Current Advances in 3D Dynamic Cell Culture Systems
Abstract
1. Introduction
2. Effects of 3D and Dynamic Culture Environment on Cell Behaviors
2.1. Cell Behaviors in 3D Culture Environment
2.1.1. Cell Proliferation and Differentiation in 3D Culture
2.1.2. Cell Apoptosis in Cancer Drug Test in 3D Culture
2.1.3. Cell Motion and Migration in 3D Culture
2.2. The Effects of Mechanical Force on Cell Behavior/Function
2.2.1. Stretching
2.2.2. Compression
2.2.3. Contraction/Relaxation
2.2.4. Shear Stress
3. Current 3D and Dynamic Cell Culture Approaches
3.1. Static 3D Cell Culture Approaches
3.1.1. Scaffold-Free 3D Static Cell Culture
- Low adhesion surface modification method.
- Hanging drop method.
3.1.2. Scaffold-Dependent 3D Static Cell Culture
- Natural ECM-derived scaffolds.
- Hydrogels scaffolds.
- Synthetic polymer scaffolds.
- Metal and ceramic scaffolds
3.2. Current 3D Dynamic Cell Culture Systems and Applications
3.2.1. Bioreactor and Microcarrier-Based Culture System
3.2.2. Microfluidic Cell Culture System (Organ-on-a-Chip)
4. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, R.G. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1906, 4, 140–143. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M. History of cell culture. In New Insights into Cell Culture Technology; IntechOpen: London, UK, 2017. [Google Scholar]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, K.; Tao, B.; Dai, L.; Yu, Y.; Mu, C.; Shen, X.; Hu, Y.; He, Y.; Cai, K. Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo. Acta Biomater. 2018, 74, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, S.; Ma, H.; Liang, X.; Ma, H.; Yan, X.; Yang, B.; Wei, J.; Liu, X. Paracrine factors from adipose-mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co-culture model. Cancer Cell Int. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Sun, K.; Lin, S.; Lin, Y.; Zhu, B.; Cai, X. MMP-2 and Notch signal pathway regulate migration of adipose-derived stem cells and chondrocytes in co-culture systems. Cell Prolif. 2017, 50, e12385. [Google Scholar] [CrossRef]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef]
- Hatherell, K.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef]
- Müller, E.K.; Gräfe, C.; Wiekhorst, F.; Bergemann, C.; Weidner, A.; Dutz, S.; Clement, J.H. Magnetic Nanoparticles Interact and Pass an In Vitro Co-Culture Blood-Placenta Barrier Model. Nanomaterials 2018, 8, 108. [Google Scholar] [CrossRef]
- Hermanns, M.I.; Unger, R.E.; Kehe, K.; Peters, K.; Kirkpatrick, C.J. Lung epithelial cell lines in coculture with human pulmonary microvascular endothe-lial cells: Development of an alveolo-capillary barrier in vitro. Lab. Investig. 2004, 84, 736–752. [Google Scholar] [CrossRef]
- Rose, S.L.; Babensee, J.E. Complimentary Endothelial Cell/Smooth Muscle Cell Co-Culture Systems with Alternate Smooth Muscle Cell Phenotypes. Ann. Biomed. Eng. 2007, 35, 1382–1390. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Fato, M.; Peñuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschlager, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar]
- Clementi, A.; Egger, D.; Charwat, V.; Kasper, C. Cell Culture Conditions: Cultivation of Stem Cells under Dynamic Conditions. In Cell Engineering and Regeneration; Gimble, J.M., Presen, D.M., Oreffo, R.O.C., Wolbank, S., Redl, H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–33. [Google Scholar]
- Kato, Y.; Kim, M.-H.; Kino-Oka, M. Comparison of growth kinetics between static and dynamic cultures of human induced pluripotent stem cells. J. Biosci. Bioeng. 2018, 125, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Grattarola, M.; Nicolini, C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. The Int. J. Biochem. Cell Biol. 2004, 36, 1447–1461. [Google Scholar] [CrossRef]
