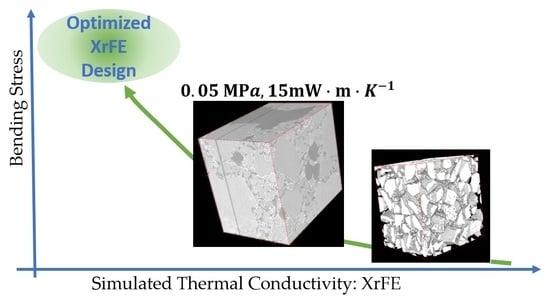
X-ray Tomography Coupled with Finite Elements, A Fast Method to Design Aerogel Composites and Prove Their Superinsulation Experimentally
Abstract
:1. Introduction
2. Results and Discussion
2.1. Statistical Results Gained on Porosity and Size Distribution
2.1.1. Compacity and Inter-Aerogel Particles Porosity (IP)
2.1.2. Pore Size Distributions
2.1.3. Binder Volume Fraction
2.2. Homogenization Computed Thermal Conductivities with Tomography Volume Fraction as Input
2.3. Image Analysis and Computation Procedure with XrFE Code for Effective Themal Conductivity
2.3.1. Aerogel Particle Size, Monomodal or Bimodal
2.3.2. Contacts
2.3.3. Binder
2.4. SAP Composite Formulated and Characterized
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Segmentation to Separate Phases

Appendix A.2. Watershed Treatment to Retrieve Contacts
Appendix A.3. Volumes to Ensure Quantitative Phase Analysis
Appendix A.4. Meshing Volume to Enable Thermal Simulation
- Binarizing the grey level images to enhance the resolution of the boundaries;
- Geometrical definition of these phases by triangular facets;
- Tetrahedral volume meshing.
Appendix A.5. Pile up with Binder, Post-Treatment Used for Aerogel Composites

- 3D segmentation of the binder phase, showing a gray level close to 225, then binarization to create a reconstructed volume with only the binder;
- 3D segmentation of the air phase, (gray level near zero, manual adjustment of the upper boundary), resulting in a reconstructed volume of only pores.
- Combination by addition of the two previous volumes, IP and binder.
- This volume is then subtracted from the raw volume to recover in a volume the last phase of aerogel grains.
- Each of the 3 volumes (particles, air and binder) is meshed with the Avizo software.
- The 3 meshed volumes are recombined into a single volume and thermal or mechanical simulations are performed.

References
- Xie, T.; He, Y.L.; Hu, Z.J. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Duan, Y.; Hu, Z. Radiative characteristics of opacifier-loaded silica aerogel composites. J. Non Cryst. Solid 2013, 375, 31–39. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Latre, S.K.; Latre, S.K.; Latre, S.K.; De Pooter, S.; Buffel, B.; Brabazon, D.; Seveno, D.; Desplentere, F. Comparative study of a Cubic, Kelvin and Weaire-Phelan Unit Cell for the Prediction of the Thermal Conductivity of Low Density Silica Aerogels. Microporous Mesoporous Mater. 2020, 301, 110206. [Google Scholar] [CrossRef]
- Wordsworth, R.; Kerber, L.; Cockell, C. Enabling Martian habitability with silica aerogel via the solid-state greenhouse effect. Nat. Astron. 2019, 3, 898–903. [Google Scholar] [CrossRef] [Green Version]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non. Cryst. Solids 2021, 562, 120770. [Google Scholar] [CrossRef]
- Berardi, U. The benefits of using aerogel-enhanced systems in building retrofits. Energy Procedia 2017, 134, 626–635. [Google Scholar] [CrossRef]
- Stahl, T.; Brunner, S.; Zimmermann, M.; Ghazi Wakili, K. Thermo-hygric properties of a newly developed aerogel based insulation rendering for both exterior and interior applications. Energy Build. 2012, 44, 114–117. [Google Scholar] [CrossRef]
