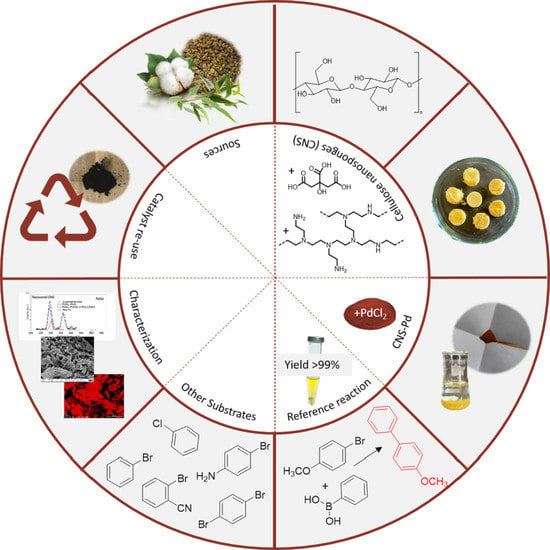
Pd-Loaded Cellulose NanoSponge as a Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. CNS-Pd Synthesis and Characterization
2.2. Suzuki–Miyaura Reaction Optimization
2.3. Suzuki–Miyaura Substrate Scope
2.4. Multiple Cross-Coupling Suzuki–Miyaura Reactions
2.5. Leaching Tests
2.6. Recyclability Tests
3. Conclusions
4. Materials and Methods
4.1. TEMPO-Oxidized Cellulose (TOC) Production and Titration and Synthesis of Cellulose NanoSponges (CNS)
4.2. Preparation of the Catalyst
4.3. Catalyst Characterization
4.4. Suzuki–Miyaura Reaction
4.5. Reaction Work-Up and Product Purification
4.6. Leaching Test
4.7. Reusability Test
4.8. NMR Product Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Molnár, Á. Efficient, Selective, and Recyclable Palladium Catalysts in Carbon-Carbon Coupling Reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at Ppm to Ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. A Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, D.; Rahman, M.; Ghosh, S.; Bagdi, A.K.; Zyryanov, G.V.; Chupakhin, O.N.; Das, P.; Hajra, A. Advances in Transition-Metal Catalyzed Carbonylative Suzuki-Miyaura Coupling Reaction: An Update. Adv. Synth. Catal. 2021, 363, 1597–1624. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective N-C/O-C Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef]
- Sau, S.C.; Hota, P.K.; Mandal, S.K.; Soleilhavoup, M.; Bertrand, G. Stable Abnormal N-Heterocyclic Carbenes and Their Applications. Chem. Soc. Rev. 2020, 49, 1233–1252. [Google Scholar] [CrossRef]
- Wang, K.; Fan, R.; Wei, X.; Fang, W. Palladacyclic N-Heterocyclic Carbene Precatalysts for Transition Metal Catalysis. Green Synth. Catal. 2022, 3, 327–338. [Google Scholar] [CrossRef]
- Wei, X.; Xue, B.; Handelmann, J.; Hu, Z.; Darmandeh, H.; Gessner, V.H.; Gooßen, L.J. Ylide-Functionalized Diisopropyl Phosphine (PrYPhos): A Ligand for Selective Suzuki-Miyaura Couplings of Aryl Chlorides. Adv. Synth. Catal. 2022, 364, 3336–3341. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. 2-Aminobiphenyl Palladacycles: The “Most Powerful” Precatalysts in C-C and C-Heteroatom Cross-Couplings. ACS Catal. 2015, 5, 1386–1396. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Espinosa, M.R.; Doppiu, A.; Hazari, N. Differences in the Performance of Allyl Based Palladium Precatalysts for Suzuki-Miyaura Reactions. Adv. Synth. Catal. 2020, 362, 5062–5078. [Google Scholar] [CrossRef] [PubMed]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem. 2017, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Orecchia, P.; Petkova, D.S.; Goetz, R.; Rominger, F.; Hashmi, A.S.K.; Schaub, T. Pd-Catalysed Suzuki-Miyaura Cross-Coupling of Aryl Chlorides at Low Catalyst Loadings in Water for the Synthesis of Industrially Important Fungicides. Green Chem. 2021, 23, 8169–8180. [Google Scholar] [CrossRef]