- Thomas, C.H.; Collier, J.H.; Sfeir, C.S.; Healy, K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 2002, 99, 1972–1977. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Yamada, K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 2002, 14, 633–640. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-dimensional (2D) and three-dimensional (3D) cell culturing in drug discovery. Cell Cult. 2018, 2, 1–22. [Google Scholar]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef]
- Farran, A.J.E.; Teller, S.S.; Jia, F.; Clifton, R.J.; Duncan, R.L.; Jia, X.; Rodney, J.C. Design and characterization of a dynamic vibrational culture system. J. Tissue Eng. Regen. Med. 2011, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Meng, X.; Shi, C.; Li, C.; Chen, Y.; Sun, H. Compressive force regulates ephrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement. Korean J. Orthod. 2014, 44, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, G.; Xu, F. Engineering Biomaterials and Approaches for Mechanical Stretching of Cells in Three Dimensions. Front. Bioeng. Biotechnol. 2020, 8, 589590. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef]
- Kagemoto, T.; Li, A.; Dos Remedios, C.; Ishiwata, S. Spontaneous oscillatory contraction (SPOC) in cardiomyocytes. Biophys. Rev. 2015, 7, 15–24. [Google Scholar] [CrossRef]
- Leverett, L.B.; Hellums, J.D.; Alfrey, C.P.; Lynch, E.C. Red Blood Cell Damage by Shear Stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef]
- Minsky, B.D.; Chlapowski, F.J. Morphometric analysis of the translocation of lumenal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J. Cell Biol. 1978, 77, 685–697. [Google Scholar] [CrossRef]
- Eke, I.; Zscheppang, K.; Dickreuter, E.; Hickmann, L.; Mazzeo, E.; Unger, K.; Krause, M.; Cordes, N. Simultaneous β1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J. Natl. Cancer Inst. 2015, 107, dju419. [Google Scholar] [CrossRef]
- Morello, V.; Cabodi, S.; Sigismund, S.; Camacho-Leal, M.P.; Repetto, D.; Volante, M.; Papotti, M.; Turco, E.; Defilippi, P. β1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene 2011, 30, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Onodera, Y.; Bissell, M.J.; Park, C.C. Breast Cancer Cells in Three-dimensional Culture Display an Enhanced Radioresponse after Coordinate Targeting of Integrin α5β1 and Fibronectinα5β1-Integrin and Fibronectin Targeting in Breast Cancer. Cancer Res. 2010, 70, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, M.; Cheng, H.C.; Elble, R.C.; Pauli, B.U. The breast cancer β4 integrin and endothelial human CLCA2 mediate lung metastasis. J. Biol. Chem. 2001, 276, 25438–25446. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.I.; Soung, Y.H.; Shaw, L.A.; Chung, J. Integrin (α6β4) Signals Through Src to Increase Expression of S100A4, a Metastasis-Promoting Factor: Implications for Cancer Cell InvasionIntegrin Regulation of S100A4 Expression. Mol. Cancer Res. 2009, 7, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, J.; Toba, Y.; Yamazaki, H.; Kanai, M.; Mizuguchi, H.; Matsui, H. Development of a 3D Cell Culture System Using Amphiphilic Polydepsipeptides and Its Application to Hepatic Differentiation. ACS Appl. Bio Mater. 2021, 4, 7290–7299. [Google Scholar] [CrossRef]
- De Smedt, A.; Steemans, M.; De Boeck, M.; Peters, A.K.; Van Der Leede, B.-J.; Van Goethem, F.; Lampo, A.; Vanparys, P. Optimisation of the cell cultivation methods in the embryonic stem cell test results in an increased differentiation potential of the cells into strong beating myocard cells. Toxicol. Vitr. 2008, 22, 1789–1796. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Jongpaiboonkit, L.; King, W.J.; Lyons, G.E.; Paguirigan, A.L.; Warrick, J.W.; Beebe, D.J.; Murphy, W.L. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials 2008, 29, 3346–3356. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Giusti, I.; Poppa, G.; D’Ascenzo, S.; Esposito, L.; Vitale, A.R.; Calvisi, G.; Dolo, V. Cancer Three-Dimensional Spheroids Mimic In Vivo Tumor Features, Displaying “Inner” Extracellular Vesicles and Vasculogenic Mimicry. Int. J. Mol. Sci. 2022, 23, 11782. [Google Scholar] [CrossRef]
