Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture
Abstract
1. Introduction
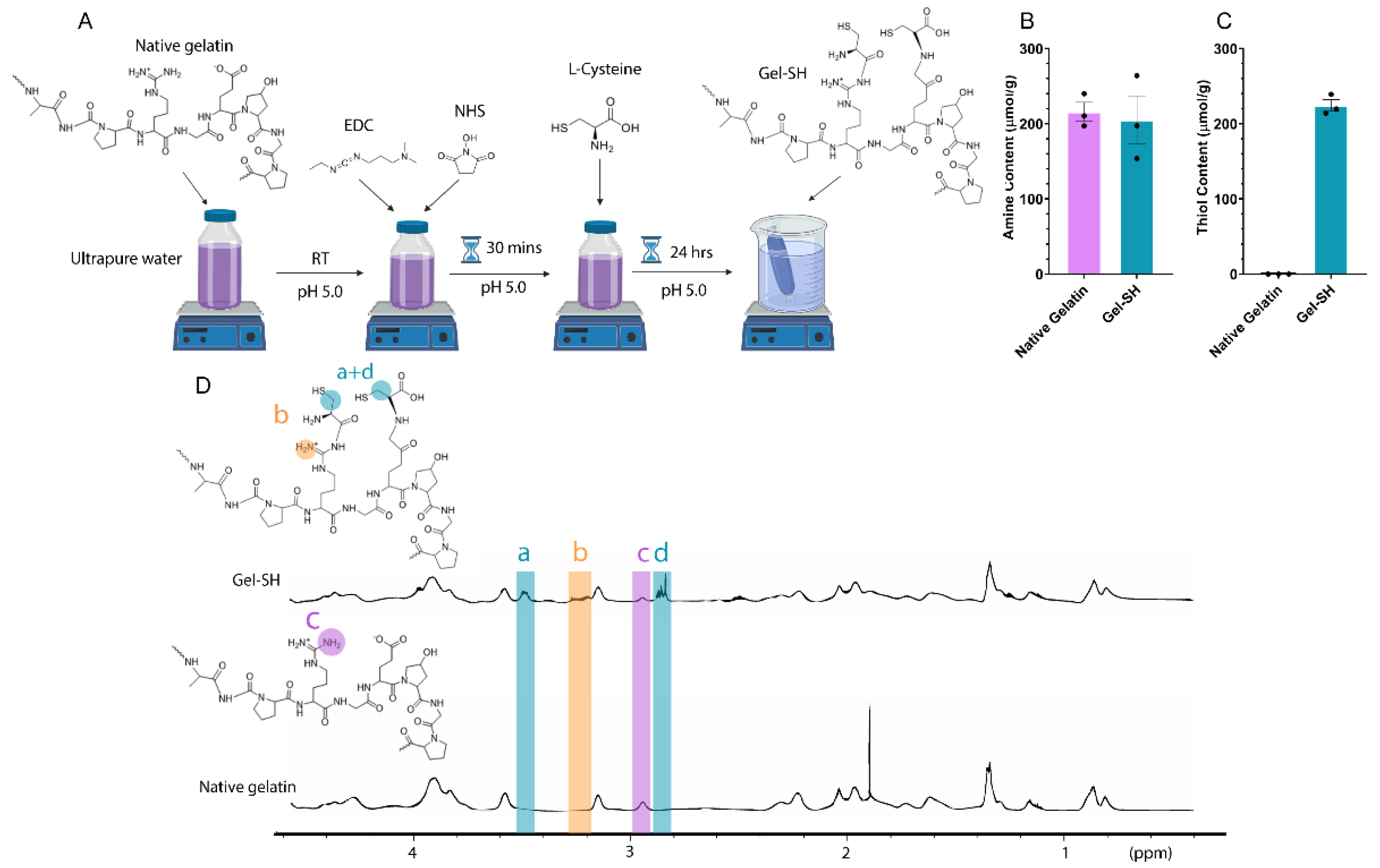
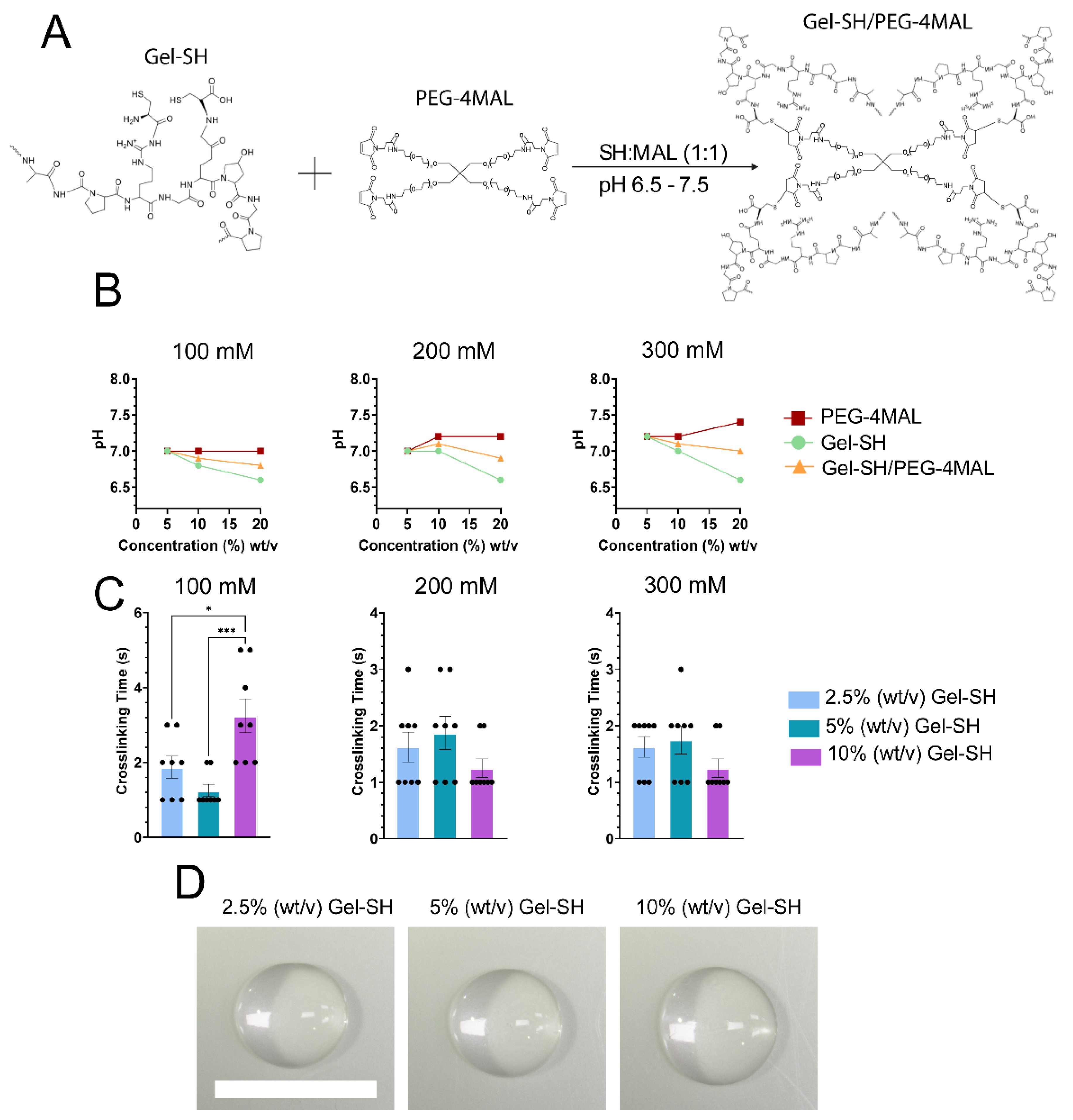
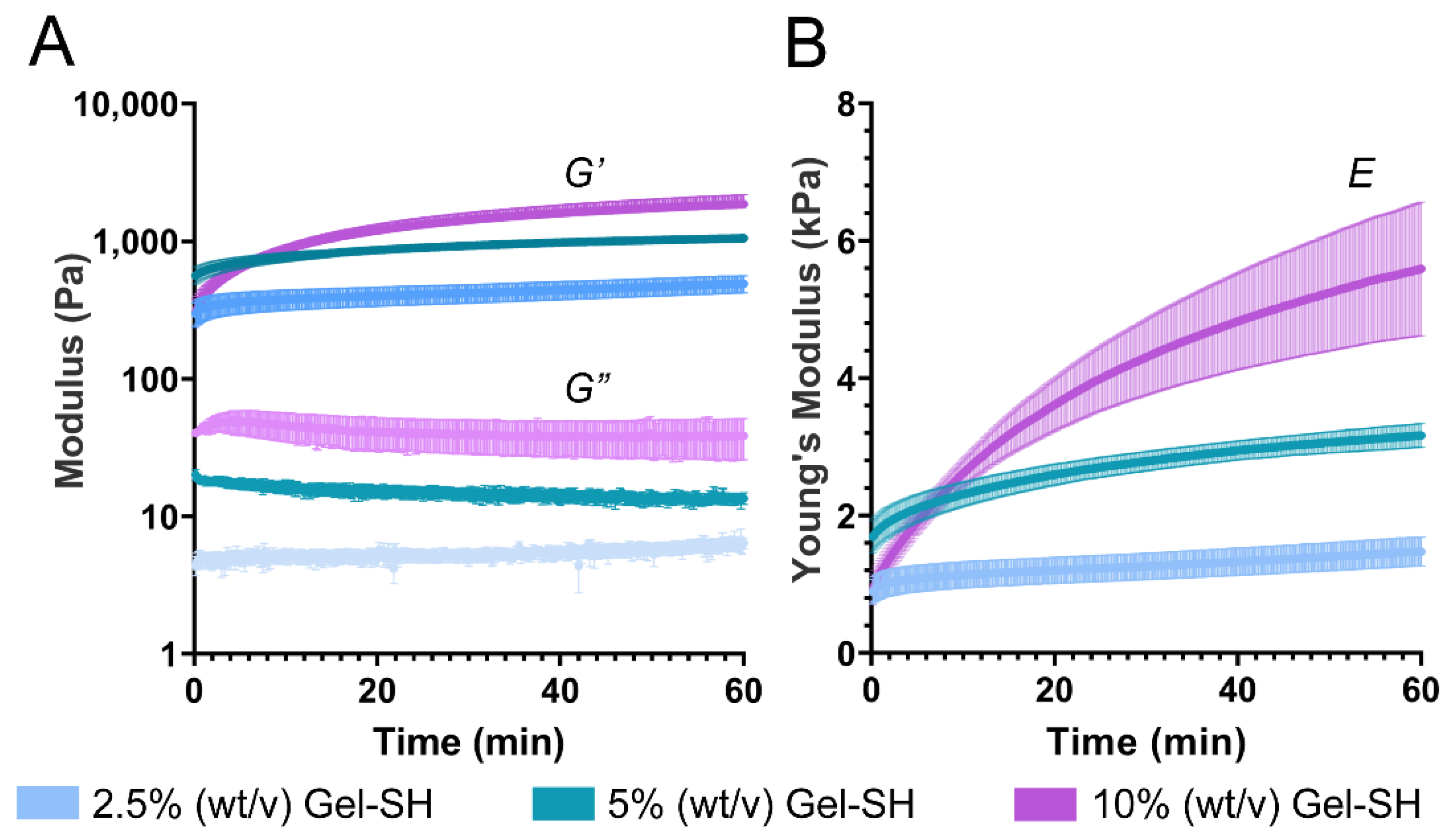
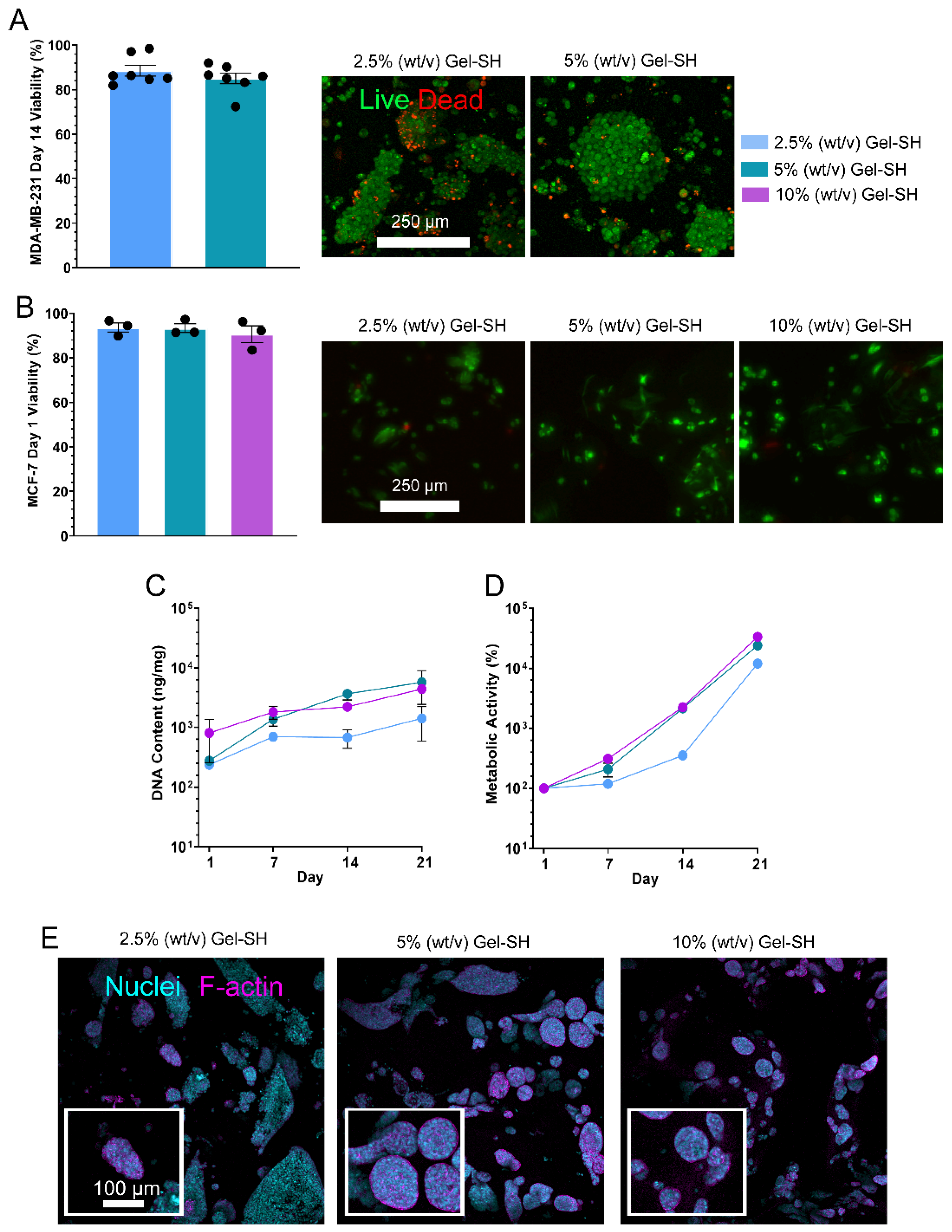
2. Results and Discussion
3. Conclusions
4. Materials and Methods
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z.; Duval, K.; Grover, H.; Han, L.-H. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Prager, J.; Adams, C.F.; Delaney, A.M.; Chanoit, G.; Tarlton, J.F.; Wong, L.F.; Chari, D.M.; Granger, N. Stiffness-matched biomaterial implants for cell delivery: Clinical, intraoperative ultrasound elastography provides a ‘target’ stiffness for hydrogel synthesis in spinal cord injury. J. Tissue Eng. 2020, 11, 2041731420934806. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.B.; Caliari, S.R.; Lampe, K.J. 3D Hyaluronic Acid Hydrogels for Modeling Oligodendrocyte Progenitor Cell Behavior as a Function of Matrix Stiffness. Biomacromolecules 2020, 21, 4962–4971. [Google Scholar] [CrossRef]
- Bonnesœur, S.; Morin-Grognet, S.; Thoumire, O.; Le Cerf, D.; Boyer, O.; Vannier, J.P.; Labat, B. Hyaluronan-based hydrogels as versatile tumor-like models: Tunable ECM and stiffness with genipin-crosslinking. J. Biomed. Mater. Res. Part A 2020, 108, 1256–1268. [Google Scholar] [CrossRef]
- Shah, L.; Latif, A.; Williams, K.J.; Mancuso, E.; Tirella, A. Invasion and secondary site colonization as a function of in vitro primary tumor matrix stiffness. Adv. Healthc. Mater. 2022, 11, 2201898. [Google Scholar] [CrossRef]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef]
- DelNero, P.; Lane, M.; Verbridge, S.S.; Kwee, B.; Kermani, P.; Hempstead, B.; Stroock, A.; Fischbach, C. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 2015, 55, 110–118. [Google Scholar] [CrossRef]
- Vantangoli, M.M.; Madnick, S.J.; Huse, S.M.; Weston, P.; Boekelheide, K. MCF-7 Human Breast Cancer Cells Form Differentiated Microtissues in Scaffold-Free Hydrogels. PLoS ONE 2015, 10, e0135426. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension-how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Menon, N.; Dang, H.X.; Datla, U.S.; Moarefian, M.; Lawrence, C.B.; Maher, C.A.; Jones, C.N. Heparin-based hydrogel scaffolding alters the transcriptomic profile and increases the chemoresistance of MDA-MB-231 triple-negative breast cancer cells. Biomater. Sci. 2020, 8, 2786–2796. [Google Scholar] [CrossRef]
- Das, V.; Bruzzese, F.; Konečný, P.; Iannelli, F.; Budillon, A.; Hajdúch, M. Pathophysiologically relevant in vitro tumor models for drug screening. Drug Discov. Today 2015, 20, 848–855. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. Available online: https://aacrjournals.org/cancerdiscovery/article-abstract/4/9/998/6397 (accessed on 13 July 2022).