- Abi Fayssal, S.; Naret, T.; Buendia, J.; Labattut, A.; Huc, V.; Martini, C.; Schulz, E. Synthesis, Catalytic Activity and Comparative Leaching Studies of Calix[8]Arene-Supported Pd-NHC Complexes for Suzuki-Miyaura Cross-Couplings. Adv. Synth. Catal. 2022, 364, 947–957. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, C.; Liu, P.; Ge, X.; Zhou, S. Covalent Organic Framework-Supported Pd Nanoparticles: An Efficient and Reusable Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reactions. Appl. Organomet. Chem. 2022, 36, 1–10. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Shang, W.; Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Zeolite-Encaged Palladium Catalysts for Heterogeneous Suzuki-Miyaura Cross-Coupling Reactions. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in Carbon Nanotubes as Efficacious Supports for Palladium-Catalysed Carbon–Carbon Cross-Coupling Reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Hoshiya, N.; Isomura, N.; Shimoda, M.; Yoshikawa, H.; Yamashita, Y.; Iizuka, K.; Tsukamoto, S.; Shuto, S.; Arisawa, M. Development of a Recyclable and Low-Leaching Palladium Catalyst Supported on Sulfur-Modified Gallium Arsenide (001) for Use in Suzuki-Miyaura Coupling. ChemCatChem 2009, 1, 279–285. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Yamada, T.; Sawama, Y.; Sajiki, H. Continuous-Flow Suzuki-Miyaura and Mizoroki-Heck Reactions under Microwave Heating Conditions. Chem. Rec. 2019, 19, 3–14. [Google Scholar] [CrossRef]
- Price, G.A.; Bogdan, A.R.; Aguirre, A.L.; Iwai, T.; Djuric, S.W.; Organ, M.G. Continuous Flow Negishi Cross-Couplings Employing Silica-Supported: Pd-PEPPSI–IPr Precatalyst. Catal. Sci. Technol. 2016, 6, 4733–4742. [Google Scholar] [CrossRef]
- Shariatipour, M.; Heydari, A. PdII Dispersed on Magnetic Partially Reduced GO/OMWCNT Non-Covalently Modified with a Vic-Dioxime: An Efficient and Magnetically Retrievable Catalyst for Suzuki-Miyaura Coupling Reaction. ChemistrySelect 2021, 6, 1107–1117. [Google Scholar] [CrossRef]
- Kandathil, V.; Kulkarni, B.; Siddiqa, A.; Kempasiddaiah, M.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Immobilized N-Heterocyclic Carbene-Palladium(II) Complex on Graphene Oxide as Efficient and Recyclable Catalyst for Suzuki–Miyaura Cross-Coupling and Reduction of Nitroarenes. Catal. Lett. 2020, 150, 384–403. [Google Scholar] [CrossRef]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. Chempluschem 2015, 80, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and Drug Release Properties of Sponges from Cross-Linked Cellulose Nanofibers. Chempluschem 2017, 82, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Liberatori, G.; Grassi, G.; Guidi, P.; Bernardeschi, M.; Fiorati, A.; Scarcelli, V.; Genovese, M.; Faleri, C.; Protano, G.; Frenzilli, G.; et al. Effect–Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks. Nanomaterials 2020, 10, 1283. [Google Scholar] [CrossRef]
- Guidi, P.; Bernardeschi, M.; Palumbo, M.; Genovese, M.; Scarcelli, V.; Fiorati, A.; Riva, L.; Punta, C.; Corsi, I.; Frenzilli, G. Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced Dna Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations. Nanomaterials 2020, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Guidi, P.; Bernardeschi, M.; Palumbo, M.; Scarcelli, V.; Genovese, M.; Protano, G.; Vitiello, V.; Pontorno, L.; Bonciani, L.; Buttino, I.; et al. Cellular Responses Induced by Zinc in Zebra Mussel Haemocytes. Loss of DNA Integrity as a Cellular Mechanism to Evaluate the Suitability of Nanocellulose-Based Materials in Nanoremediation. Nanomaterials 2021, 11, 2219. [Google Scholar] [CrossRef]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-Design of Nanostructured Cellulose Sponges for Sea-Water Decontamination from Heavy Metal Ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life Cycle Assessment of Emerging Environmental Technologies in the Early Stage of Development: A Case Study on Nanostructured Materials. J. Ind. Ecol. 2020, 24, 101–115. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-Linked Cellulose Nano-Sponges: A Small Angle Neutron Scattering (SANS) Study. Cellulose 2019, 26, 9005–9019. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Crupi, V.; Majolino, D.; Fiorati, A.; Punta, C. FTIR-ATR Analysis of the H-Bond Network of Water in Branched Polyethyleneimine/TEMPO-Oxidized Cellulose Nano-Fiber Xerogels. Cellulose 2020, 27, 8605–8618. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Crupi, V.; Majolino, D.; Fiorati, A.; Punta, C. 2D Correlation Spectroscopy (2Dcos) Analysis of Temperature-Dependent Ftir-Atr Spectra in Branched Polyethyleneimine/Tempo-Oxidized Cellulose Nano-Fiber Xerogels. Polymers 2021, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Pastori, N.; Panozzo, A.; Antonelli, M.; Punta, C. Nanostructured Cellulose-Based Sorbent Materials for Water Decontamination from Organic Dyes. Nanomaterials 2020, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Melone, L.; Bonafede, S.; Tushi, D.; Punta, C.; Cametti, M. Dip in Colorimetric Fluoride Sensing by a Chemically Engineered Polymeric Cellulose/ BPEI Conjugate in the Solid State. RSC Adv. 2015, 5, 83197–83205. [Google Scholar] [CrossRef] [Green Version]
- Riva, L.; Fiorati, A.; Sganappa, A.; Melone, L.; Punta, C.; Cametti, M. Naked-Eye Heterogeneous Sensing of Fluoride Ions by Co-Polymeric Nanosponge Systems Comprising Aromatic-Imide-Functionalized Nanocellulose and Branched Polyethyleneimine. Chempluschem 2019, 84, 1512–1518. [Google Scholar] [CrossRef]
- Riva, L.; Punta, C.; Sacchetti, A. Co-Polymeric Nanosponges from Cellulose Biomass as Heterogeneous Catalysts for Amine-Catalyzed Organic Reactions. ChemCatChem 2020, 12, 6214–6222. [Google Scholar] [CrossRef]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc-and Copper-Loaded Nanosponges from Cellulose Nanofibers Hydrogels: New Heterogeneous Catalysts for the Synthesis of Aromatic Acetals. Gels 2022, 8, 54. [Google Scholar] [CrossRef]
- Bashar, M.M.; Zhu, H.; Yamamoto, S.; Mitsuishi, M. Highly Carboxylated and Crystalline Cellulose Nanocrystals from Jute Fiber by Facile Ammonium Persulfate Oxidation. Cellulose 2019, 26, 3671–3684. [Google Scholar] [CrossRef]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on Biopolymers: Cellulose Studies. Surf. Interface Anal. 2004, 36, 1018–1022. [Google Scholar] [CrossRef]
- Awada, H.; Monplaisir, D.; Daneault, C. Growth of Polyelectrolyte on Lignocellulosic Fibres: Study by ζ-Potential, FTIR, and XPS. BioResources 2012, 7, 2090–2104. [Google Scholar] [CrossRef] [Green Version]
- Benkaddour, A.; Journoux-Lapp, C.; Jradi, K.; Robert, S.; Daneault, C. Study of the Hydrophobization of TEMPO-Oxidized Cellulose Gel through Two Routes: Amidation and Esterification Process. J. Mater. Sci. 2014, 49, 2832–2843. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MA, USA, 2012.
