Sodium Alginate-Quaternary Polymethacrylate Composites: Characterization of Dispersions and Calcium Ion Cross-Linked Gel Beads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Appearance of SA-QPM Composite Dispersions
2.2. Characteristics of the SA-QPM Composite Dispersions
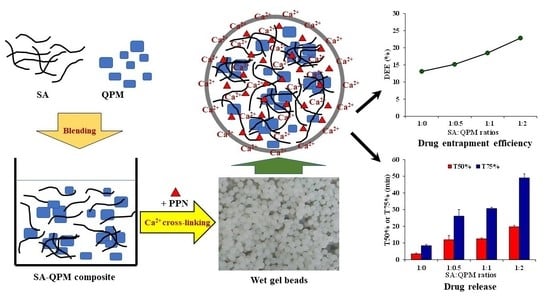
2.3. Particle Morphology and DEE of PPN-Loaded CA-QPM Gel Beads
2.4. Particle Strength of PPN-Loaded CA-QPM Gel Beads
2.5. Water Uptake and Drug Release of PPN-Loaded CA-QPM Gel Beads
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of SA-QPM Composite Dispersions
4.3. Characterization of SA-QPM Composite Dispersions
4.3.1. Microscopic Morphology Studies
4.3.2. Particle Size Determination
4.3.3. Zeta Potential Measurement
4.3.4. Rheological Studies of Composite Dispersions
4.4. Preparation of Calcium Ion Cross-Linked Gel Beads
4.5. Characterization of PPN-Loaded CA-QPM Gel Beads
4.5.1. Particle Size and Morphology Studies
4.5.2. Determination of Drug Entrapment Efficiency (DEE)
4.5.3. Mechanical Property of Gel Beads
4.5.4. Water Uptake Measurement
4.5.5. In Vitro Drug Release Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological Interaction between Polysaccharides and Divalent Cation: The Egg-Box Model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Østberg, T.; Lund, E.M.; Graffner, C. Calcium Alginate Matrices for Oral Multiple Unit Administration. IV. Release Characteristics in Different Media. Int. J. Pharm. 1994, 112, 241–248. [Google Scholar] [CrossRef]
- Sugawara, S.; Imai, T.; Otagiri, M. The Controlled Release of Prednisolone Using Alginate Gel. Pharm. Res. 1994, 11, 272–277. [Google Scholar] [CrossRef]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Hwang, S.J.; Rhee, G.J.; Lee, K.M.; Oh, K.H.; Kim, C.K. Release Characteristics of Ibuprofen from Excipient-Loaded Alginate Gel Beads. Int. J. Pharm. 1995, 116, 125–128. [Google Scholar] [CrossRef]
- Fernández-Hervás, M.J.; Holgado, M.A.; Fini, A.; Fell, J.T. In Vitro Evaluation of Alginate Beads of a Diclofenac Salt. Int. J.Pharm. 1998, 163, 23–34. [Google Scholar] [CrossRef]
- Ha, B.B.; Jo, E.H.; Cho, S.; Kim, S.B. Production Optimization of Flying Fish Roe Analogs Using Calcium Alginate Hydrogel Beads. Fish. Aquat. Sci. 2016, 19, 30. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.Y.; Chen, X.; Park, H.J. Calcium-Alginate Beads Loaded with Gallic Acid: Preparation and Characterization. LWT Food Sci. Technol. 2016, 68, 667–673. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control. 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Petzold, G.; Gianelli, M.P.; Bugueño, G.; Celan, R.; Pavez, C.; Orellana, P. Encapsulation of Liquid Smoke Flavoring in Ca-Alginate and Ca-Alginate-Chitosan Beads. J. Food Sci. Technol. 2014, 51, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Miyamoto, E.; Kawashima, S. Additive Effect of Chondroitin Sulfate and Chitosan on Drug Release from Calcium-Induced Alginate Gel Beads. J. Control. Release 1996, 38, 101–108. [Google Scholar] [CrossRef]
- Wang, K.; He, Z. Alginate-Konjac Glucomannan—Chitosan Beads as Controlled Release Matrix. Int. J. Pharm. 2002, 244, 117–126. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Khunkitti, W.; Pongjanyakul, T. Alginate-Caseinate Composites: Molecular Interactions and Characterization of Cross-Linked Beads for the Delivery of Anticandidals. Int. J. Biol. Macromol. 2018, 115, 483–493. [Google Scholar] [CrossRef]
- Khoder, M.; Gbormoi, H.K., Sr.; Ryan, A.; Karam, A.; Alany, R.G. Potential Use of the Maillard Reaction for Pharmaceutical Applications: Gastric and Intestinal Controlled Release Alginate-Albumin Beads. Pharmaceutics 2019, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Khlibsuwan, R.; Khunkitti, W.; Pongjanyakul, T. Alginate-Poloxamer Beads for Clotrimazole Delivery: Molecular Interactions, Mechanical Properties, and Anticandidal Activity. Int. J. Biol. Macromol. 2020, 148, 1061–1071. [Google Scholar] [CrossRef]