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymer 2017, 9, 328. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J. Biomed. Mater. Res. Part A 2017, 105, 236–252. [Google Scholar] [CrossRef]
- Kleinman, H.K. Preparation of Basement Membrane Components from EHS Tumors. Curr. Protoc. Cell Biol. 1998, 10, 10.2.1–10.2.10. [Google Scholar] [CrossRef]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The elastic modulus of MatrigelTM as determined by atomic force microscopy. J. Struct. Biol. 2009, 167, 216–219. Available online: https://www.sciencedirect.com/science/article/pii/S1047847709001385 (accessed on 7 October 2022). [CrossRef]
- Tsao, C.T.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. Chitosan-based thermoreversible hydrogel as an in Vitro tumor microenvironment for testing breast cancer therapies. Mol. Pharm. 2014, 11, 2134–2142. [Google Scholar] [CrossRef]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix. Exp. Cell Res. 1992, 202, 1–8. Available online: https://www.sciencedirect.com/science/article/pii/001448279290397Q (accessed on 7 October 2022). [CrossRef]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Akhmetzhan, A.; Myrzakhmetova, N.; Amangeldi, N.; Kuanyshova, Z.; Akimbayeva, N.; Dosmaganbetova, S.; Toktarbay, Z.; Longinos, S.N. A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels. Gels 2021, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.; Yeligbayeva, G.; Nakan, U.; Amitova, A.; Suleimenov, I.; Zh Yeligbayeva, G. Some peculiarities of the kinetics of interaction of cationic hydrogels based on copolymers of vinyl esters of monoethanolamine and ethylene glycol with copper ions. Orig. Res. 2021, 9, 291–304. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X.; Yao, F.; Luo, Z.; Jin, L.; Xie, B.; Shi, S.; Ma, H.; Li, X.; Chen, H. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. Int. J. Pharm. 2014, 470, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Utama, R.H.; Tan, V.T.; Tjandra, K.C.; Sexton, A.; Nguyen, D.H.; O’Mahony, A.P.; Ribeiro, J.C.; Kavallaris, M.; Gooding, J.J. A covalently crosslinked bioink for multi-materials drop-on-demand 3D bioprinting of three-dimensional cell cultures. Biomater. Sci. 2021, 10, 5876–5887. [Google Scholar] [CrossRef]
- Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubois, P.; Mason, A.F.; Hedrick, J.L.; Liao, Q.; Frank, C.W.; Kingsbury, K.; et al. Synthesis of well-defined hydrogel networks using Click chemistry. Chem. Commun. 2006, 26, 2774–2776. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Abd Elgadir, M.; Mirghani, M.E.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ 2005, 11, 73–79. Available online: https://www.researchgate.net/profile/Mohamed-Saeed-12/publication/312332853_WFL_Publisher_Science_and_Technology_Fish_gelatin_and_its_applications_in_selected_pharmaceutical_aspects_as_alternative_source_to_pork_gelatin/links/58848642aca272b7b448a353/WFL-Publisher-Science-and-Technology-Fish-gelatin-and-its-applications-in-selected-pharmaceutical-aspects-as-alternative-source-to-pork-gelatin.pdf (accessed on 3 July 2022).
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymer 2020, 12, 3051. [Google Scholar] [CrossRef]
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- He, L.; Li, S.; Xu, C.; Wei, B.; Zhang, J.; Xu, Y.; Zhu, B.; Cao, Y.; Wu, X.; Xiong, Z.; et al. A New Method of Gelatin Modified Collagen and Viscoelastic Study of Gelatin-Collagen Composite Hydrogel. Macromol. Res. 2020, 28, 861–868. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Li, Y.; Khuu, N.; Prince, E.; Tao, H.; Zhang, N.; Chen, Z.; Gevorkian, A.; McGuigan, A.P.; Kumacheva, E. Matrix Stiffness-Regulated Growth of Breast Tumor Spheroids and Their Response to Chemotherapy. Biomacromolecules 2021, 22, 419–429. [Google Scholar] [CrossRef]
- Cavo, M.; Fato, M.; Peñuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef]
- Meinert, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W.; Loessner, D. A method for prostate and breast cancer cell spheroid cultures using gelatin methacryloyl-based hydrogels. Methods Mol. Biol. 2018, 1786, 175–194. [Google Scholar]
- Soliman, B.G.; Major, G.S.; Atienza-Roca, P.; Murphy, C.A.; Longoni, A.; Alcala-Orozco, C.R.; Rnjak-Kovacina, J.; Gawlitta, D.; Woodfield, T.B.F.; Lim, K.S. Development and Characterization of Gelatin-Norbornene Bioink to Understand the Interplay between Physical Architecture and Micro-Capillary Formation in Biofabricated Vascularized Constructs. Adv. Healthc. Mater. 2022, 11, 2101873. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef]
- Gu, Z.; Huang, J.; Chen, L.; Wu, J. Red Jujube-Incorporated Gelatin Methacryloyl (GelMA) Hydrogels with Anti-Oxidation and Immunoregulation Activity for Wound Healing. Artic. J. Biomed. Nanotechnol. 2019, 15, 1357–1370. [Google Scholar] [CrossRef]
