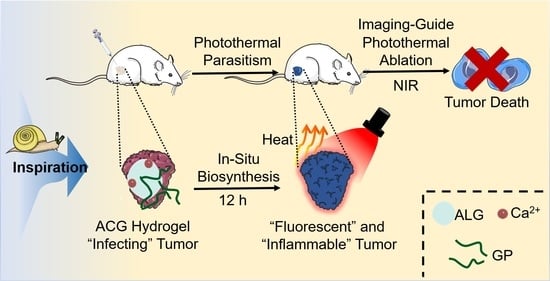
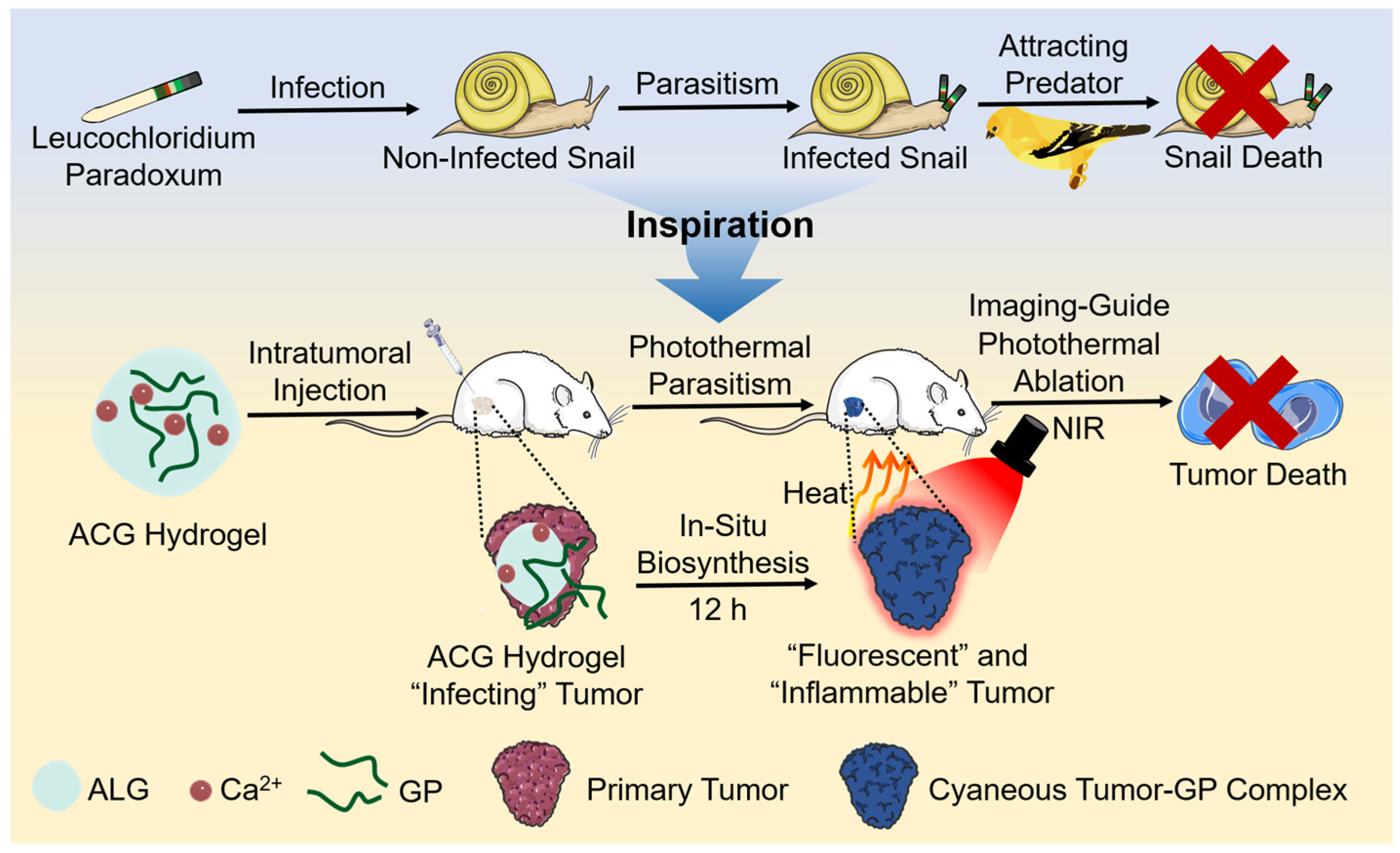
In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of ACG Hydrogel
2.2. In Vitro Evaluation of ACG Hydrogel in a Simulated Protein-Rich Environment
2.3. Cytotoxicity of ACG Hydrogel
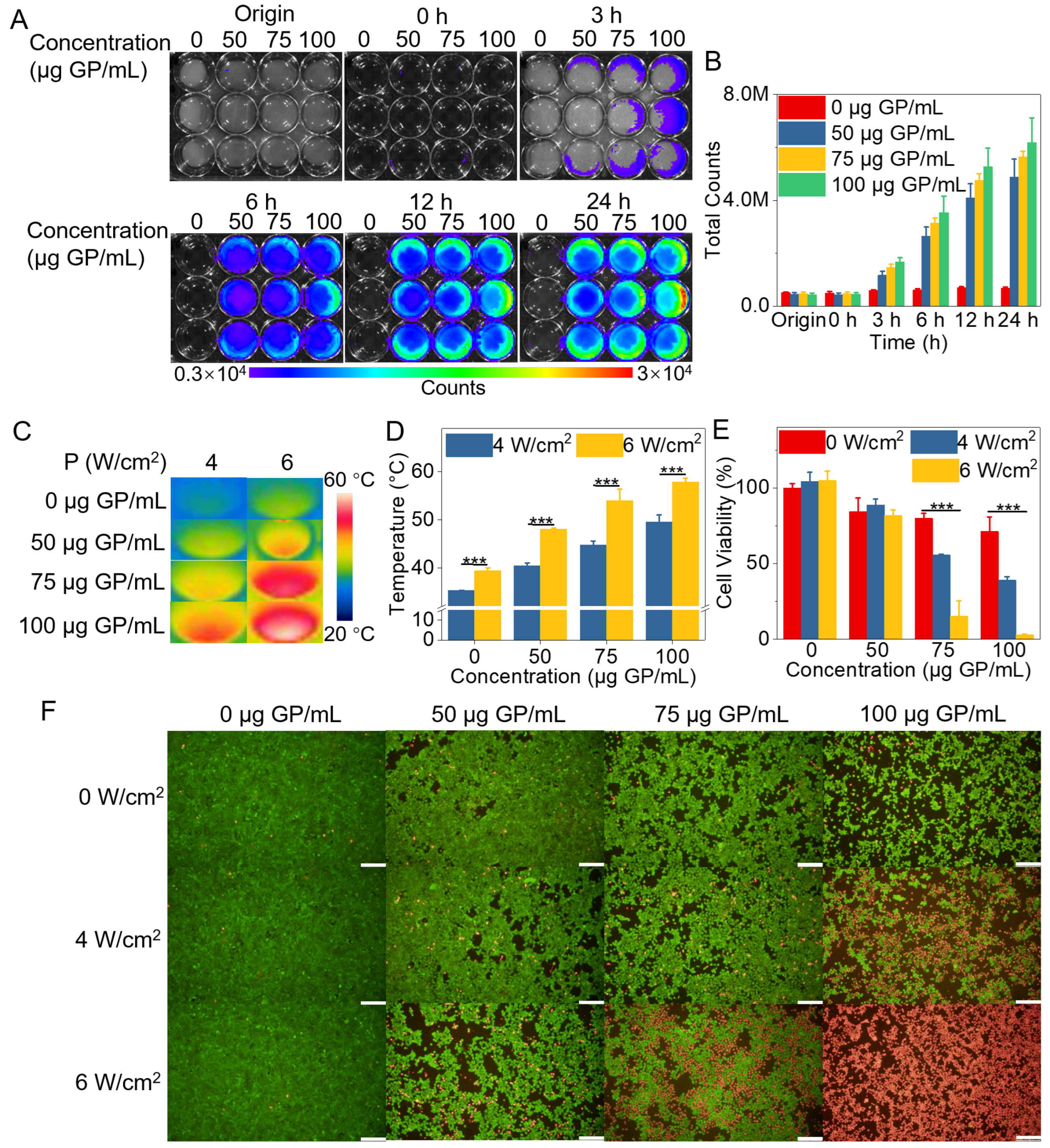
2.4. In Vitro Evaluation of PTT Effect of GP-Based Complex
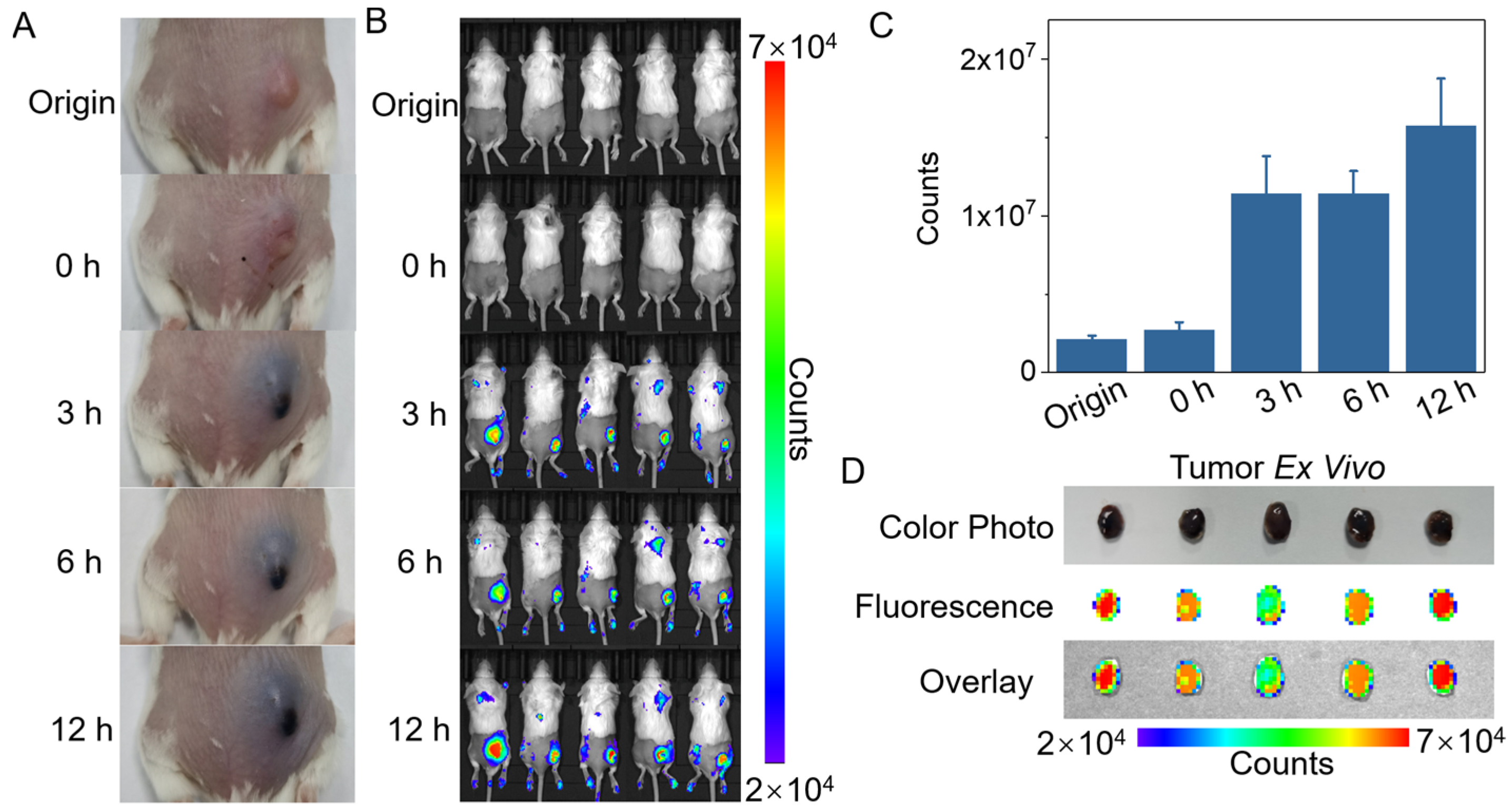
2.5. Time Optimization of Intratumoral Injection of ACG Hydrogel in Mice
2.6. In Vivo PTT Assisted by the “Parasitism” of ACG Hydrogel in Tumor
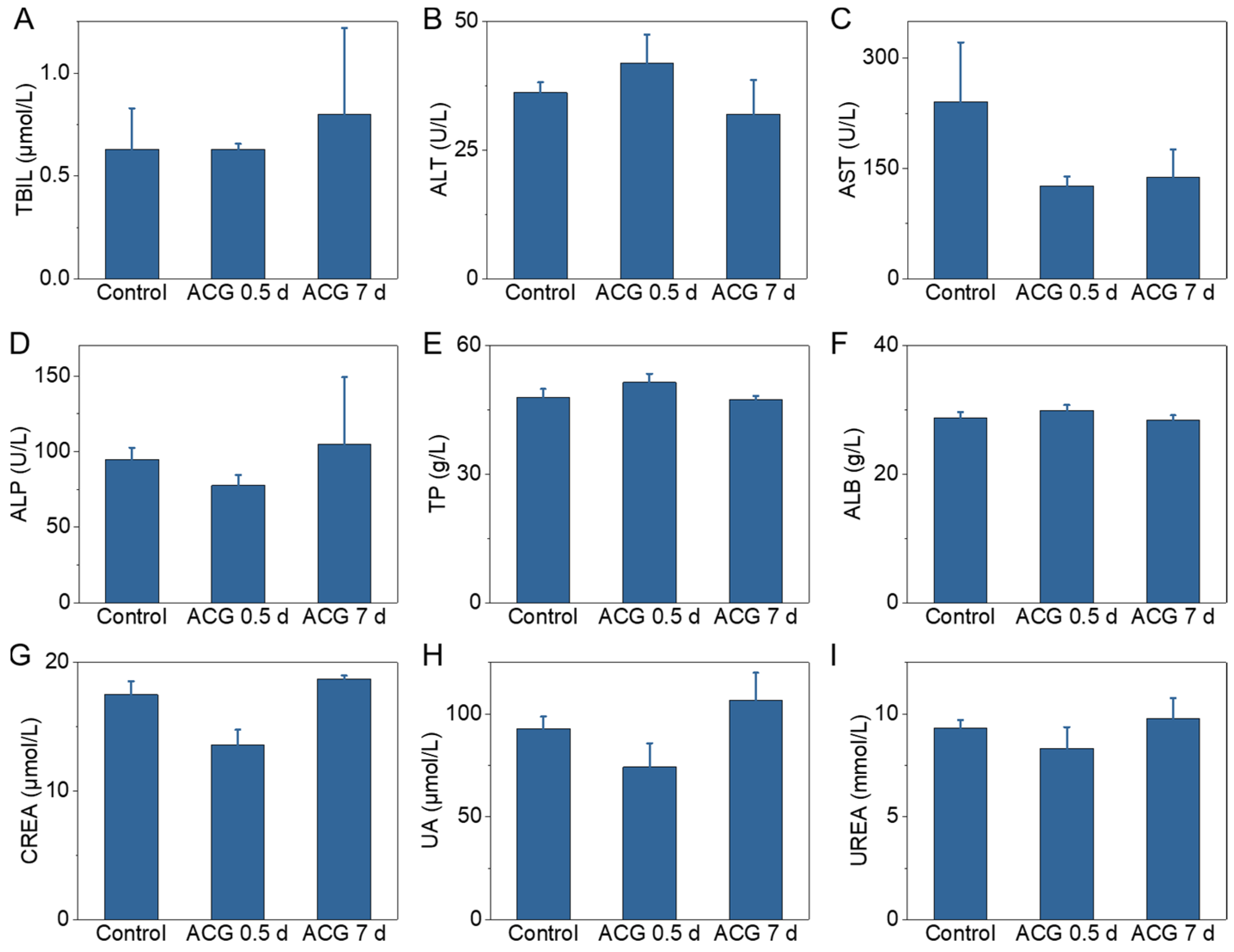
2.7. In Vivo Toxicity Assessment of ACG Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Characterizations
4.3. Preparation of ACG Hydrogel
4.4. In Vitro Evaluation of GP-Based Complex
4.5. In Vitro Cytotoxicity Assay
4.6. Optimization of Incubation Time of ACG Hydrogel and 4T1 Cells
4.7. Evaluation of In Vitro PTT Effect of GP-Based Complex
4.8. Animal Model
4.9. Optimization of Incubation Time of ACG Hydrogel and Tumor Tissues In Vivo
4.10. In Vivo PTT of the “Parasitism” of ACG Hydrogel in Tumor
4.11. In Vivo Toxicity Assessment of ACG Hydrogel
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Ning, C.Y.; Zhou, Z.N.; Yu, P.; Zhu, Y.; Tan, G.X.; Mao, C.B. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Hola, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots-highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Kenney, M.; Chen, Y.S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon Dots for In Vivo Bioimaging and Theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo. Adv. Mater. 