- Xiong, G.F.; Xu, R. Function of cancer cell-derived extracellular matrix in tumor progression. J. Cancer Metastasis Treat. 2016, 2, 357–364. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C.; et al. The Role of Tumor Microenvironment in Chemoresistance: 3D Extracellular Matrices as Accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef] [PubMed]
- Szczepny, A.; Hogarth, C.A.; Young, J.; Loveland, K.L. Identification of Hedgehog Signaling Outcomes in Mouse Testis Development Using a Hanging Drop-Culture System1. Biol. Reprod. 2009, 80, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.K.; Yarborough, O., III; Lifton, R.P.; Cantley, L.G.; Caplan, M.J. Epithelial morphogenesis of MDCK cells in three-dimensional collagen culture is modulated by interleukin-8. Am. J. Physiol. Cell Physiol. 2013, 304, C966–C975. [Google Scholar] [CrossRef][Green Version]
- Burr, D.; Milgrom, C.; Fyhrie, D.; Forwood, M.; Nyska, M.; Finestone, A.; Hoshaw, S.; Saiag, E.; Simkin, A. In vivo measurement of human tibial strains during vigorous activity. Bone 1996, 18, 405–410. [Google Scholar] [CrossRef]
- Konstantonis, D.; Papadopoulou, A.; Makou, M.; Eliades, T.; Basdra, E.; Kletsas, D. The role of cellular senescence on the cyclic stretching-mediated activation of MAPK and ALP expression and activity in human periodontal ligament fibroblasts. Exp. Gerontol. 2014, 57, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lessa, T.B.; de Abreu, D.K.; Rodrigues, M.N.; Brólio, M.P.; Miglino, M.A.; Ambrósio, C.E. Morphological and ultrastructural evaluation of the golden retriever muscular dystrophy trachea, lungs, and diaphragm muscle. Microsc. Res. Tech. 2014, 77, 857–861. [Google Scholar] [CrossRef]
- Rabello, F.B.; Souza, C.D.; Júnior, J.A.F. Update on hypertrophic scar treatment. Clinics 2014, 69, 565–573. [Google Scholar] [CrossRef]
- Dolbow, J.; Throckmorton, Z. Neuroanatomy, Spinal Cord Myotatic Reflex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Le Li, M.; Yi, J.; Yang, Y.; Zhang, X.; Zheng, W.; Li, Y.; Zhao, Z. Compression and hypoxia play independent roles while having combinative effects in the osteoclastogenesis induced by periodontal ligament cells. Angle Orthod. 2016, 86, 66–73. [Google Scholar] [CrossRef]
- Wu, B.-H.; Kou, X.-X.; Zhang, C.; Zhang, Y.-M.; Cui, Z.; Wang, X.-D.; Liu, Y.; Liu, D.-W.; Zhou, Y.-H. Stretch force guides finger-like pattern of bone formation in suture. PLoS ONE 2017, 12, e0177159. [Google Scholar] [CrossRef]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Human periodontal ligament fibroblasts stimulate osteoclasto-genesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J. Cell. Biochem. 2011, 112, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Gao, F.; Wang, K.; Dong, F. ClC-3 Promotes Osteogenic Differentiation in MC3T3-E1 Cell After Dynamic Compression. J. Cell. Biochem. 2017, 118, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Puig, F.; Rico, F.; Almendros, I.; Montserrat, J.M.; Navajas, D.; Farre, R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep 2005, 28, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Reinhart-King, C.A.; Fujiwara, K.; Berk, B.C. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol. 2008, 443, 25–44. [Google Scholar] [PubMed]
- Zhou, J.; Li, Y.-S.; Chien, S. Shear Stress–Initiated Signaling and Its Regulation of Endothelial Function. Arter. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Usami, S.; Chien, S. Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Ann. Med. 2009, 41, 19–28. [Google Scholar] [CrossRef]
