Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Polymer Synthesis Conditions
2.2. Characterization of AADN
2.3. Performance Evaluation of Polymer AADN in WBDFs
2.3.1. Rheological and Filtration Properties of AADN in BT-DF
2.3.2. Salt Resistance of AADN
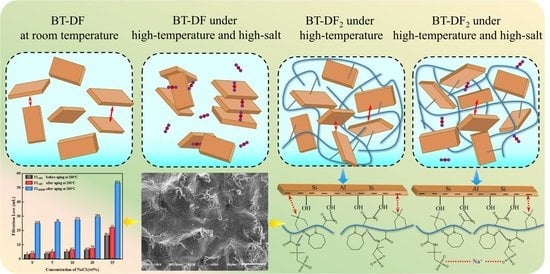
2.4. Mechanism Analysis of Filtration Loss Control
2.4.1. FTIR Analysis
2.4.2. XRD Analysis
2.4.3. Zeta Potential Analysis
2.4.4. Particle Size Distribution Analysis
2.4.5. Micro-Morphology of Filter Cake
2.4.6. Filtration Control Mechanism
2.5. Comparison with Other Filtration Loss Reducers
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Polymer AADN
4.3. AADN Characterization
4.4. Preparation and Performance Evaluation of WBDFs
4.4.1. Preparation of WBDFs
4.4.2. Performance Evaluation and Mechanism Analysis of WBDFs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beg, M.; Sharma, S.; Ojha, U. Effect of cationic copolyelectrolyte additives on drilling fluids for shales. J. Pet. Sci. Eng. 2018, 161, 506–514. [Google Scholar] [CrossRef]
- Aghdam, S.B.; Moslemizadeh, A.; Kowsari, E.; Asghari, N. Synthesis and performance evaluation of a novel polymeric fluid loss controller in water-based drilling fluids: High-temperature and high-salinity conditions. J. Nat. Gas Sci. Eng. 2020, 83, 103576. [Google Scholar] [CrossRef]
- Ricky, E.; Mpelwa, M.; Wang, C.; Hamad, B.; Xu, X. Modified Corn Starch as an Environmentally Friendly Rheology Enhancer and Fluid Loss Reducer for Water-Based Drilling Mud. SPE J. 2022, 27, 1064–1080. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, J.; He, Y.; Cui, K.; Dong, T.; Yang, L.; Yang, X.; Wang, X. Novel water-based drilling and completion fluid technology to improve wellbore quality during drilling and protect unconventional reservoirs. Engineering, 2021; in press. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Lv, K.; Ji, Y.; Ji, J.; Bai, Y.; Wang, J.; Jin, J.; Shi, S.; Huang, X. A zwitterionic copolymer as fluid loss reducer for water-based drilling fluids in high temperature and high salinity conditions. J. Pet. Sci. Eng. 2022, 111200, in press. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C. Optimal synthesis, characterization, and performance evaluation of high-pressure high-temperature polymer-based drilling fluid: The effect of viscoelasticity on cutting transport, filtration loss, and lubricity. SPE J. 2020, 25, 1333–1350. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Wang, R.; Qu, Y.; Liu, F.; Yang, J.; Cheng, R.; Gao, S.; Huang, H. Synthesis of a new high temperature and salt resistant zwitterionic filtrate reducer and its application in water-based drilling fluid. Colloids Surf. Physicochem. Eng. Asp. 2022, 651, 129730. [Google Scholar] [CrossRef]
- Cao, J.; Meng, L.; Yang, Y.; Zhu, Y.; Wang, X.; Yao, C.; Sun, M.; Zhong, H. Novel acrylamide/2-acrylamide-2-methylpropanesulfonic acid/4-vinylpyridine terpolymer as an anti-calcium contamination fluid-loss additive for water-based drilling fluids. Energy Fuels 2017, 31, 11963–11970. [Google Scholar] [CrossRef]
- Longde, S.; Caineng, Z.; Rukai, Z.; Zhang, Y.; Zhang, S.; Zhang, B.; Guangyou, Z.; Zhiyong, G. Formation, distribution and potential of deep hydrocarbon resources in China. Pet. Explor. Dev. 2013, 40, 687–695. [Google Scholar]