- Srikhao, N.; Chirochrapas, K.; Kwansanei, N.; Kasemsiri, P.; Ounkaew, A.; Okhawilai, M.; Likitaporn, C.; Theerakulpisut, S.; Uyama, H. Multi-Responsive Optimization of Novel pH-Sensitive Hydrogel Beads Based on Basil Seed Mucilage, Alginate, and Magnetic Particles. Gels 2022, 8, 274. [Google Scholar] [CrossRef]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Miletić Kovačević, M.; Gazdić Janković, M.; Stanić, Z.D. pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, G.D.; Jun, S.W.; Lee, S.; Park, J.S.; Hwang, S.J. Controlled Release Tamsulosin Hydrochloride from Alginate Beads with Waxy Materials. J. Pharm. Pharmacol. 2005, 57, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Pongjanyakul, T.; Sungtongjeen, S.; Puttipipatkhachorn, S. Modulation of Drug Release from Glyceryl Palmitostearate-Alginate Beads via Heat Treatment. Int. J. Pharm. 2006, 319, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Puttipipatkhachorn, S.; Pongjanyakul, T.; Priprem, A. Molecular Interaction in Alginate Beads Reinforced with Sodium Starch Glycolate or Magnesium Aluminum Silicate, and Their Physical Characteristics. Int. J. Pharm. 2005, 293, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Datta, M. Montmorillonite-Alginate Microspheres as a Delivery Vehicle for Oral Extended Release of Venlafaxine Hydrochloride. J. Drug Delivery Sci. Technol. 2016, 33, 149–156. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Rongthong, T. Enhanced Entrapment Efficiency and Modulated Drug Release of Alginate Beads Loaded with Drug–Clay Intercalated Complexes as Microreservoirs. Carbohydr. Polym. 2010, 81, 409–419. [Google Scholar] [CrossRef]
- Murata, Y.; Tsumoto, K.; Kofuji, K.; Kawashima, S. Effect of Natural Polysaccharide Addition on Drug Release from Calcium Induced Alginate Gel Beads. Chem. Pharm. Bull. 2002, 51, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, K.O.R. Chemistry and application properties of polymethacrylatecoating systems. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; McGinity, J.W., Ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 101–179. [Google Scholar]
- Bodmeier, R.; Paeratakul, O. Dry and Wet Strengths of Polymericfilms Prepared from an Aqueous Colloidal Polymer Dispersion, Eudragit RS30D. Int. J. Pharm. 1993, 96, 129–138. [Google Scholar] [CrossRef]
- Semdé, R.; Amighi, K.; Devleeschouwer, M.J.; Moës, A.J. Effect of Pectinolytic Enzymes on the Theophylline Release from Pellets Coated with Water Insoluble Polymers Containing Pectin HM or Calcium Pectinate. Int. J. Pharm. 2000, 197, 169–179. [Google Scholar] [CrossRef]
- Khuathan, N.; Pongjanyakul, T. Modification of Quaternary Polymethacrylate Films Using Sodium Alginate: Film Characterization and Drug Permeability. Int. J. Pharm. 2014, 460, 63–72. [Google Scholar] [CrossRef]
- Rongthong, T.; Sungthongjeen, S.; Siepmann, J.; Pongjanyakul, T. Quaternary Polymethacrylate-Magnesium Aluminum Silicate Films: Molecular Interactions, Mechanical Properties and Tackiness. Int. J. Pharm. 2013, 458, 57–64. [Google Scholar] [CrossRef]
- Günister, E.; Pestreli, D.; Ünlü, C.H.; Atıcı, O.; Güngör, N. Synthesis and Characterization of Chitosan–MMT Biocomposite Systems. Carbohydr. Polym. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Khunawattanakul, W.; Puttipipatkhachorn, S.; Rades, T.; Pongjanyakul, T. Chitosan-Magnesium Aluminum Silicate Composite Dispersions: Characterization of Rheology, Flocculate Size and Zeta Potential. Int. J. Pharm. 2008, 351, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Liu, Y.; Jiang, S.; Nie, J.; Ma, G. Effect of Intermolecular Interaction on Electrospinning of Sodium Alginate. Carbohydr. Polym. 2011, 85, 276–279. [Google Scholar] [CrossRef]