- Ur Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin methacryloyl hydrogels control the localized delivery of albumin-bound paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; O’connell, C.D.; Cometta, S.; Hutmacher, D.W.; Meinert, C.; Bock, N. Gelatin Methacryloyl Hydrogels for the Localized Delivery of Cefazolin. Polymer 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Chen, Z.; Li, S.; Yan, C. Thiol-Ene Click Reaction Initiated Rapid Gelation of PEGDA/Silk Fibroin Hydrogels. Polymers 2019, 11, 2102. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Schacht, E.; Dubruel, P. Reversible gelatin-based hydrogels: Finetuning of material properties. Eur. Polym. J. 2011, 47, 1039–1047. [Google Scholar] [CrossRef]
- Göckler, T.; Haase, S.; Kempter, X.; Pfister, R.; Maciel, B.R.; Grimm, A.; Molitor, T.; Willenbacher, N.; Schepers, U.; Göckler, T.; et al. Tuning Superfast Curing Thiol-Norbornene-Functionalized Gelatin Hydrogels for 3D Bioprinting. Adv. Healthc. Mater. 2021, 10, 2100206. [Google Scholar] [CrossRef]
- Dong, Y.; Sigen, A.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619. [Google Scholar] [CrossRef]
- Dong, R.; Krishnan, S.; Baird, B.A.; Lindau, M.; Ober, C.K. Patterned biofunctional poly (acrylic acid) brushes on silicon surfaces. ACS Publ. 2007, 8, 3082–3092. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Nguyen, M.N.; Thorel, A.; Boudou, J.P.; Chehimi, M.M.; Mangeney, C. Protein-functionalized hairy diamond nanoparticles. Langmuir 2009, 25, 9633–9638. [Google Scholar] [CrossRef]
- Wissink, M.J.B.; Beernink, R.; Pieper, J.S.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; Van Aken, W.G.; Feijen, J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Sohn, Y.-S.; Lee, Y.K. Site-directed immobilization of antibody using EDC-NHS-activated protein A on a bimetallic-based surface plasmon resonance chip. J. Biomed. Opt. 2014, 19, 051209. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Xu, J.; Duan, Z.; Qi, X.; Ou, Y.; Guo, X.; Zi, L.; Wei, Y.; Liu, H.; Ma, L.; Li, H.; et al. Injectable Gelatin Hydrogel Suppresses Inflammation and Enhances Functional Recovery in a Mouse Model of Intracerebral Hemorrhage. Front. Bioeng. Biotechnol. 2020, 8, 785. [Google Scholar] [CrossRef]
- Deng, L.; Li, X.; Miao, K.; Mao, X.; Han, M.; Li, D.; Mu, C.; Ge, L. Development of Disulfide Bond Crosslinked Gelatin/ε-Polylysine Active Edible Film with Antibacterial and Antioxidant Activities. Food Bioprocess Technol. 2020, 13, 577–588. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Xu, Y.; Chen, M.; Lu, Y.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Effects of the bonding intensity between hyaluronan and gelatin on chondrogenic phenotypic maintenance. J. Mater. Chem. B 2020, 8, 9062–9074. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Gharaie, S.S.; Dabiri, S.M.H.; Akbari, M. Smart Shear-Thinning Hydrogels as Injectable Drug Delivery Systems. Polymer 2018, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Madhamuthanalli, C.V.; Bangalore, S.A. Rheological and physico-chemical properties of gelatin extracted from the skin of a few species of freshwater carp. Int. J. Food Sci. Technol. 2014, 49, 1758–1764. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold Water Fish Gelatin Methacryloyl Hydrogel for Tissue Engineering Application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [PubMed]
- Persikov, A.V.; Ramshaw, J.A.M.; Kirkpatrick, A.; Brodsky, B. Amino acid propensities for the collagen triple-helix. Biochemistry 2000, 39, 14960–14967. [Google Scholar] [CrossRef]
- Gudmundsson, M. Rheological Properties of Fish Gelatins. J. Food Sci. 2002, 67, 2172–2176. [Google Scholar] [CrossRef]
- Heggli, M.; Tirelli, N.; Zisch, A.; Hubbell, J.A. Michael-type addition as a tool for surface functionalization. Bioconjug. Chem. 2003, 14, 967–973. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Rahmawati, D.; Setyadewi, N.M. Extraction and characterization of gelatin from skin trimming pickled waste of tannery. IOP Conf. Ser. Earth Environ. Sci. 2019, 306, 012022. [Google Scholar] [CrossRef]
- Lin, J.; Pan, D.; Sun, Y.; Ou, C.; Wang, Y.; Cao, J. The modification of gelatin films: Based on various cross-linking mechanism of glutaraldehyde at acidic and alkaline conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef]
- Phelps, E.A.; Enemchukwu, N.O.; Sulchek, T.A.; García, A.J.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Barker, T.H. Maleimide cross-linked bioactive peg hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 2012, 24, 64–70. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Li, S.; Song, J.; Song, B.; Lu, W.F. Shear stress analysis and its effects on cell viability and cell proliferation in drop-on-demand bioprinting. Biomed. Phys. Eng. Express 2018, 4, 045028. [Google Scholar] [CrossRef]