- Guo, X.; Hu, D.; Li, Y.; Duan, J.; Zhang, X.; Fan, X.; Duan, H.; Li, W. Theoretical progress and key technologies of onshore ultra-deep oil/gas exploration. Engineering 2019, 5, 458–470. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.; Peng, S.; He, Y.; Wang, J. Amphoteric polymer as an anti-calcium contamination fluid-loss additive in water-based drilling fluids. Energy Fuels 2016, 30, 7221–7228. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, G.; Yang, J.; Yang, L.; Li, X.; He, Y.; Chang, X. Novel N, N-dimethylacrylamide copolymer containing multiple rigid comonomers as a filtrate reducer in water-based drilling fluids and mechanism study. J. Appl. Polym. Sci. 2021, 138, 51001. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, X.; Shi, J.; Wang, Y. A high-temperature resistant colloid gas aphron drilling fluid system prepared by using a novel graft copolymer xanthan gum-AA/AM/AMPS. J. Pet. Sci. Eng. 2021, 205, 108821. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Liu, F.; Wang, J.; Qu, Y.; Wang, P.; Huang, H.; Liu, L.; Zhao, Z. Laponite-polymer composite as a rheology modifier and filtration loss reducer for water-based drilling fluids at high temperature. Colloids Surf. Physicochem. Eng. Asp. 2022, 655, 130261. [Google Scholar] [CrossRef]
- Guo, W.Y.; Peng, B. Highly effective utilization of vinyl copolymer as filtrate reducer of water-bentonite drilling fluid under ultrasonic oscillations. J. Appl. Polym. Sci. 2022, 139, 51831. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, G.; Shi, Y.; Lin, X.; Yang, X. Application of ionic liquid to a high-performance calcium-resistant additive for filtration control of bentonite/water-based drilling fluids. J. Mater. Sci. 2017, 52, 6362–6375. [Google Scholar] [CrossRef]
- Nagre, R.; Owusu, P.; Tchameni, A.P.; Kyei, S.; Azanu, D. Synthesis and assessment of a hydrophobically associating heteropolymer in water-based mud. Chem. Pap. 2021, 75, 1197–1209. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Jin, J.; Zhou, X.; Dai, Z. Environmentally friendly and salt-responsive polymer brush based on lignin nanoparticle as fluid-loss additive in water-based drilling fluids. Colloids Surf. Physicochem. Eng. Asp. 2021, 621, 126482. [Google Scholar] [CrossRef]
- Zhong, H.; Kong, X.; Chen, S.; Grady, B.P.; Qiu, Z. Preparation, characterization and filtration control properties of cross-linked starch nanospheres in water-based drilling fluids. J. Mol. Liq. 2021, 325, 115221. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Zhang, X.; Chen, A.; Qiu, Z.; Kong, X.; Huang, W. Minimizing the filtration loss of water-based drilling fluid with sustainable basil seed powder. Petroleum 2022, 8, 39–52. [Google Scholar] [CrossRef]
- Li, M.-C.; Ren, S.; Zhang, X.; Dong, L.; Lei, T.; Lee, S.; Wu, Q. Surface-chemistry-tuned cellulose nanocrystals in a bentonite suspension for water-based drilling fluids. ACS Appl. Nano Mater. 2018, 1, 7039–7051. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Huang, X.; Bai, Y.; Wang, J.; Jin, J. Synthesis of hydrophobic associative polymers to improve the rheological and filtration performance of drilling fluids under high temperature and high salinity conditions. J. Pet. Sci. Eng. 2022, 209, 109808. [Google Scholar] [CrossRef]