- Chintavalakorn, R.; Khantachawana, A.; Viravaidya-Pasuwat, K.; Santiwong, P.; Surarit, R. In vitro effects of mechanical stimulation and photobiomodulation on osteoblastic cell function: A proof of concept study. Pediatr. Dent. J. 2017, 27, 29–41. [Google Scholar] [CrossRef]
- Banerjee, M.; Bhonde, R.R. Application of hanging drop technique for stem cell differentiation and cytotoxicity studies. Cytotechnology 2006, 51, 1–5. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2010, 136, 473–478. [Google Scholar] [CrossRef]
- Wu, L.Y.; Di Carlo, D.; Lee, L.P. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed. Microdevices 2008, 10, 197–202. [Google Scholar] [CrossRef]
- Lee, S.W.; Hong, S.; Jung, B.; Jeong, S.Y.; Byeon, J.H.; Jeong, G.S.; Choi, J.; Hwang, C. In vitro lung cancer multicellular tumor spheroid formation using a microfluidic device. Biotechnol. Bioeng. 2019, 116, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor spheroid-on-a-chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, D.M.; Roberge, C.L.; Rudkouskaya, A.; Faulkner, D.E.; Barroso, M.; Intes, X.; Corr, D.T. Laser-based 3D bioprinting for spatial and size control of tumor spheroids and embryoid bodies. Acta Biomater. 2019, 95, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. SLAS Discov. 2006, 11, 922–932. [Google Scholar] [CrossRef]
- Hampel, U.; Garreis, F.; Burgemeister, F.; Eßel, N.; Paulsen, F. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul. Surf. 2018, 16, 341–351. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Karzbrun, E.; Khankhel, A.H.; Megale, H.C.; Glasauer, S.M.K.; Wyle, Y.; Britton, G.; Warmflash, A.; Kosik, K.S.; Siggia, E.D.; Shraiman, B.I.; et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 2021, 599, 268–272. [Google Scholar] [CrossRef]
- Pineda, E.T.; Nerem, R.M.; Ahsan, T. Differentiation Patterns of Embryonic Stem Cells in Two- versus Three-Dimensional Culture. Cells Tissues Organs 2013, 197, 399–410. [Google Scholar] [CrossRef]
- Keller, G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995, 7, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.Y.; Tung, Y.-C.; Kuo, C.-H.; Mosadegh, B.; Bedenis, R.; Pienta, K.J.; Takayama, S. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed. Microdevices 2011, 14, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Timmins, N.; Dietmair, S.; Nielsen, L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 2004, 7, 97–103. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Dolder, J.V.D.; Spauwen, P.H.; Jansen, J.A. Evaluation of Various Seeding Techniques for Culturing Osteogenic Cells on Titanium Fiber Mesh. Tissue Eng. 2003, 9, 315–325. [Google Scholar] [CrossRef]
- Smith, D.J., Jr. Use of Biobrane in wound management. J. Burn. Care Rehabil. 1995, 16, 317–320. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef]
- Meng, F.W.; Slivka, P.F.; Dearth, C.L.; Badylak, S.F. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials 2015, 46, 131–140. [Google Scholar] [CrossRef]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Maity, P.P.; Manchikanti, P.; Chaudhury, K.; Dhara, S. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl. Mater. Interfaces 2018, 10, 16977–16991. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2019, 42, 107421. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Gerecht, S.; Burdick, J.A.; Ferreira, L.S.; Townsend, S.A.; Langer, R.; Vunjak-Novakovic, G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11298–11303. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; A Burdick, J. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mauck, R.L.; Gerecht, S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 2016, 18, 13–15. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2019, 573, 118803. [Google Scholar] [CrossRef]