- Jansen, L.E.; Negrón-Piñeiro, L.J.; Galarza, S.; Peyton, S.R. Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 2018, 70, 120–128. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, K.; Zheng, X.; Giacomin, A.J.; Mix, A.W.; Kao, W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials 2012, 33, 48–58. [Google Scholar] [CrossRef]
- Owen, S.C.; Fisher, S.A.; Tam, R.Y.; Nimmo, C.M.; Shoichet, M.S. Hyaluronic acid click hydrogels emulate the extracellular matrix. ACS Publ. 2013, 29, 7393–7400. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.; Groll, J. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- April Kloxin, B.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S.; Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic moduli of normal and pathological human breast tissues: An inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 2007, 52, 1565. [Google Scholar] [CrossRef]
- Briot, N.; Chagnon, G.; Connesson, N.; Payan, Y. In vivo measurement of breast tissues stiffness using a light aspiration device. Clin. Biomech. 2022, 99, 105743. [Google Scholar] [CrossRef]
- Xydeas, T.; Siegmann, K.; Sinkus, R.; Krainick-Strobel, U.; Miller, S.; Claussen, C.D. Magnetic resonance elastography of the breast: Correlation of signal intensity data with viscoelastic properties. Investig. Radiol. 2005, 40, 412–420. Available online: https://journals.lww.com/investigativeradiology/Fulltext/2005/07000/Further_Signs_in_the_Evaluation_of_Magnetic.4.aspx?casa_token=GhkJs0jhkeYAAAAA:pb1JuIa0Brzubc_--R-ACf-Q6pY2yY5nZRc34OQ717c_imCzUil_uUA6c549WC_307oABhZyQqGIQDxecm6SEib5vA (accessed on 31 October 2022). [CrossRef]
- Sinkus, R.; Tanter, M.; Xydeas, T.; Catheline, S.; Bercoff, J.; Fink, M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 2005, 23(PT0), 159–165. Available online: https://www.sciencedirect.com/science/article/pii/S0730725X05000391?casa_token=_Q4GsYZcerMAAAAA:6DKQB8E7LgXYTMAHPT0Eyxf7NXhTgkiY5n0Qz-XaJ_fRTDJTV5QdjYN3ZCZFGp-bp4ikBjUdlBA (accessed on 31 October 2022). [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Bock, N.; Dargaville, B.L.; Hutmacher, D.W. Deciphering the molecular mechanism of water interaction with gelatin Methacryloyl hydrogels: Role of ionic strength, pH, drug loading and hydrogel network. Biomedicines 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lee, B.H.; Peled, H.B.; Venkatraman, S.S. Synthesis of stiffness-tunable and cell-responsive Gelatin–poly(ethylene glycol) hydrogel for three-dimensional cell encapsulation. J. Biomed. Mater. Res. Part A 2016, 104, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.; Rastogi, S.K.; Dominguez, J.; Cantu, T.; Brittain, W.; Irvin, J.; Betancourt, T. Biodegradable DNA-enabled poly(ethylene glycol) hydrogels prepared by copper-free click chemistry. J. Biomater. Sci. Polym. Ed. 2016, 27, 22–39. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Eun, J.B.; Wierenga, P.A.; Gruppen, H. Antioxidative activity and emulsifying properties of cuttlefish skin gelatin modified by oxidised phenolic compounds. Food Chem. 2009, 117, 160–168. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-Anan, M. An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin. J. Food Eng. 2012, 108, 94–102. [Google Scholar] [CrossRef]
- Truong, V.; Blakey, I.; Whittaker, A.K. Hydrophilic and amphiphilic polyethylene glycol-based hydrogels with tunable degradability prepared by “click” chemistry. Biomacromolecules 2012, 13, 4012–4021. [Google Scholar] [CrossRef]
- Hegab, R.A.; Pardue, S.; Shen, X.; Kevil, C.; Peppas, N.A.; Caldorera-Moore, M.E. Effect of network mesh size and swelling to the drug delivery from pH responsive hydrogels. J. Appl. Polym. Sci. 2020, 137, 48767. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Greene, T.; Lin, C.C. Modular Cross-Linking of Gelatin-Based Thiol-Norbornene Hydrogels for in Vitro 3D Culture of Hepatocellular Carcinoma Cells. ACS Biomater. Sci. Eng. 2015, 1, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.D.; Yeung, O.W.H.; Qi, X.; Kao, W.J.; Man, K. The Anti-Tumor Effects of M1 Macrophage-Loaded Poly (ethylene glycol) and Gelatin-Based Hydrogels on Hepatocellular Carcinoma. Theranostics 2017, 7, 3732. [Google Scholar] [CrossRef] [PubMed]
- James-Bhasin, M.; Siegel, P.M.; Nazhat, S.N. A three-dimensional dense collagen hydrogel to model cancer cell/osteoblast interactions. J. Funct. Biomater. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Friedrichs, J.; Song, Y.H.; Werner, C.; Estroff, L.A.; Fischbach, C. Intrafibrillar, bone-mimetic collagen mineralization regulates breast cancer cell adhesion and migration. Biomaterials 2019, 198, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Jeon, H.L.; Oh, I.S.; Yang, H.; Park, J.; Shin, J.Y. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: A 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019, 61, 104–110. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Pulze, L.; Congiu, T.; Brevini, T.A.; Grimaldi, A.; Tettamanti, G.; D’Antona, P.; Baranzini, N.; Acquati, F.; Ferraro, F.; de Eguileor, M. MCF7 spheroid development: New insight about spatio/temporal arrangements of TNTs, amyloid fibrils, cell connections, and cellular bridges. Int. J. Mol. Sci. 2020, 21, 5400. [Google Scholar] [CrossRef]