- Laschewsky, A.; Rosenhahn, A. Molecular design of zwitterionic polymer interfaces: Searching for the difference. Langmuir 2018, 35, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Sun, J.; Zhang, F.; Lv, K.; Zhou, X.; Wang, J.; Zhao, J. A novel zwitterionic quaternary copolymer as a fluid-loss additive for water-based drilling fluids. Energy Sources Part A 2020, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Wang, R.; Liu, F.; Gao, S.; Yang, J.; Ren, H.; Qu, Y.; Cheng, R.; Geng, Y. New Zwitterionic Polymer as a Highly Effective Salt-and Calcium-Resistant Fluid Loss Reducer in Water-Based Drilling Fluids. Gels 2022, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, Y.; Xiao, D.; Pu, X.; Wang, X. Synthesis, characterization, and performance evaluation of the AM/AMPS/DMDAAC/SSS quadripolymer as a fluid loss additive for water-based drilling fluid. J. Appl. Polym. Sci. 2015, 132, 41762. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure-property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Lv, K.; Ji, Y.; Liu, J.; Huang, X.; Bai, Y.; Wang, J.; Jin, J.; Shi, S. Temperature-and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids. Gels 2022, 8, 289. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, M.; Xu, Y.; Xia, X.; Zhang, C.; Yu, Y.; Feng, Y.; Guo, J. Inhibitory effects of functionalized polycarboxylate retarder on aberrant thickening phenomena of oil well cement at high temperature. Constr. Build. Mater. 2021, 274, 121994. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Pei, J.; Yu, P.; Xia, B. A novel star-shaped copolymer as a rheology modifier in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 168, 98–106. [Google Scholar] [CrossRef]
- Lin, L.; Luo, P. Amphoteric hydrolyzed poly (acrylamide/dimethyl diallyl ammonium chloride) as a filtration reducer under high temperatures and high salinities. J. Appl. Polym. Sci. 2015, 132, 41581. [Google Scholar] [CrossRef]
- Thaemlitz, C.J. Synthetic Filtration Control Polymers for Wellbore Fluids. U.S. Patent 7,098,171, 29 August 2006. [Google Scholar]
- Jia, X.; Zhan, X.; Xie, J.; Gao, B.; Zhang, Y. Thermal stability of poly (diallyldimethylammonium chloride) with different molecular weight. J. Macromol. Sci. Part A 2020, 57, 83–90. [Google Scholar] [CrossRef]
- Mao, H.; Wang, W.; Ma, Y.; Huang, Y. Synthesis, characterization and properties of an anionic polymer for water-based drilling fluid as an anti-high temperature and anti-salt contamination fluid loss control additive. Polym. Bull. 2021, 78, 2483–2503. [Google Scholar] [CrossRef]
- Loh, X.J.; Scherman, O.A. Polymeric and Self Assembled Hydrogels: From Fundamental Understanding to Applications; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Enhanced mechanical stability and sensitive swelling performance of chitosan/yeast hybrid hydrogel beads. New J. Chem. 2016, 40, 3350–3362. [Google Scholar] [CrossRef]
- Kulkarni, A.; Bambole, V.; Mahanwar, P. Electrospinning of polymers, their modeling and applications. Polym.-Plast. Technol. Eng. 2010, 49, 427–441. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pooley, S.A.; Luna, M.; Geckeler, K.E. Synthesis of water-soluble polymers containing sulfonic acid and amine moieties for the recovery of metal ions using ultrafiltration. J. Appl. Polym. Sci. 2001, 82, 22–30. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, D.; Zhang, B.; Zhang, C.G. Properties of high-temperature drilling fluids incorporating disodium itaconate/acrylamide/sodium 2-acrylamido-2-methylpropanesulfonate terpolymers as fluid-loss reducers. J. Appl. Polym. Sci. 2002, 83, 3068–3075. [Google Scholar] [CrossRef]
- Liu, L.; Pu, X.; Rong, K.; Yang, Y. Comb-shaped copolymer as filtrate loss reducer for water-based drilling fluid. J. Appl. Polym. Sci. 2018, 135, 45989. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, X. Effective Modified Xanthan Gum Fluid Loss Agent for High-Temperature Water-Based Drilling Fluid and the Filtration Control Mechanism. ACS Omega 2021, 6, 23788–23801. [Google Scholar] [CrossRef]