- Song, H.; Cai, G.-H.; Liang, J.; Ao, D.-S.; Wang, H.; Yang, Z.-H. Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials. J. Nanobiotechnol. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.S.; Zhao, D.H.; Jin, H.F.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 226. [Google Scholar] [CrossRef]
- Caetano, G.F.; Bártolo, P.J.; Domingos, M.; Oliveira, C.C.; Leite, M.N.; Frade, M.A.C. Osteogenic differentiation of adipose-derived mesenchymal stem cells into Polycaprolac-tone (PCL) scaffold. Procedia Eng. 2015, 110, 59–66. [Google Scholar] [CrossRef]
- Rodrigues, N.; Benning, M.; Ferreira, A.M.; Dixon, L.; Dalgarno, K. Manufacture and Characterisation of Porous PLA Scaffolds. Procedia CIRP 2016, 49, 33–38. [Google Scholar] [CrossRef]
- Howard, D.; Partridge, K.; Yang, X.; Clarke, N.M.; Okubo, Y.; Bessho, K.; Howdle, S.M.; Shakesheff, K.; Oreffo, R.O. Immunoselection and adenoviral genetic modulation of human osteoprogenitors: In vivo bone formation on PLA scaffold. Biochem. Biophys. Res. Commun. 2002, 299, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Milovac, D.; Ferrer, G.G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C 2014, 34, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Thimm, B.W.; Wechsler, O.; Bohner, M.; Müller, R.; Hofmann, S. In vitro ceramic scaffold mineralization: Comparison between histological and micro-computed tomographical analysis. Ann. Biomed. Eng. 2013, 41, 2666–2675. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Chudinova, E.A.; Koptioug, A.; Tkachev, M.S.; Gorodzha, S.N.; Rännar, L.-E. Fabrication of multiple-layered gradient cellular metal scaffold via electron beam melting for segmental bone reconstruction. Mater. Des. 2017, 133, 195–204. [Google Scholar] [CrossRef]
- Kirsch, A.; Hortobagyi, D.; Stachl, T.; Karbiener, M.; Grossmann, T.; Gerstenberger, C.; Gugatschka, M. Development and validation of a novel phonomimetic bioreactor. PLoS ONE 2019, 14, e0213788. [Google Scholar] [CrossRef]
- Badenes, S.M.; Fernandes, T.G.; Rodrigues, C.A.; Diogo, M.M.; Cabral, J.M. Microcarrier-based platforms for in vitro expansion and differentiation of human pluripotent stem cells in bioreactor culture systems. J. Biotechnol. 2016, 234, 71–82. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Choquette, D.T.; Fisher, J.P. Bioreactors to influence stem cell fate: Augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim. Biophys. Acta 2013, 1830, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.; Childs, P.; Pemberton, G.D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; González-García, C.; Blackburn, G.; Thomas, D.; et al. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 2017, 1, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Shannag, M.; Al-Busoul, M.; Penchev, I.; Orfali, W. Immobilized enzymes bioreactors utilizing a magnetic field: A review. Biochem. Eng. J. 2017, 121, 94–106. [Google Scholar] [CrossRef]
- Diban, N.; Sánchez-González, S.; Lázaro-Díez, M.; Ramos-Vivas, J.; Urtiaga, A. Facile fabrication of poly (ε-caprolactone)/graphene oxide membranes for bioreactors in tis-sue engineering. J. Membr. Sci. 2017, 540, 219–228. [Google Scholar] [CrossRef]
- Van Wezel, A.L. Growth of Cell-strains and Primary Cells on Micro-carriers in Homogeneous Culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.; Oh, S.K.W.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, present, and future of microcarrier-based tissue engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef]
- Chen, A.K.-L.; Reuveny, S.; Oh, S.K.W. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol. Adv. 2013, 31, 1032–1046. [Google Scholar] [CrossRef]
- Kiesslich, S.; Kamen, A.A. Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnol. Adv. 2020, 44, 107608. [Google Scholar] [CrossRef]