- Natas Bussador Do Amaral, J.; Urabayashi, M.S.; Ucia, G.; Machado-Santelli, M. Cell death and lumen formation in spheroids of MCF-7 cells. Cell Biol. Int. 2010, 34, 267–274. [Google Scholar] [CrossRef]
- Herheliuk, T.; Perepelytsina, O.; Ugnivenko, A.; Ostapchenko, L.; Sydorenko, M. Investigation of multicellular tumor spheroids enriched for a cancer stem cell phenotype. Stem Cell Investig. 2019, 6, 21. [Google Scholar] [CrossRef]
- Boo, L.; Yeap, S.K.; Ali, N.M.; Ho, W.Y.; Ky, H.; Satharasinghe, D.A.; Liew, W.C.; Tan, S.W.; Wang, M.L.; Cheong, S.K.; et al. Phenotypic and microRNA characterization of the neglected CD24+ cell population in MCF-7 breast cancer 3-dimensional spheroid culture. J. Chin. Med. Assoc. 2020, 83, 67–76. [Google Scholar] [CrossRef]
- Singh, A.; Tayalia, P. Three-dimensional cryogel matrix for spheroid formation and anti-cancer drug screening. J. Biomed. Mater. Res. Part A 2020, 108, 365–376. [Google Scholar] [CrossRef]
- Yang, W.; Cai, S.; Chen, Y.; Liang, W.; Lai, Y.; Yu, H.; Wang, Y.; Liu, L. Modular and Customized Fabrication of 3D Functional Microgels for Bottom-Up Tissue Engineering and Drug Screening. Adv. Mater. Technol. 2020, 5, 1900847. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zheng, L.; Wang, L.; Zhang, X.; Xu, F. Polyacrylamide/GelMA hydrogel templates for breast cancer cell spheroids fabrication. J. Nanotechnol. Eng. Med. 2015, 6, 034501. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Nakata, J.; Akiba, Y.; Nihara, J.; Thant, L.; Eguchi, K.; Kato, H.; Izumi, K.; Ohkura, M.; Otake, M.; Kakihara, Y.; et al. ROCK inhibitors enhance bone healing by promoting osteoclastic and osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2020, 526, 547–552. [Google Scholar] [CrossRef]
- Hsieh, M.K.; Wu, C.J.; Chen, C.C.; Tsai, T.T.; Niu, C.C.; Wu, S.C.; Lai, P.L. BMP-2 gene transfection of bone marrow stromal cells to induce osteoblastic differentiation in a rat calvarial defect model. Mater. Sci. Eng. C 2018, 91, 806–816. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Modi, P.K.; Prabhu, A.; Bhandary, Y.P.; Sudheer Shenoy, P.; Hegde, A.; Sindhu Priya, E.S.; Johnson, R.P.; Das, S.P.; Vazirally, S.; Rekha, P.D. Effect of calcium glucoheptonate on proliferation and osteogenesis of osteoblast-like cells in vitro. PLoS ONE 2019, 14, e0222240. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J. Biomed. Mater. Res. Part A 2021, 109, 2425–2437. [Google Scholar] [CrossRef]
- Choi, D.; Qiu, M.; Hwang, Y.C.; Oh, W.M.; Koh, J.T.; Park, C.; Lee, B.N. The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials 2022, 15, 2170. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Shi, X.; Wang, Y. Injectable alendronate-functionalized GelMA hydrogels for mineralization and osteogenesis. RSC Adv. 2018, 8, 22764–22776. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.; Mondal, P.; Yadav, P.; Chatterjee, K. Light-based 3D bioprinting of bone tissue scaffolds with tunable mechanical properties and architecture from photocurable silk fibroin. Int. J. Biol. Macromol. 2022, 202, 644–656. [Google Scholar] [CrossRef]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Polio, S.R.; Kundu, A.N.; Dougan, C.E.; Birch, N.P.; Ezra Aurian-Blajeni, D.; Schiffman, J.D.; Crosby, A.J.; Peyton, S.R. Cross-platform mechanical characterization of lung tissue. PLoS ONE 2018, 13, e0204765. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hipwood, L.; Clegg, J.; Weekes, A.; Davern, J.W.; Dargaville, T.R.; Meinert, C.; Bock, N. Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels 2022, 8, 821. https://doi.org/10.3390/gels8120821
Hipwood L, Clegg J, Weekes A, Davern JW, Dargaville TR, Meinert C, Bock N. Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels. 2022; 8(12):821. https://doi.org/10.3390/gels8120821
Chicago/Turabian StyleHipwood, Luke, Julien Clegg, Angus Weekes, Jordan W. Davern, Tim R. Dargaville, Christoph Meinert, and Nathalie Bock. 2022. "Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture" Gels 8, no. 12: 821. https://doi.org/10.3390/gels8120821
APA StyleHipwood, L., Clegg, J., Weekes, A., Davern, J. W., Dargaville, T. R., Meinert, C., & Bock, N. (2022). Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels, 8(12), 821. https://doi.org/10.3390/gels8120821