2017, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Jin, J.; Wang, D.W.; Lv, J.W.; Hou, K.; Liu, Y.L.; Chen, C.Y.; Tang, Z.Y. Coordination-responsive drug release inside gold nanorod@metal-organic framework core-shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy. Nano Res. 2018, 11, 3294–3305. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, X.W.; Zhang, H.; Huang, H.X.; Sun, L.A.; Ma, L.; Du, Y.H.; Pei, C.J.; Zhang, Q.H.; Li, H.; et al. Activating Layered Metal Oxide Nanomaterials via Structural Engineering as Biodegradable Nanoagents for Photothermal Cancer Therapy. Small 2021, 17, 12. [Google Scholar] [CrossRef]
- Li, W.; Rong, P.; Yang, K.; Huang, P.; Sun, K.; Chen, X. Semimetal nanomaterials of antimony as highly efficient agent for photoacoustic imaging and photothermal therapy. Biomaterials 2015, 45, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, A.; Zhao, C.; Yang, K.; Chen, X.; Li, W. Ultrasmall Semimetal Nanoparticles of Bismuth for Dual-Modal Computed Tomography/Photoacoustic Imaging and Synergistic Thermoradiotherapy. ACS Nano 2017, 11, 3990–4001. [Google Scholar] [CrossRef]
- Hu, K.; Xie, L.; Zhang, Y.; Hanyu, M.; Yang, Z.; Nagatsu, K.; Suzuki, H.; Ouyang, J.; Ji, X.; Wei, J.; et al. Marriage of black phosphorus and Cu(2+) as effective photothermal agents for PET-guided combination cancer therapy. Nat. Commun. 2020, 11, 2778. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; Shi, Y.; Huang, W.; Shao, J.; Dong, X. Nano-black phosphorus for combined cancer phototherapy: Recent advances and prospects. Nanotechnology 2018, 29, 222001. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xiao, Y.; Li, W.; Yang, Q.; Tan, L.; Jia, Y.; Qu, Y.; Qian, Z. Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv. Sci. 2018, 5, 1700891. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; He, Q.X.; Li, X.S.; Yoon, J.; Huang, J.D. Phthalocyanines as contrast agents for photothermal therapy. Coord. Chem. Rev. 2021, 426, 14. [Google Scholar] [CrossRef]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 1057–1061. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Xiao, X.; Fan, X.F.; Yang, Y.; Song, C.W.; Fan, Y.F.; Liu, Y.M. Low cost, facile, environmentally friendly all biomass-based squid ink-starch hydrogel for efficient solar-steam generation. J. Mater. Chem. A 2020, 8, 24108–24116. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Tian, J.; Liu, Y.K.X.; Xu, L.Q.; Wang, Y.; Fei, X.; Li, Y. A novel floatable composite hydrogel for solar evaporation enhancement. Environ. Sci. Water Res. Technol. 2020, 6, 221–230. [Google Scholar] [CrossRef]
- Chu, M.; Peng, J.; Zhao, J.; Liang, S.; Shao, Y.; Wu, Q. Laser light triggered-activated carbon nanosystem for cancer therapy. Biomaterials 2013, 34, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yin, W.; Yu, J.; Dou, R.; Bao, T.; Zhang, X.; Yan, L.; Yong, Y.; Su, C.; Wang, Q.; et al. Mesoporous Bamboo Charcoal Nanoparticles as a New Near-Infrared Responsive Drug Carrier for Imaging-Guided Chemotherapy/Photothermal Synergistic Therapy of Tumor. Adv. Healthc. Mater. 2016, 5, 1627–1637. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Yu, H.; Tian, H.; Chen, X. Multifunctional Theranostic Nanoparticles Derived from Fruit-Extracted Anthocyanins with Dynamic Disassembly and Elimination Abilities. ACS Nano 2018, 12, 8255–8265. [Google Scholar] [CrossRef]