- Chal, B.; Foray, G.; Yrieix, B.; Roiban, L.; Chenal, J. Hygrothermal durability of silica aerogels dedicated to superinsulation. Microporous Mesoporous Mater. 2018, 272, 61–69. [Google Scholar] [CrossRef]
- Hamelin, G.; Jauffrés, D.; Martin, C.L.; Meille, S.; Foray, G. Mechanical properties of milimetric silica aerogel particles produced through evaporative drying: A coupled experimental and discrete element approach. J. Non. Cryst. Solids 2021, 560, 120727. [Google Scholar] [CrossRef]
- Perret, A.; Foray, G.; Masenelli-Varlot, K.; Maire, E.; Yrieix, B. Study of the surfactant role in latex–aerogel systems by scanning transmission electron microscopy on aqueous suspensions. J. Microsc. 2018, 269, 3–13. [Google Scholar] [CrossRef]
- Zhao, J.J.; Duan, Y.Y.; Wang, X.D.; Wang, B.X. Radiative properties and heat transfer characteristics of fiber-loaded silica aerogel composites for thermal insulation. Int. J. Heat Mass Transf. 2012, 55, 5196–5204. [Google Scholar] [CrossRef]
- Hao-Qiang, P.; Zeng-Yao, L. Experimental investigations on the thermal insulation performance of SiC opacifier doped silica aerogel at large temperature difference. Int. J. Therm. Sci. 2021, 160, 106681. [Google Scholar] [CrossRef]
- Yuan, B.; Ding, S.; Wang, D.; Wang, G.; Li, H. Heat insulation properties of silica aerogel/glass fiber composites fabricated by press forming. Mater. Lett. 2012, 75, 204–206. [Google Scholar] [CrossRef]
- Hrubesh Lawrence, W.; Richard, W.; Pekala, A. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Spagnol, S.B.; Lartigue, A.; Trombe, V.G. Modeling of thermal conduction in granular silica aerogel. J. Sol-Gel Sci. Technol. 2008, 48, 40–46. [Google Scholar] [CrossRef]
- Dai, Y.J.; Tang, Y.Q.; Fang, W.Z.; Zhang, H.; Tao, W.Q. A theoretical model for the effective thermal conductivity of silica aerogel composites. Appl. Therm. Eng. 2018, 128, 1634–1645. [Google Scholar] [CrossRef]
- Garnett, J.C.M.; Larmor, J. Colours in metal glasses and in metallic films. Proc. R. Soc. London 1904, 73, 443–445. [Google Scholar] [CrossRef] [Green Version]
- Hashin, Z.; Shtrikman, S. A Variational approach to the theory of the effective magnetic permeability of multiphase materials. J. Appl. Phys. 1962, 33, 3125–3131. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Wang, F.; Li, X. The stagnant thermal conductivity of porous media predicted by the random walk theory. Int. J. Heat Mass Transf. 2017, 107, 520–533. [Google Scholar] [CrossRef]
- Costeux, S.; Kalantar, T.H.; Ya, A.; Zhang, H. Nanoporous Particles in a Hollow Latex Matrix. Patent, WO2012065288 A1, 24 May 2012. Available online: https://patents.google.com/patent/WO2012065288A1/en (accessed on 26 September 2022).
- Wol, I.B.; Seybold, G. Fritz Ernst Krueckau, Insulating Material with a Density of 0.1 to 0.4 g/cm3. Patent, EP0340707 A3, 23 June 1992. Available online: https://patents.google.com/patent/EP0340707A3/en (accessed on 26 September 2022).
- Gerhard Geiss, A.Z.; Müller, H.K. Werner Prass, Ude Scheunemann, Composition Contenant un Aerogel, son Procede de Fabrication et son Utilisation. Patent, EP0787112 B1, 9 July 1999. Available online: https://patents.google.com/patent/EP0787112B1/fr (accessed on 26 September 2022).