- Criado, J.J.; Fernandez, I.; Macias, B.; Salas, J.M.; Medarde, M. Novel Chelates of Pd(II) Dithiocarbamates. Spectroscopic Studies and Thermal Behaviour. Inorg. Chim. Acta 1990, 174, 67–75. [Google Scholar] [CrossRef]
- Bertolini, J.C.; Delichere, P.; Khanra, B.C.; Massardier, J.; Noupa, C.; Tardy, B. Electronic Properties of Supported Pd Aggregates in Relation with Their Reactivity for 1,3-Butadiene Hydrogenation. Catal. Lett. 1990, 6, 215–223. [Google Scholar] [CrossRef]
- Kishi, K.; Ikeda, S. X-ray Photoelectron Spectroscopic Study of the Reaction of Evaporated Metal Films with Chlorine Gas. J. Phys. Chem. 1974, 78, 107–112. [Google Scholar] [CrossRef]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; De Matteis, F.; Casciardi, S.; et al. Plasmonic Sensor Based on Interaction between Silver Nanoparticles and Ni2+ or Co2+ in Water. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, I.A.; Salyn, J.V.; Tatjanenko, L.V.; Mashkovsky, Y.S.; Ponticelli, G. Inhibitory Activity of Palladium(II) and Platinum(II) Complexes with Isoxazole and Its Derivatives. J. Inorg. Biochem. 1981, 15, 89–92. [Google Scholar] [CrossRef]
- Wei, C.S.; Davies, G.H.M.; Soltani, O.; Albrecht, J.; Gao, Q.; Pathirana, C.; Hsiao, Y.; Tummala, S.; Eastgate, M.D. The Impact of Palladium(II) Reduction Pathways on the Structure and Activity of Palladium(0) Catalysts. Angew. Chem. Int. Ed. 2013, 52, 5822–5826. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-Mediated Oxidation of Polysaccharides: An Ongoing Story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef]
- Secchi, V.; Franchi, S.; Dettin, M.; Zamuner, A.; Beranová, K.; Vladescu, A.; Battocchio, C.; Graziani, V.; Tortora, L.; Iucci, G. Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, Rairs and Nexafs Study. Nanomaterials 2020, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Boston, MA, USA, 1992; ISBN 978-0-9627026-2-4. [Google Scholar]
- Castle, J.E. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; Briggs, D., Seah, M.P., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1983; ISBN 047126279X. [Google Scholar]
- Isfahani, A.L.; Mohammadpoor-Baltork, I.; Mirkhani, V.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Kia, R. Palladium Nanoparticles Immobilized on Nano-Silica Triazine Dendritic Polymer (Pdnp-NSTDP): An Efficient and Reusable Catalyst for Suzuki-Miyaura Cross-Coupling and Heck Reactions. Adv. Synth. Catal. 2013, 355, 957–972. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Hsu, Y.C.; Shih, W.C.; Lee, C.Y.; Chuang, W.H.; Tsai, Y.F.; Chen, P.P.Y.; Ong, T.G. Metal-Free Arylation of Benzene and Pyridine Promoted by Amino-Linked Nitrogen Heterocyclic Carbenes. Chem. Commun. 2012, 48, 6702–6704. [Google Scholar] [CrossRef] [PubMed]
- Budén, M.E.; Guastavino, J.F.; Rossi, R.A. Room-Temperature Photoinduced Direct C-H-Arylation via Base-Promoted Homolytic Aromatic Substitution. Org. Lett. 2013, 15, 1174–1177. [Google Scholar] [CrossRef]