- Couto, P.S.; Rotondi, M.; Bersenev, A.; Hewitt, C.; Nienow, A.; Verter, F.; Rafiq, Q. Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: Lessons learnt and what the future holds. Biotechnol. Adv. 2020, 45, 107636. [Google Scholar] [CrossRef]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.H.; Nishino, T.; et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014, 2, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Stoecklein, D.; Kommajosula, A.; Lin, J.; Owsley, K.; Ganapathysubramanian, B.; Di Carlo, D. Shaped 3D microcarriers for adherent cell culture and analysis. Microsyst. Nanoeng. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, J.; Gorr, I.H.; Schwald, C.; Beaucamp, N.; Wiechmann, K.; Kuentzer, K.; Huss, R.; Rieger, B.; Neubauer, M.; Wegmeyer, H. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol. Bioeng. 2014, 111, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Lim, J.F.Y.; Lee, J.; Choolani, M.; Chan, J.K.Y.; Reuveny, S.; Oh, S.K.W. Expansion in microcarrier-spinner cultures improves the chondrogenic potential of human early mesenchymal stromal cells. Cytotherapy 2016, 18, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.K.-P.; Zhang, Z.-Y.; Chen, A.K.-L.; Reuveny, S.; Choolani, M.; Chan, J.K.Y.; Oh, S.K.-W. Microcarrier Culture for Efficient Expansion and Osteogenic Differentiation of Human Fetal Mesenchymal Stem Cells. BioResearch Open Access 2013, 2, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; Sim, E.; Tan, K.Y.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Oh, S. Enhanced in vitro osteogenic differentiation of human fetal MSCs attached to 3D micro-carriers versus harvested from 2D monolayers. BMC Biotechnol. 2015, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Lewis, S.; Tsai, A.-C.; Sanders, K.; Liu, C.; Yuan, X.; Li, Y. Agitation in a microcarrier-based spinner flask bioreactor modulates homeostasis of human mesenchymal stem cells. Biochem. Eng. J. 2021, 168, 107947. [Google Scholar] [CrossRef]
- Chen, S.; Sato, Y.; Tada, Y.; Suzuki, Y.; Takahashi, R.; Okanojo, M.; Nakashima, K. Facile bead-to-bead cell-transfer method for serial subculture and large-scale expansion of human mesenchymal stem cells in bioreactors. Stem Cells Transl. Med. 2021, 10, 1329–1342. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, W.; Fang, J.; Yin, J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and car-tilage tissue engineering. Int. Mater. Rev. 2021, 66, 77–113. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Rodrigues, C.A.; Gomes, A.R.; Vieira, S.F.; Badenes, S.M.; Diogo, M.M.; Cabral, J.M. Dissolvable Microcarriers Allow Scalable Expansion and Harvesting of Human In-duced Pluripotent Stem Cells Under Xeno-Free Conditions. Biotechnol. J. 2019, 14, 1800461. [Google Scholar] [CrossRef]
- Tamura, A.; Kobayashi, J.; Yamato, M.; Okano, T. Temperature-responsive poly(N-isopropylacrylamide)-grafted microcarriers for large-scale non-invasive harvest of anchorage-dependent cells. Biomaterials 2012, 33, 3803–3812. [Google Scholar] [CrossRef]
- Steinhilber, D.; Rossow, T.; Wedepohl, S.; Paulus, F.; Seiffert, S.; Haag, R. A Microgel Construction Kit for Bioorthogonal Encapsulation and pH-Controlled Release of Living Cells. Angew. Chem. Int. Ed. 2013, 52, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Dosta, P.; Ferber, S.; Zhang, Y.; Wang, K.; Ros, A.; Uth, N.; Levinson, Y.; Abraham, E.; Artzi, N. Scale-up manufacturing of gelatin-based microcarriers for cell therapy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Lauth, V.; Maas, M.; Rezwan, K. Coacervate-directed synthesis of CaCO3 microcarriers for pH-responsive delivery of biomolecules. J. Mater. Chem. B 2014, 2, 7725–7731. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiao, L.; Wang, J.; Mu, Y.; Mendhi, J.; Gao, W.; Li, Z.Y.; Yarlagadda, P.; Wu, C.; Xiao, Y. The Hollow Porous Sphere Cell Carrier for the Dynamic Three-Dimensional Cell Culture. Tissue Eng. Part C Methods 2022, 28, 610–622. [Google Scholar] [CrossRef]
