of Gellan Gum on the Thermogelation Property and Drug Release Profile of Poloxamer 407 Based Ophthalmic Formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef]
- Rajendran, S.; Kumar, K.S.; Ramesh, S.; Rao, S.R. Thermoreversible in Situ Gel for Subgingival Delivery of Simvastatin for Treatment of Periodontal Disease. Int. J. Pharm. Investig. 2017, 7, 101. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; Rotonda, M.I.L. A Novel Poloxamers/Hyaluronic Acid in Situ Forming Hydrogel for Drug Delivery: Rheological, Mucoadhesive and in Vitro Release Properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable Injectable in Situ Forming Drug Delivery Systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Hanafy, A.S.; El-Ganainy, S.O. Thermoresponsive Hyalomer Intra-Articular Hydrogels Improve Monoiodoacetate-Induced Osteoarthritis in Rats. Int. J. Pharm. 2020, 573, 118859. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, M.; Patel, S.; MacFarlane, R.J.; Haddad, F.S. Biodegradable Antibiotic Delivery Systems. J. Bone Jt. Surg. Ser. B 2011, 93 B, 151–157. [Google Scholar] [CrossRef]
- Casadidio, C.; Butini, M.E.; Trampuz, A.; Di Luca, M.; Censi, R.; Di Martino, P. Daptomycin-Loaded Biodegradable Thermosensitive Hydrogels Enhance Drug Stability and Foster Bactericidal Activity against Staphylococcus Aureus. Eur. J. Pharm. Biopharm. 2018, 130, 260–271. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, Q.; Yan, D.; Zheng, G.; Gu, M.; Luo, Z.; Mao, C.; Qian, Z.; Ni, W.; Shen, L. A Multi-Functionalized Calcitriol Sustainable Delivery System for Promoting Osteoporotic Bone Regeneration Both in Vitro and in Vivo. Appl. Mater. Today 2021, 22, 100906. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vazquez, B.; Román, J.S. Smart Polymers and Their Applications as Biomaterials. In Topics in Tissue Engineering; Smart Polymers: Alicante, Spain, 2007; Volume 3, pp. 1–27. ISBN 9780081024164. [Google Scholar]
- Ruan, H.; Yu, Y.; Guo, X.; Jiang, Q.; Luo, Y. The Possibility of Healing Alveolar Bone Defects with Simvastatin Thermosensitive Gel: In Vitro/in Vivo Evaluation. Drug Des. Devel. Ther. 2018, 12, 1997–2003. [Google Scholar] [CrossRef]
- Thassu, D.; Deleers, M.; Pathak, Y. Nanoparticulate Drug Delivery Systems. In Nanoparticulate Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–376. ISBN 9781420008449. [Google Scholar]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Gel-One (Cross-Linked Hyaluronate Viscoelastic Hydrogel): Uses, Dosage, Side Effects, Interactions, Warning. Available online: https://www.rxlist.com/gel-one-drug.htm (accessed on 31 October 2022).
- Osteoarthritis Knee Pain Relief Treatment|Synvisc-One® Official Site. Available online: https://www.synviscone.com/ (accessed on 31 October 2022).


| NSAIDs | Hyaluronic Acid | Corticosteroids | Platelet-Rich Plasma | Mesenchymal Stem Cells | |
|---|---|---|---|---|---|
| Constituents | Aspirin, Ibuprofen, Naproxen, Celecoxib, Diclofenac, Ketoprofen | Hyaluronic acid | Betamethasone acetate, Dexamethasone acetate, Triamcinolone hexacetonide, Betamethasone sodium phosphate, Prednisone tebutate | Cells and coagulation factors, anticoagulants, fibrinogen, activators, platelet-rich fibrin, leukocyte-rich plasma | Suspension of mesenchymal stem cells |
| Advantages | Inexpensive Noninfectious Used as monotherapy | Relatively safe Benefit up to 60 days | Low dose is required: eg. triamcinolone acetonide (5 mg). Adverse effect is very low Provide pain relief and reduce joint effusions | Simple Low cost Minimally invasive Reduce inflammation, pain relief, improved function, and possible cartilage regeneration | Safe and encouraging results for articular cartilage repair and regeneration |
| Disadvantages | Dose-dependent toxicity. Using NSAIDs in the short term. Long-term usage may cause liver toxicity | Risk of infection Local adverse events after injection due to injection technique, partially flexed knee, etc. Other side effects are acute synovitis, joint swelling for up to 3 weeks, haemarthrosis, pseudogout, muscle pain | Short-lived beneficial effect: not more than one week. IA corticosteroids seem to produce time and dose-dependent deleterious effects on articular cartilage with erosion, decreased glycosaminoglycans content, and joint narrowing Local side effects include post-injection flare, infection, and skin hypopigmentation | PRP formulations are complex Mechanisms of action in a joint with OA remain unanswered. More clinical evidence required Optimal therapeutic protocol not yet been established related to timing, dosage, volume, frequency, and composition | Preparation is complex Exact mechanism of action of MSCs is debated Long-term clinical trial studies are required |