- Ao, T.; Yang, L.; Xie, C.; Jiang, G.; Wang, G.; Liu, Z.; He, X. Zwitterionic Silica-Based Hybrid Nanoparticles for Filtration Control in Oil Drilling Conditions. ACS Appl. Nano Mater. 2021, 4, 11052–11062. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Zhou, X.; Zhao, J. Salt-responsive zwitterionic copolymer as tackifier in brine drilling fluids. J. Mol. Liq. 2020, 319, 114345. [Google Scholar] [CrossRef]
- Li, Z.; Pu, X.; Tao, H.; Liu, L.; Su, J. Synthesis and properties of acrylamide 2-acrylamido-2-methypropane sulfonic acid sodium styrene sulfonate N-vinyl pyrrolidone quadripolymer and its reduction of drilling fluid filtration at high temperature and high salinity. J. Polym. Eng. 2014, 34, 125–131. [Google Scholar] [CrossRef]
- Tombacz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Institute, A.P. Recommended Practice for Laboratory Testing of Drilling Fluids; American Petroleum Institute: Washington, DC, USA, 2009. [Google Scholar]













| Number | Mole Ratio AM: AMPS: DMDAAC: NVCL | Temperature °C | Initiator % | Monomer Concentration % | FLAPI mL |
|---|---|---|---|---|---|
| 1 | 8:2:1:2 | 45 | 0.3 | 20 | 4.9 |
| 2 | 55 | 0.4 | 25 | 7.5 | |
| 3 | 65 | 0.5 | 30 | 6.2 | |
| 4 | 6:4:1:2 | 45 | 0.4 | 30 | 4.5 |
| 5 | 55 | 0.5 | 20 | 5.6 | |
| 6 | 65 | 0.3 | 25 | 4.0 | |
| 7 | 4:6:1:2 | 45 | 0.5 | 25 | 6.3 |
| 8 | 55 | 0.3 | 30 | 7.0 | |
| 9 | 65 | 0.4 | 20 | 5.4 | |
| K1 | 6.200 | 5.233 | 5.233 | 5.300 | |
| K2 | 4.633 | 6.700 | 5.800 | 5.867 | |
| K3 | 6.233 | 5.133 | 6.033 | 5.900 | |
| R | 1.600 | 1.567 | 0.800 | 0.600 |
| Mole Ratio AM: AMPS: DMDAAC: NVCL | Temperature °C | Initiator % | Monomer Concentration % | FLAPI mL |
|---|---|---|---|---|
| 4: 6: 1: 2 | 55 | 0.5 | 30 | 3.8 |
| Formulation | Test Conditions | AV mPa·s | PV mPa·s | YP Pa | YP/PV Pa/(mPa·s) |
|---|---|---|---|---|---|
| BT-DF | before aging at 200 °C | 11.5 | 7 | 4.5 | 0.64 |
| after aging at 200 °C | 8.5 | 5 | 3.5 | 0.70 | |
| BT-DF + 0.5 wt% AADN | before aging at 200 °C | 30.5 | 17 | 13.5 | 0.79 |
| after aging at 200 °C | 16.5 | 12 | 4.5 | 0.38 | |
| BT-DF + 1 wt% AADN | before aging at 200 °C | 56.5 | 31 | 25.5 | 0.82 |
| after aging at 200 °C | 35 | 24 | 11 | 0.46 | |
| BT-DF + 1.5 wt% AADN | before aging at 200 °C | 69 | 37 | 32 | 0.86 |
| after aging at 200 °C | 55 | 32 | 23 | 0.72 | |
| BT-DF + 2 wt% AADN | before aging at 200 °C | 83.5 | 45 | 38.5 | 0.86 |
| after aging at 200 °C | 76.5 | 50 | 26.5 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, J.; Liu, L.; Wang, J.; Feng, Z.; Zhang, D.; Gao, S.; Wang, J.; Ren, H.; Hui, B. Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids. Gels 2022, 8, 826. https://doi.org/10.3390/gels8120826
Wang R, Yang J, Liu L, Wang J, Feng Z, Zhang D, Gao S, Wang J, Ren H, Hui B. Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids. Gels. 2022; 8(12):826. https://doi.org/10.3390/gels8120826
Chicago/Turabian StyleWang, Ren, Jie Yang, Luman Liu, Jianlong Wang, Zhenbo Feng, Die Zhang, Shan Gao, Jiao Wang, Han Ren, and Baotong Hui. 2022. "Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids" Gels 8, no. 12: 826. https://doi.org/10.3390/gels8120826
APA StyleWang, R., Yang, J., Liu, L., Wang, J., Feng, Z., Zhang, D., Gao, S., Wang, J., Ren, H., & Hui, B. (2022). Investigation on Filtration Control of Zwitterionic Polymer AADN in High Temperature High Pressure Water-Based Drilling Fluids. Gels, 8(12), 826. https://doi.org/10.3390/gels8120826