- Gao, W. Novel 3D Printed Hollow Porous Sphere (HPS) for Cell Dynamic Culture to Investigate the Effect of Hydrodynamic Force on Cell Behaviours. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2022. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K. Microcarrier Cell Culture. Biotechnol. Genet. Eng. Rev. 1988, 6, 404–439. [Google Scholar] [CrossRef]
- Hua, J.; Erickson, L.E.; Yiin, T.-Y.; Glasgow, L.A. A Review of the Effects of Shear and Interfacial Phenomena on Cell Viability. Crit. Rev. Biotechnol. 1993, 13, 305–328. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2020, 20, 345–361. [Google Scholar] [CrossRef]
- Shrestha, J.; Bazaz, S.R.; Es, H.A.; Azari, D.Y.; Thierry, B.; Warkiani, M.E.; Ghadiri, M. Lung-on-a-chip: The future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Jalili-Firoozinezhad, S.; Solati-Hashjin, M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 2020, 116, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, V.; Sabater-Lleal, M.; Middelkamp, H.; Vivas, A.; Johansson, S.; van der Meer, A.; Tenje, M.; Maegdefessel, L. Organ-on-a-chip technology: A novel approach to investigate cardiovascular diseases. Cardiovasc. Res. 2021, 117, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; Macdonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.; et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 2018, 3, e123673. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain J. Neurol. 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Holloway, P.M.; Willaime-Morawek, S.; Siow, R.; Barber, M.; Owens, R.M.; Sharma, A.D.; Rowan, W.; Hill, E.; Zagnoni, M. Advances in microfluidic in vitro systems for neurological disease modeling. J. Neurosci. Res. 2021, 99, 1276–1307. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Nasiri, R.; de Barros, N.R.; Tebon, P.; Thakor, J.; Goudie, M.; Shamloo, A.; Martin, M.G.; Khademhosseini, A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 2020, 255, 120196. [Google Scholar] [CrossRef]
- Sokolowska, P.; Janikiewicz, J.; Jastrzebska, E.; Brzozka, Z.; Dobrzyn, A. Combinations of regenerative medicine and Lab-on-a-chip systems: New hope to re-storing the proper function of pancreatic islets in diabetes. Biosens. Bioelectron. 2020, 167, 112451. [Google Scholar] [CrossRef]
- McCarthy, M.; Brown, T.; Alarcon, M.A.; Williams, C.; Wu, X.; Abbott, R.D.; Gimble, J.M.; Frazier, T. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng. Part B Rev. 2020, 26, 586–595. [Google Scholar] [CrossRef]
- Arrigoni, C.; Lopa, S.; Candrian, C.; Moretti, M. Organs-on-a-chip as model systems for multifactorial musculoskeletal diseases. Curr. Opin. Biotechnol. 2020, 63, 79–88. [Google Scholar] [CrossRef]
- Mansoorifar, A.; Gordon, R.; Bergan, R.C.; Bertassoni, L.E. Bone-on-a-Chip: Microfluidic Technologies and Microphysiologic Models of Bone Tissue. Adv. Funct. Mater. 2021, 31, 2006796. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Biomimetic 3D-Bone Tissue Model. In Next Generation Culture Platforms for Reliable In Vitro Models; Springer: Berlin/Heidelberg, Germany, 2021; pp. 239–250. [Google Scholar]
- Vurat, M.T.; Şeker, Ş.; Lalegül-Ülker, Ö.; Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Development of a multicellular 3D-bioprinted microtissue model of human periodontal ligament-alveolar bone biointerface: Towards a pre-clinical model of periodontal diseases and personalized periodontal tissue engineering. Genes Dis. 2020, 9, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Young, R.E.; Huh, D.D. Organ-on-a-chip technology for the study of the female reproductive system. Adv. Drug Deliv. Rev. 2021, 173, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Del Piccolo, N.; Shirure, V.S.; Bi, Y.; Goedegebuure, S.P.; Gholami, S.; Hughes, C.C.; Fields, R.C.; George, S.C. Tumor-on-chip modeling of organ-specific cancer and metastasis. Adv. Drug Deliv. Rev. 2021, 175, 113798. [Google Scholar] [CrossRef] [PubMed]