- Guo, C.; Sun, J.; Dong, J.; Cai, W.; Zhao, X.; Song, B.; Zhang, R. A natural anthocyanin-based multifunctional theranostic agent for dual-modal imaging and photothermal anti-tumor therapy. J. Mater. Chem. B 2021, 9, 7447–7460. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, R.; Zhu, Y.; Peng, M.; Fan, T.; Ren, P.; Wang, B.; Kang, L.; Liu, X.; Li, S.; et al. Liquid-phase exfoliation of black sesame to create a nanoplatform for in vitro photoluminescence and photothermal therapy. Nanomedicine 2020, 15, 2041–2052. [Google Scholar] [CrossRef]
- Chu, M.; Hai, W.; Zhang, Z.; Wo, F.; Wu, Q.; Zhang, Z.; Shao, Y.; Zhang, D.; Jin, L.; Shi, D. Melanin nanoparticles derived from a homology of medicine and food for sentinel lymph node mapping and photothermal in vivo cancer therapy. Biomaterials 2016, 91, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Sasaki, M.; Waki, T.; Iwaki, T.; Morii, Y.; Yanagida, K.; Watanabe, M.; Tsuchitani, Y.; Saito, T.; Asakawa, M. Distribution records of three species of Leucochloridium (Trematoda: Leucochloridiidae) in Japan, with comments on their microtaxonomy and ecology. Parasitol. Int. 2019, 72, 101936. [Google Scholar] [CrossRef]
- Wesołowska, W.; Wesołowski, T. Do Leucochloridium sporocysts manipulate the behaviour of their snail hosts? J. Zool. 2013, 292, 151–155. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef]