- Raza, F.; Yim, D.; Park, J.H.; Kim, H.I.; Jeon, S.J.; Kim, J.H. Structuring Pd Nanoparticles on 2H-WS2 Nanosheets Induces Excellent Photocatalytic Activity for Cross-Coupling Reactions under Visible Light. J. Am. Chem. Soc. 2017, 139, 14767–14774. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.J.M.; Dinh, A.H.; Ton, N.N.H.; Crocker, R.D.; Mai, B.K.; Nguyen, T.V. Ring Contraction of Tropylium Ions into Benzenoid Derivatives. Org. Lett. 2022, 24, 2520–2525. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Dong, L.; Chen, X.; Xin, G.; Zhang, Y.; Zang, J. One-step synthesis of shell/core structural boron and nitrogen co-doped graphitic carbon/nanodiamond as efficient electrocatalyst for the oxygen reduction reaction in alkaline media. Electrochim. Acta 2016, 194, 161–167. [Google Scholar] [CrossRef]







| Entry | T (°C) | T (h) | Yield (%) |
|---|---|---|---|
| 1 | 25 (RT) | 48 | 19 |
| 2 | 80 | 0.5 | 57 |
| 3 | 100 | 0.33 | 72 |
| 4 | 100 | 0.5 | 92 |
| 5 b | 100 | 0.5 | 19 |
| Entry | CNS-Pd (%) | Pd (%) | Yield (%) |
|---|---|---|---|
| 1 | 0 | 0.00 | 0 |
| 2 | 2 | 0.45 | 92 |
| 3 | 5 | 1.14 | 95 |
| 4 | 10 | 2.27 | 94 |
| 5 b | 10 | - | 0 |
| Entry | Base | Yield (%) |
|---|---|---|
| 1 | - | 15 |
| 2 | NaOAc | 54 |
| 3 | Na2CO3 | 25 |
| 4 | K2CO3 | 25 |
| 5 | KOH | 92 |
| Entry | TBAB Equivalents | Conversion (%) |
|---|---|---|
| 1 | 0.60 | 92 |
| 2 | 0.30 | 96 |
| 3 | 0.15 | 97 |
| 4 | 0 | 90 |
| 5 b | 0 | 60 |
| 6 b | 0.15 | 95 |

| Entry | ArX | R | R′ | R″ | R‴ | X | Conversion (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1a | -H | -OCH3 | -H | -H | -Br | 99 |
| 2 | 1b | -H | -H | -OCH3 | -H | -Br | 98 |
| 3 | 1c | -H | -H | -H | -OCH3 | -Br | 54 |
| 4 c | 1c | -H | -H | -H | -OCH3 | -Br | 38 |
| 5 b | 1d | -H | -CH3 | -H | -H | -Br | 72 |
| 6 b | 1e | -H | -CHO | -H | -H | -Br | 13 |
| 7 b,c | 1e | -H | -CHO | -H | -H | -Br | 80 |
| 8 | 1f | -H | -H | -H | -H | -Br | 93 |
| 9 | 1g | -H | -NH2 | -H | -H | -Br | 76 |
| 10 | 1h | -CN | -H | -H | -H | -Br | 99 |
| 11 | 1i | -H | -H | -H | -H | -Cl | 55 |
| Entry | Cycle Number | Yield (%) |
|---|---|---|
| 1 | I | 97 |
| 2 | II | 99 |
| 3 | III | 98 |
| 4 | IV | 99 |
| 5 | V | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, L.; Nicastro, G.; Liu, M.; Battocchio, C.; Punta, C.; Sacchetti, A. Pd-Loaded Cellulose NanoSponge as a Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reactions. Gels 2022, 8, 789. https://doi.org/10.3390/gels8120789
Riva L, Nicastro G, Liu M, Battocchio C, Punta C, Sacchetti A. Pd-Loaded Cellulose NanoSponge as a Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reactions. Gels. 2022; 8(12):789. https://doi.org/10.3390/gels8120789
Chicago/Turabian StyleRiva, Laura, Gloria Nicastro, Mingchong Liu, Chiara Battocchio, Carlo Punta, and Alessandro Sacchetti. 2022. "Pd-Loaded Cellulose NanoSponge as a Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reactions" Gels 8, no. 12: 789. https://doi.org/10.3390/gels8120789