- Picollet-D’Hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach to Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Rennert, K.; Steinborn, S.; Gröger, M.; Ungerböck, B.; Jank, A.-M.; Ehgartner, J.; Nietzsche, S.; Dinger, J.; Kiehntopf, M.; Funke, H.; et al. A microfluidically perfused three dimensional human liver model. Biomaterials 2015, 71, 119–131. [Google Scholar] [CrossRef]
- Kostrzewski, T.; Maraver, P.; Ouro-Gnao, L.; Levi, A.; Snow, S.; Miedzik, A.; Rombouts, K.; Hughes, D. A Microphysiological System for Studying Nonalcoholic Steatohepatitis. Hepatol. Commun. 2019, 4, 77–91. [Google Scholar] [CrossRef]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L.; et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Phan, D.T.T.; Wang, X.; Craver, B.M.; Sobrino, A.; Zhao, D.; Chen, J.C.; Lee, L.Y.N.; George, S.C.; Lee, A.P.; Hughes, C.C. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening ap-plications. Lab Chip 2017, 17, 511–520. [Google Scholar] [CrossRef]
- Hassan, S.; Sebastian, S.; Maharjan, S.; Lesha, A.; Carpenter, A.M.; Liu, X.; Xie, X.; Livermore, G.; Zhang, Y.S.; Zarrinpar, A. Liver-on-a-Chip Models of Fatty Liver Disease. Hepatology 2020, 71, 733–740. [Google Scholar] [CrossRef]
- Delon, L.C.; Guo, Z.; Oszmiana, A.; Chien, C.-C.; Gibson, R.; Prestidge, C.; Thierry, B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials 2019, 225, 119521. [Google Scholar] [CrossRef] [PubMed]
- Raasch, M.; Rennert, K.; Jahn, T.; Peters, S.; Henkel, T.; Huber, O.; Schulz, I.; Becker, H.; Lorkowski, S.; Funke, H.; et al. Microfluidically supported biochip design for culture of endothelial cell layers with im-proved perfusion conditions. Biofabrication 2015, 7, 015013. [Google Scholar] [CrossRef] [PubMed]
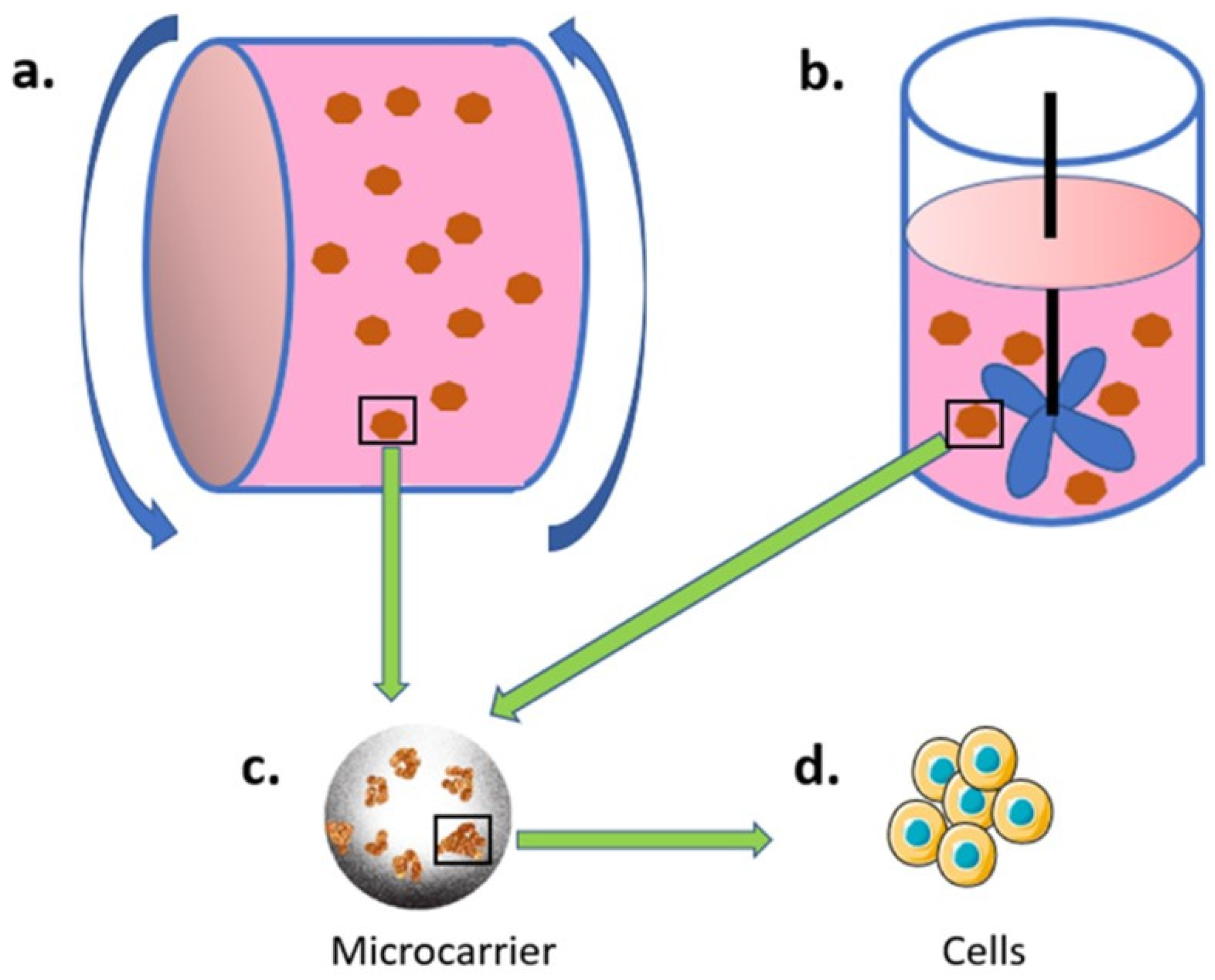

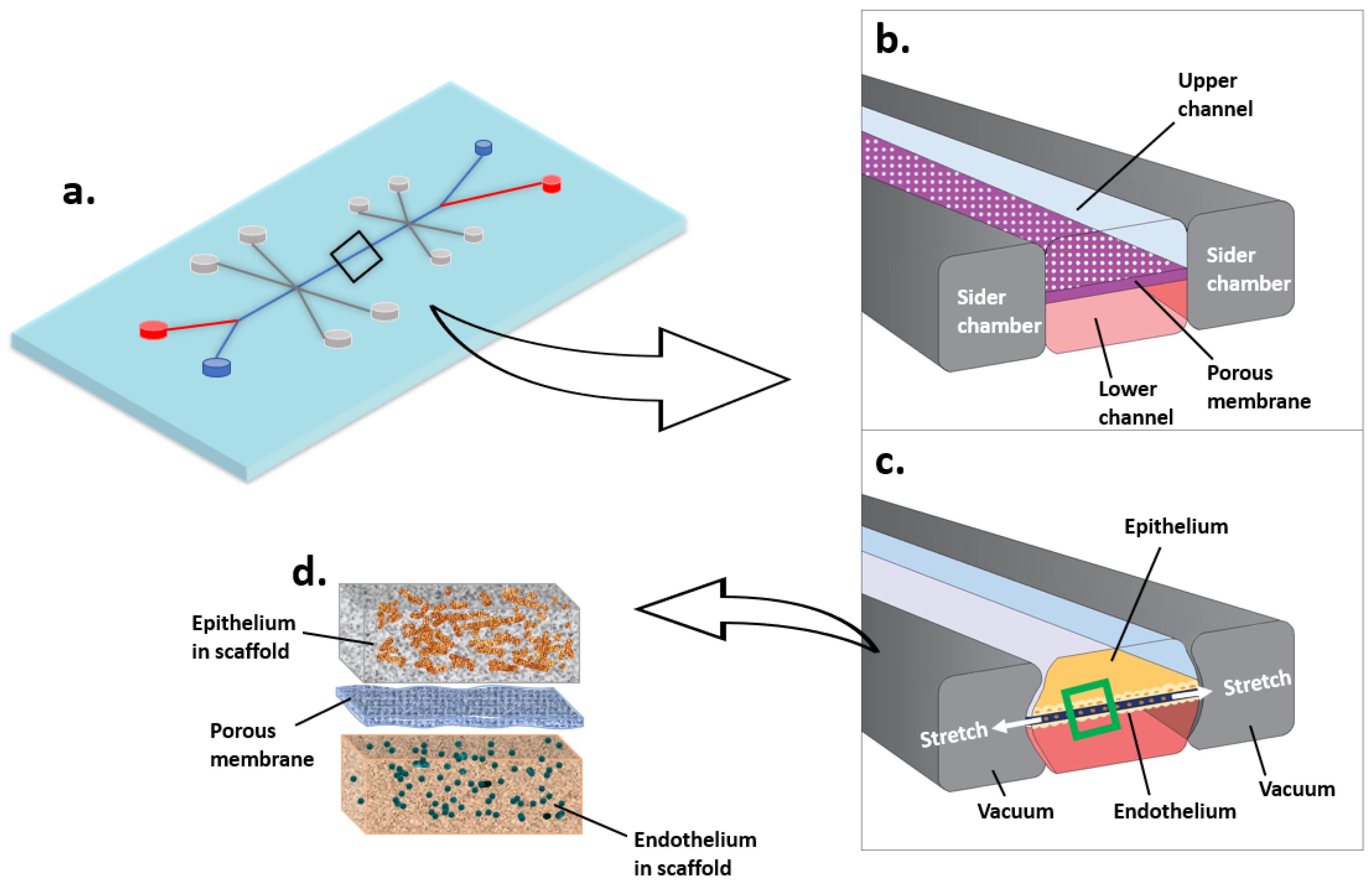
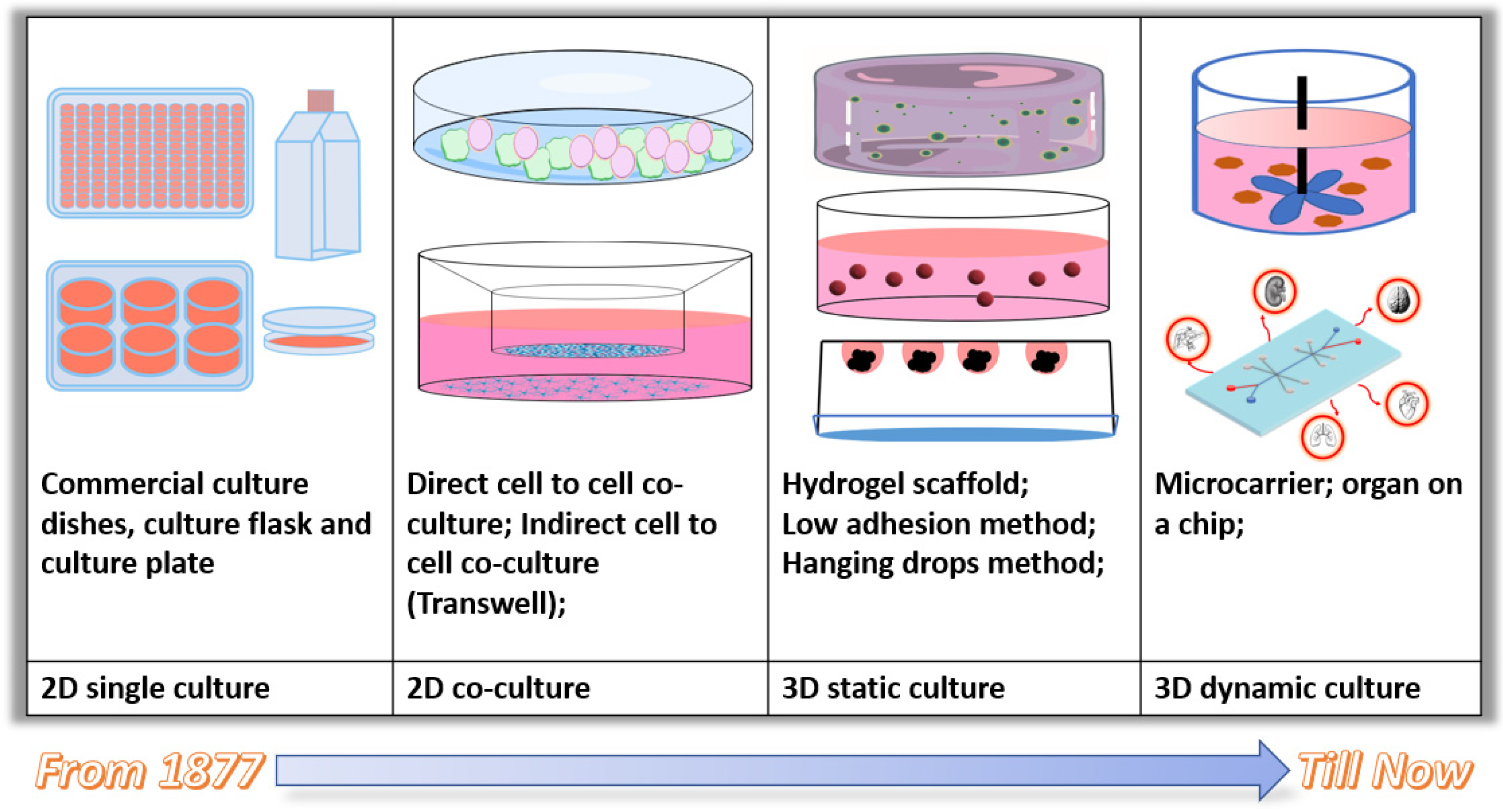
| Able to Provide a 3D Environment? | Able to Perform Static Culture? | Able to Perform Dynamic Culture? | Harvest a Large Number of Cells for Classic Molecular Biology Analysis? | Large-Scale Production? | Easy to Handle? | Cost? | |
|---|---|---|---|---|---|---|---|
| Flask/plate/dish | N | Y | N | Y | Y | Easy | Low |
| Transwell system | N | Y | N | Y | N | Easy | High |
| Scaffold | Y | Y | Y | Y | N | Moderate | High |
| Low adhesion | Y | Y | N | N | N | Easy | Low |
| Hanging drops | Y | Y | N | N | N | Easy | Low |
| Microcarrier and bioreactor | Y | N | Y | Y | Y | Moderate | Moderate |
| Organ-on-a-chip | Y | Y | Y | N | N | Hard | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Huang, Z.; Gao, W.; Gao, W.; He, R.; Li, Y.; Crawford, R.; Zhou, Y.; Xiao, L.; Xiao, Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels 2022, 8, 829. https://doi.org/10.3390/gels8120829
Huang X, Huang Z, Gao W, Gao W, He R, Li Y, Crawford R, Zhou Y, Xiao L, Xiao Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels. 2022; 8(12):829. https://doi.org/10.3390/gels8120829
Chicago/Turabian StyleHuang, Xin, Zhengxiang Huang, Weidong Gao, Wendong Gao, Ruiying He, Yulin Li, Ross Crawford, Yinghong Zhou, Lan Xiao, and Yin Xiao. 2022. "Current Advances in 3D Dynamic Cell Culture Systems" Gels 8, no. 12: 829. https://doi.org/10.3390/gels8120829
APA StyleHuang, X., Huang, Z., Gao, W., Gao, W., He, R., Li, Y., Crawford, R., Zhou, Y., Xiao, L., & Xiao, Y. (2022). Current Advances in 3D Dynamic Cell Culture Systems. Gels, 8(12), 829. https://doi.org/10.3390/gels8120829