- Almog, J.; Cohen, Y.; Azoury, M.; Hahn, T.R. Genipin—A novel fingerprint reagent with colorimetric and fluorogenic activity. J. Forensic Sci. 2004, 49, 255–257. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Koyanagi, M.; Swartz, C.; Davis, J.; Maronpot, R.; Recio, L.; Hayashi, S.M. Genotoxicity evaluation of the naturally-derived food colorant, gardenia blue, and its precursor, genipin. Food Chem. Toxicol. 2018, 118, 695–708. [Google Scholar] [CrossRef]
- Guo, Z.C.; Zhang, T.Z.; Chen, X.X.; Fang, K.; Hou, M.; Gu, N. The effects of porosity and stiffness of genipin cross-linked egg white simulating aged extracellular matrix on proliferation and aggregation of ovarian cancer cells. Colloid Surf. A 2017, 520, 649–660. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 28–37. [Google Scholar] [CrossRef]
- Li, K.D.; Yan, K.; Wang, Q.S.; Tian, J.S.; Xu, D.; Zhang, W.Y.; Cui, Y.L. Antidepressant-like effects of dietary gardenia blue pigment derived from genipin and tyrosine. Food Funct. 2019, 10, 4533–4545. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, S.; Fukui, Y.; Koga, K.; Kumada, J.-I. Brilliant skyblue pigment formation from gardenia fruits. J. Ferment. Technol. 1987, 65, 419–424. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shen, G.Z.; Zhao, L.Y.; Zou, Q.L.; Jiao, T.F.; Yan, X.H. Robust Photothermal Nanodrugs Based on Covalent Assembly of Nonpigmented Biomolecules for Antitumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41898–41905. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and characterization of IPN hydrogels composed of chitosan and gelatin cross-linked by genipin. Carbohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.K. Biocompatible therapeutic albumin/genipin bioglue for postoperative wound adhesion and residual tumor ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef]