- Tabeei, A.; Samimi, A.; Khorram, M.; Moghadam, H. Study Pulsating Electrospray of Non-Newtonian and Thixotropic Sodium Alginate Solution. J. Electrostat. 2012, 70, 77–82. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Andrzejewska, E. Photocurable Polymethacrylate-Silica Nanocomposites: Correlation between Dispersion Stability, Curing Kinetics, Morphology and Properties. J. Polym. Res. 2016, 23, 116. [Google Scholar] [CrossRef] [Green Version]
- Pongjanyakul, T. Characterization of Microcrystalline Cellulose Loaded Diclofenac Calcium Alginate Gel Beads In Vitro. Pharmazie 2007, 62, 493–498. [Google Scholar]
- Dashevsky, A. Protein Loss by the Microencapsulation of an Enzyme (Lactase) in Alginate Beads. Int. J. Pharm. 1998, 161, 1–5. [Google Scholar] [CrossRef]
- Remuñán-López, C.; Bodmeier, R. Mechanical, Water Uptake and Permeability Properties of Crosslinked Chitosan Glutamate and Alginate Films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Puttipipatkhachorn, S. Xanthan-Alginate Composite Gel Beads: Molecular Interaction and In Vitro Characterization. Int. J. Pharm. 2007, 331, 61–71. [Google Scholar] [CrossRef]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological Investigations of Water-Soluble Polysaccharides from the Tunisian Brown Seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Edwards-Lévy, F.; Lévy, M.C. Serum Albumin–Alginate Coated Beads: Mechanical Properties and Stability. Biomaterials 1999, 20, 2069–2084. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release. I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]








| Dispersions | Exponential Constant, N | Viscosity Coefficient, η ((dyne cm−2)N s) | Viscosity at 22.4 s−1 Shear Rate (cP) |
|---|---|---|---|
| SA:QPM ratios | |||
| 1:0 | 1.00 ± 0.01 | 2.70 ± 0.08 | 270.39 ± 1.12 |
| 1:0.5 | 0.95 ± 0.02 | 3.80 ± 0.20 | 326.16 ± 1.86 |
| 1:1 | 0.98 ± 0.01 | 3.07 ± 0.11 | 289.58 ± 0.52 |
| 1:2 | 0.94 ± 0.01 | 3.64 ± 0.08 | 311.76 ± 1.80 |
| Gel Beads | Particle Size a (mm) | Drug Content b (%w/w) | DEE b (%) |
|---|---|---|---|
| SA:QPM ratios | |||
| 1:0 | 1.03 ± 0.12 | 3.27 ± 0.06 | 13.10 ± 0.25 |
| 1:0.5 | 1.17 ± 0.13 | 2.76 ± 0.10 | 15.17 ± 0.56 |
| 1:1 | 1.29 ± 0.18 | 2.64 ± 0.01 | 18.49 ± 0.07 |
| 1:2 | 1.33 ± 0.14 | 2.29 ± 0.02 | 22.82 ± 0.25 |
| Gel Beads | Burst Release a (%) | T50% a (min) | T75% a (min) | Release Exponent, n | Kinetic Constant, k |
|---|---|---|---|---|---|
| SA:QPM ratios | |||||
| 1:0 | 37.23 ± 5.63 | 3.41 ± 0.52 | 8.34 ± 0.74 | 0.483 | 0.274 |
| 1:0.5 | 10.01 ± 3.01 | 11.94 ± 2.52 | 26.10 ± 3.97 | 0.689 | 0.089 |
| 1:1 | 13.15 ± 0.58 | 12.49 ± 0.55 | 30.67 ± 0.72 | 0.694 | 0.086 |
| 1:2 | 9.50 ± 1.56 | 19.70 ± 0.82 | 49.02 ± 2.39 | 0.735 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khunawattanakul, W.; Jaipakdee, N.; Rongthong, T.; Chansri, N.; Srisuk, P.; Chitropas, P.; Pongjanyakul, T. Sodium Alginate-Quaternary Polymethacrylate Composites: Characterization of Dispersions and Calcium Ion Cross-Linked Gel Beads. Gels 2022, 8, 739. https://doi.org/10.3390/gels8110739
Khunawattanakul W, Jaipakdee N, Rongthong T, Chansri N, Srisuk P, Chitropas P, Pongjanyakul T. Sodium Alginate-Quaternary Polymethacrylate Composites: Characterization of Dispersions and Calcium Ion Cross-Linked Gel Beads. Gels. 2022; 8(11):739. https://doi.org/10.3390/gels8110739
Chicago/Turabian StyleKhunawattanakul, Wanwisa, Napaphak Jaipakdee, Thitiphorn Rongthong, Narin Chansri, Pathomthat Srisuk, Padungkwan Chitropas, and Thaned Pongjanyakul. 2022. "Sodium Alginate-Quaternary Polymethacrylate Composites: Characterization of Dispersions and Calcium Ion Cross-Linked Gel Beads" Gels 8, no. 11: 739. https://doi.org/10.3390/gels8110739