- Dierk, F.; Zimmermann, A.; Stuhler, H.G. Aerogelhaltiges Verbundmaterial, Verfahren zu Seiner Herstellung Sowie Seine Verwendung. Patent, DE4441567 A1, 30 May 1995. Available online: https://patents.google.com/patent/DE4441567A1/de (accessed on 26 September 2022).
- Fickler, S.; Milow, B.; Ratke, L.; Schnellenbach-Held, M.; Welsch, T. Development of high performance aerogel concrete. Energy Procedia 2015, 78, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Jelle, B.P.; Gustavsen, A.; Jacobsen, S. Aerogel-incorporated concrete: An experimental study. Constr. Build. Mater. 2014, 52, 130–136. [Google Scholar] [CrossRef]
- Ng, S.; Jelle, B.P.; Sandberg, L.I.C.; Gao, T.; Wallevik, Ó.H. Experimental investigations of aerogel-incorporated ultra-high performance concrete. Constr. Build. Mater. 2015, 77, 307–316. [Google Scholar] [CrossRef]
- Westgate, P.; Paine, K.; Ball, R.J. Physical and mechanical properties of plasters incorporating aerogel granules and polypropylene monofilament fibres. Constr. Build. Mater. 2018, 158, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Biwole, P.H.; Achard, P. Energy Sustainability Through Green Energy; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Berkefeld, A.; Heyer, M.; Milow, B. Silica aerogel paper honeycomb composites for thermal insulations. J. Sol-Gel Sci. Technol. 2017, 84, 486–495. [Google Scholar] [CrossRef]
- Weigold, L.; Mohite, D.P.; Mahadik-Khanolkar, S.; Leventis, N.; Reichenauer, G. Correlation of microstructure and thermal conductivity in nanoporous solids: The case of polyurea aerogels synthesized from an aliphatic tri-isocyanate and water. J. Non. Cryst. Solids 2013, 368, 105–111. [Google Scholar] [CrossRef]
- Li, C.; Cheng, X.; Li, Z.; Pan, Y.; Huang, Y.; Gong, L. Mechanical, thermal and flammability properties of glass fiber film/silica aerogel composites. J. Non. Cryst. Solids 2017, 457, 52–59. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non. Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, J.H.; Kim, J.D.; Lee, D.H.; Kim, S.K.; Lee, J.M. Influence of silica-aerogel on mechanical characteristics of polyurethane-based composites: Thermal conductivity and strength. Materials 2021, 14, 1790. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, K.; Achard, P.; Biwole, P. Hygro-thermal properties of silica aerogel blankets dried using microwave heating for building thermal insulation. Energy Build. 2018, 158, 14–22. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, X.; Pan, Y.; Li, C.; Gong, L.; Zhang, H. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel composites based on co-precursor method by freeze drying. Appl. Surf. Sci. 2018, 437, 321–328. [Google Scholar] [CrossRef]
- Yan, Q.; Meng, Z.; Luo, J.; Wu, Z. Experimental study on improving the properties of rock wool and glass wool by silica aerogel. Energy Build. 2021, 247, 111146. [Google Scholar] [CrossRef]
- Wernery, J.; Brunner, S.; Weber, B.; Knuth, C.; Koebel, M.M. Superinsulation materials for energy-efficient train envelopes. Appl. Sci. 2021, 11, 2939. [Google Scholar] [CrossRef]
- Iswar, S.; Griffac, M.; Kaufmannd, R.; Huber, M.; Brunner, S.; Lattuadab, M.; Koebel, M.; Malfait, W. Effect of aging on thermal conductivity of fiber-reinforced aerogel composites: An X-ray tomography study. Microporous Mesoporous Mater. 2019, 278, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, A.; Chen, K.; Tang, A.; Allgeier, A.; Glicksman, L.-R.; Gibson, L.-J. Thermal conductivity and characterization of compacted, granular silica aerogel. Energy Build. 2014, 79, 47–57. [Google Scholar] [CrossRef]
- Guesnet, E.; Bénane, B.; Jauffrès, D.; Martin, C.L.; Baeza, G.P.; Foray, G.; Meille, S.; Olagnon, C.; Yrieix, B. Why fumed and precipitated silica have different mechanical behavior: Contribution of discrete element simulations. J. Non. Cryst. Solids 2019, 524, 119646. [Google Scholar] [CrossRef]
- Chal, B.; Roiban, L.; Masenelli-Varlot, K.; Baeza, G.P.; Yrieix, B.; Foray, G. 3D multi-scale quantification of industrially relevant ultra-porous silicas by low-dose electron tomography combined with SANS. J. Non. Cryst. Solids 2021, 557, 120577. [Google Scholar] [CrossRef]
- Yrieix, B.; Morel, B.; Foray, G.; Bogner, A.; Van de mootelle, B. Aerogel-Based Material That Is Super-Insulating at Athmospheric Pressure. Patent W02012168617 A1, 13 December 2012. Available online: https://patents.google.com/patent/WO2012168617A1/en (accessed on 26 September 2022).