- Popova, N.V.; Juecker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.M.; Kearney, J.F.; Yeh, J.J. The PDAC Extracellular Matrix: A Review of the ECM Protein Composition, Tumor Cell Interaction, and Therapeutic Strategies. Front. Oncol. 2021, 11, 751311. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.G.; Yu, X.Y.; Zhao, X.H.; Wang, W.G.; Wang, K.L. Nanocrystallization of the Pharmaceutically Active Agent Genipin by an Emulsion Solvent Evaporation Method. J. Nanomater. 2014, 2014, 13. [Google Scholar] [CrossRef]
- Xu, W.; Wen, M.; Su, W.; Dushkin, A.V.; Suntsova, L.P.; Markova, I.D.; Selyutina, O.Y.; Polyakov, N.E. Physicochemical and Toxic Properties of Novel Genipin Drug Delivery Systems Prepared by Mechanochemistry. Curr. Drug Deliv. 2018, 15, 727–736. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, L.; Pan, J.; Zhang, X.; Sun, S.K. Green and Kilogram-Scale Synthesis of Fe Hydrogel for Photothermal Therapy of Tumors in Vivo. ACS Biomater. Sci. Eng. 2020, 6, 4276–4284. [Google Scholar] [CrossRef]
- Sun, S.K.; Wu, J.C.; Wang, H.; Zhou, L.; Zhang, C.; Cheng, R.; Kan, D.; Zhang, X.; Yu, C. Turning solid into gel for high-efficient persistent luminescence-sensitized photodynamic therapy. Biomaterials 2019, 218, 119328. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, C.; Wang, T.; Chen, J.; Sun, S.K. In Situ Fabrication of Intelligent Photothermal Indocyanine Green-Alginate Hydrogel for Localized Tumor Ablation. ACS Appl. Mater. Interfaces 2019, 11, 2782–2789. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. A Visible Codelivery Nanovaccine of Antigen and Adjuvant with Self-Carrier for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2019, 11, 4876–4888. [Google Scholar] [CrossRef]
- Lian, W.; Fan, M.; Li, T.; Zhang, X.; Rao, Z.; Li, Y.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. A Novel Green Synthesis Approach for Natural Bluish-Violet Pigments Derived from Water Extracts of Vaccinium bracteatum Thunb. Leaves. Ind. Crops Prod. 2019, 142, 111862. [Google Scholar] [CrossRef]
- Madhavan, K.; Belchenko, D.; Tan, W. Roles of Genipin Crosslinking and Biomolecule Conditioning in Collagen-Based Biopolymer: Potential for Vascular Media Regeneration. J. Biomed. Mater. Res. A 2011, 97, 16–26. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Pan, H.; Meng, Z.; Zhang, C. In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors. Gels 2022, 8, 754. https://doi.org/10.3390/gels8110754
Wang Y, Pan H, Meng Z, Zhang C. In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors. Gels. 2022; 8(11):754. https://doi.org/10.3390/gels8110754
Chicago/Turabian StyleWang, Yaqiong, Haiyan Pan, Zhaowei Meng, and Cai Zhang. 2022. "In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors" Gels 8, no. 11: 754. https://doi.org/10.3390/gels8110754
APA StyleWang, Y., Pan, H., Meng, Z., & Zhang, C. (2022). In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors. Gels, 8(11), 754. https://doi.org/10.3390/gels8110754