| References | [21,22,26,27] | [26,31,32,33] | [19,23,46,47] | [26,39,41,47] | [26,37,40,42] |
| Type of Stimuli-Responsive Hydrogel | Advantages | Limitations | References |
|---|---|---|---|
| Thermosensitive | Compatible with both hydrophilic and hydrophobic drugs, high drug loading capacity, drug sustained-release carriers, easy to formulate with novel drug delivery carrier systems | Long responsive time and low biocompatibility | [51] |
| Magnetic-responsive | Biocompatibility, controlled architectures, smart response to the magnetic field | Long response time and less precision in drug release | [59] |
| Ultrasound-activated | Suitable with various drug delivery carriers | Damage to the surrounding tissue | [60] |
| Enzyme-responsive | Offers specificity towards degrading enzymes. Quick degradation and drug release in presence of specific enzymes | Quick degradation limits the usefulness of long-term therapy | [61] |
| pH-responsive | Altered tissue pH in pathological conditions is effectively utilized for gelation at selective sites of tissue inflammation such as cancer | pH variations in pathological conditions may adversely affect the gel | [61] |
| Nanosystems | Composition | Applications | References |
|---|---|---|---|
| Collagomers | Collagen type I, dipalmitoyl phosphatidylethanolamine, glutaraldehyde | Osteoarthritis | [80] |
| Hydrogel + exosomes | Pluronic F 127, hyaluronic acid | Osteoarthritis | [81] |
| Microspheres | Poloxamer 407, alginate sodium, chitosan, β glycerophosphate | Rheumatoid arthritis | [10] |
| Hydrogel | Chitosan, β glycerophosphate | Rheumatoid arthritis | [82] |
| Microparticles | Polyethylene glycol methacrylate methyl ether (PEGMA 246, 188, 475) | Rheumatoid arthritis | [83] |
| Hydrogel | PEG1500, ε-caprolactone, tri ethyl amine, toluene | Osteoarthritis | [84] |
| Polymeric-nanoparticle-based hydrogel system | Tetrahydrofuran, trimethyl- amine, Methoxy poly (ethylene glycol) | Osteoarthritis | [85] |
| Hydrogel loaded in nanocapsules | Soya lecithin, oleylamine, olive oil, ethanol, | Joint diseases | [86] |
| Hydrogel nanoparticles | Polyethyleneimine, Pluronic F 127 and 68, propylene sulfide | Rheumatoid arthritis | [87] |
| PLGA-based nanoparticles | GE11, PLGA, PEG, chitosan, β-glycerophosphate | Joint diseases | [88] |
| Polymeric nanoparticles | Chitosan, clodronate, TPP, glutaraldehyde | Rheumatoid arthritis | [89] |
| Ethosomes | Phospholipon 90 G, ethanol | Joint diseases | [90] |
| Hydrogel | Poloxamer 407, hyaluronic acid | Osteoarthritis | [64] |
| NLC-based gels | Cetyl palmitate, Labrafac PG, Captex 200, Tween 80, Labrasol, Pluronic F 68 and 127 | Rheumatoid arthritis | [91] |
| In situ hydrogel + nanoparticles | Hyaluronic acid, chitosan, Pluronic F 127 | Osteoarthritis | [92] |
| Hydrogel | Chitosan, quaternaryammonium chitosan, β-glycerol phosphate disodium | Osteomylitis | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koland, M.; Narayanan Vadakkepushpakath, A.; John, A.; Tharamelveliyil Rajendran, A.; Raghunath, I. Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery. Gels 2022, 8, 723. https://doi.org/10.3390/gels8110723
Koland M, Narayanan Vadakkepushpakath A, John A, Tharamelveliyil Rajendran A, Raghunath I. Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery. Gels. 2022; 8(11):723. https://doi.org/10.3390/gels8110723
Chicago/Turabian StyleKoland, Marina, Anoop Narayanan Vadakkepushpakath, Anish John, Arunraj Tharamelveliyil Rajendran, and Indu Raghunath. 2022. "Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery" Gels 8, no. 11: 723. https://doi.org/10.3390/gels8110723
APA StyleKoland, M., Narayanan Vadakkepushpakath, A., John, A., Tharamelveliyil Rajendran, A., & Raghunath, I. (2022). Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery. Gels, 8(11), 723. https://doi.org/10.3390/gels8110723