- Foray, G.; Masenelli-Varlot, K.; Malchère, A.; Maire, E.; Lucian, R.; Randrianalisoa, J.; Martin, L.-C.; Gonçalves, W.; Morthomas, J.; Julien Amadéo, P.; et al. Yrieix, les « grains de vide » des aérogels, matière à construire. In Inven. l’avenir l’ingénierie Se Met Au Vert. 2018. Available online: https://www.researchgate.net/publication/345253462_Les_%27grains_de_vide%27_des_aerogels_matiere_a_construire (accessed on 23 August 2022).
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Foray, G.; Chal, B.; Perret, A.; Roiban, L.; Masenelli-Varlot, K.; Maire, E. Mechanical properties of monolithic and granular-based aerogels, measurement and analysis. In Translucent Silica Aerogel: Properites, Preparations, and Applications; Nova Science and Technologies, 2019; Available online: https://novapublishers.com/shop/translucent-silica-aerogel-properties-preparation-and-applications/ (accessed on 23 August 2022).
- Maire, É.; Adrien, J.; Petit, C. Liquid and solid foams / Mousses liquides et solides Structural characterization of solid foams Caractérisation structurale des mousses solides. Comptes Rendus Phys. 2014, 15, 674–682. [Google Scholar] [CrossRef]
- Payraudeau-Le Roux, N.; Meille, S.; Chevalier, J.; Maire, E.; Adrien, J. In situ observation of plaster microstructure evolution during thermal loading. Fire Mater. 2016, 40, 973–984. [Google Scholar] [CrossRef]
- Ulrich, D.J.O.; van Rietbergen, B.; Weinans, H.H.; Rüegsegger, P. Finite element analysis of trabecular bone structure: A comparison of image-based meshing techniques. J. Biomech. 1998, 31, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Amani, Y.; Takahashi, A.; Chantrenne, P.; Maruyama, S.; Dancette, S.; Maire, E. Thermal conductivity of highly porous metal foams: Experimental and image based finite element analysis. Int. J. Heat Mass Transf. 2018, 122, 1–10. [Google Scholar] [CrossRef]
- Glicksman, L.-R. Heat transfert in foams. In Low Density Cellular Plastics: Physical vasis of Behaviour; Hilyard, N.C., Cunningham, A., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994; pp. 105–152. [Google Scholar]
- Randrianalisoa, J.; Coquard, R.; Baillis, D. Microscale direct calculation of solid phase conductivity of voronoi’s foams. J. Porous Media 2013, 16, 411–426. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Snyder, K.A.; François, D.J. Geometrical percolation threshold of overlapping ellipsoids. Phys. Rev. E 1995, 52, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Maire, E.; Meille, S.; Adrien, J. Two scale study of the fracture of an aluminium foam by X-ray tomography and finite element modeling. Mater. Des. 2017, 120, 117–127. [Google Scholar] [CrossRef]








| Aerogel Pile-up_Binder | Ref. Vol. (1) | Resolution (2) (µm) | IP | Air | Specific Phase | ||
|---|---|---|---|---|---|---|---|
| Monomodal_No Binder | 1 | 4.30 | 0.069 ± 0.005 | 0.483 | 0.517 | 0.483 | No |
| 2 | 4.30 | 0.076 ± 0.005 | 0.360 | 0.640 | 0.360 | No | |
| Monomodal Compaction | L | 13.5 | 0.09 ± 0.01 | 0.410 | 0.590 | 0.410 | No |
| LC | 13.5 | 0.11 ± 0.01 | 0.370 | 0.630 | 0.370 | No | |
| Bimodal No Binder | 4 | 3.00 | 0.069 ± 0.005 | 0.483 | 0.516 | 0.482 | Vfc = 0.001 |
| 3 | 4.30 | 0.076 ± 0.005 | 0.360 | 0.640 | 0.358 | Vfc = 0.002 | |
| Bimodal_Organic_Binder | T | 1.00 | 0.149 ± 0.005 | 0.190 | 0.790 | 0.190 | Vfb = 0.020 |
| X | 1.00 | 0.163 ± 0.005 | 0.120 | 0.860 | 0.120 | Vfb = 0.020 | |
| Bimodal Organic Binder SiC | TSiC | 1.00 | 0.155 ± 0.005 | 0.060 | 0.060 | not meas. |
| Volume Name and Features | Values Measured by Grain Growth within 3 D Tomography Volumes | |
|---|---|---|
| Large SAP | 650 | 380 |
| Small SAP | 30 | 22 |
| Pileup with 60% Large SAP | ||
| +40% Small SAP | ||
| XrFE Volume Used as Input | Ref. Vol | IP (%) | |||
|---|---|---|---|---|---|
| (1) | |||||
| Monomodal Pile-up | L | 12 | 25 | 0.48 | 17.1 |
| S | 0.36 | 15.5 | |||
| Bimodal Pile-up | L80 | 12 | 25 | 0.42 | 16.5 |
| L60 | 0.38 | 16.1 | |||
| XrFE Volume Used as Input | ||||
|---|---|---|---|---|
| No contacts | 12 | 25 | 0 | 17.4 |
| Low contacts | 12 | 17.4 | ||
| High contacts | 1000 | 17.7 | ||
| XrFE Volume Used as Input (1) | IP Measured (%) | XrFE Thermal Conductivity | |||||
|---|---|---|---|---|---|---|---|
| Upper Bound | 12 | 25 | 200–1000 | 14.0 | None | 21.5 | 45.3 |
| Lower Bound | None | 14.0 | 12.9 | 12.9 | |||
| Vertical defect band | 14 | 25 | 200–1000 | 1.0 + 1.0 | 17.4 | 17.2 | - |
| No defect band | 1.3 | 17.4 | 13.8 | 15.1 | |||
| Moderate | 12 | 25 | 200–1000 | 1.3 | 14.0 | 13.6 | 14.8 |
| Low | 0.3 | 12.7 | 13.7 | 13.8 | |||
| Tenuous | 0.1 | 17.5 | 13.2 | 13.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foray, G.; Randrianalisoa, J.H.; Adrien, J.; Maire, E. X-ray Tomography Coupled with Finite Elements, A Fast Method to Design Aerogel Composites and Prove Their Superinsulation Experimentally. Gels 2022, 8, 732. https://doi.org/10.3390/gels8110732
Foray G, Randrianalisoa JH, Adrien J, Maire E. X-ray Tomography Coupled with Finite Elements, A Fast Method to Design Aerogel Composites and Prove Their Superinsulation Experimentally. Gels. 2022; 8(11):732. https://doi.org/10.3390/gels8110732
Chicago/Turabian StyleForay, Genevieve, Jaona Harifidy Randrianalisoa, Jerome Adrien, and Eric Maire. 2022. "X-ray Tomography Coupled with Finite Elements, A Fast Method to Design Aerogel Composites and Prove Their Superinsulation Experimentally" Gels 8, no. 11: 732. https://doi.org/10.3390/gels8110732