Novel Hydrogel Material with Tailored Internal Architecture Modified by “Bio” Amphiphilic Components—Design and Analysis by a Physico-Chemical Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Crosslinking
2.1.1. Rheology
2.1.2. Drying and Rehydration Measurements
2.1.3. Morphological Characterization of Xerogels
2.2. Ionic Crosslinking
2.2.1. Rheology
2.2.2. Drying and Rehydration Measurements
2.2.3. Morphological Characterization of Xerogels
2.3. Chemical Crosslinking
2.3.1. Rheology
2.3.2. Drying and Rehydration Measurements
2.3.3. Morphological Characterization of Xerogels
3. Conclusions
4. Materials and Methods
4.1. Water Loss during Drying and Rehydration Measurements
4.2. Rheology
4.3. Morphological Characterization of Xerogels
4.3.1. Scanning Electron Microscopy
4.3.2. Gas Sorption
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Gadjanski, I. Recent advances on gradient hydrogels in biomimetic cartilage tissue engineering. F1000Research 2017, 6, 2158. [Google Scholar] [CrossRef]
- Pekař, M. Hydrogels with Micellar Hydrophobic (Nano)Domains. Front. Mater. 2015, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Q.; Dai, Z.; Dai, Y.; Xia, F.; Zhang, X. Nanocomposite adhesive hydrogels: From design to application. J. Mater. Chem. B 2021, 9, 585–593. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Algi, M.P.; Okay, O. Autonomic self-healing in covalently crosslinked hydrogels containing hydrophobic domains. Polymer 2013, 54, 6381–6388. [Google Scholar] [CrossRef]
- Gu, S.; Duan, L.; Ren, X.; Gao, G.H. Robust, tough and anti-fatigue cationic latex composite hydrogels based on dual physically cross-linked networks. J. Colloid Interface Sci. 2017, 492, 119–126. [Google Scholar] [CrossRef]
- Li, A.; Jia, Y.; Sun, S.; Xu, Y.; Minsky, B.B.; Stuart, M.A.C.; Cölfen, H.; von Klitzing, R.; Guo, X. Mineral-Enhanced Polyacrylic Acid Hydrogel as an Oyster-Inspired Organic–Inorganic Hybrid Adhesive. ACS Appl. Mater. Interfaces 2018, 10, 10471–10479. [Google Scholar] [CrossRef]
- Cui, C.; Wu, T.; Gao, F.; Fan, C.; Xu, Z.; Wang, H.; Liu, B.; Liu, W. An Autolytic High Strength Instant Adhesive Hydrogel for Emergency Self-Rescue. Adv. Funct. Mater. 2018, 28, 1804925. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hao, R.; Ren, X.; Yu, L.; Yang, H.; Yu, H. PEG/lecithin–liquid-crystalline composite hydrogels for quasi-zero-order combined release of hydrophilic and lipophilic drugs. RSC Adv. 2013, 3, 22927–22930. [Google Scholar] [CrossRef]
- Shchipunov, Y.A. Lecithin. In Encyclopedia of Surface and Colloid Science, 3rd ed.; Somasundaran, P., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 3, pp. 3674–3693. [Google Scholar]
- Elnaggar, Y.S.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.R.; Zarket, B.C.; Lauten, E.H.; Amin, S.; Muthukrishnan, S.; Raghavan, S.R. Liposomes Entrapped in Biopolymer Hydrogels Can Spontaneously Release into the External Solution. Langmuir 2020, 36, 7268–7276. [Google Scholar] [CrossRef]
- Li, D.; An, X.; Mu, Y. A liposomal hydrogel with enzyme triggered release for infected wound. Chem. Phys. Lipids 2019, 223, 104783. [Google Scholar] [CrossRef]
- Talaat, S.M.; Elnaggar, Y.S.R.; Abdalla, O.Y. Lecithin Microemulsion Lipogels Versus Conventional Gels for Skin Targeting of Terconazole: In Vitro, Ex Vivo, and In Vivo Investigation. AAPS PharmSciTech 2019, 20, 161. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Trudicova, M.; Smilek, J.; Kalina, M.; Smilkova, M.; Adamkova, K.; Hrubanova, K.; Krzyzanek, V.; Sedlacek, P. Multiscale Experimental Evaluation of Agarose-Based Semi-Interpenetrating Polymer Network Hydrogels as Materials with Tunable Rheological and Transport Performance. Polymers 2020, 12, 2561. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M.; Velasquillo, C.; García-Carvajal, Z.; Garcia-Lopez, J.; Ortega-Sánchez, C.; Ibarra, C.; Luna-Barcenas, G.; Solís-Arrieta, L. Mechanical and structural response of a hybrid hydrogel based on chitosan and poly(vinyl alcohol) cross-linked with epichlorohydrin for potential use in tissue engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.L.; Shekarforoush, E.; Engwer, C.; Beeren, S.; Gorzelanny, C.; Goycoolea, F.M.; Chronakis, I.S. Co-assembly of chitosan and phospholipids into hybrid hydrogels. Pure Appl. Chem. 2016, 88, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Smilek, J.; Jarábková, S.; Velcer, T.; Pekař, M. Compositional and Temperature Effects on the Rheological Properties of Polyelectrolyte–Surfactant Hydrogels. Polymers 2019, 11, 927. [Google Scholar] [CrossRef] [Green Version]
- Mourycová, J.; Datta, K.K.R.; Procházková, A.; Plotěná, M.; Enev, V.; Smilek, J.; Másilko, J.; Pekař, M. Facile synthesis and rheological characterization of nanocomposite hyaluronan-organoclay hydrogels. Int. J. Biol. Macromol. 2018, 111, 680–684. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT-Food Sci. Technol. 2015, 63, 612–619. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Bonhome-Espinosa, A.B.; Kuzhir, P.; Zubarev, A.; Duran, J.D.G.; Lopez-Lopez, M.T. Rheology of magnetic alginate hydrogels. J. Rheol. 2018, 62, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Gradzielski, M.; Hoffmann, I. Polyelectrolyte-surfactant complexes (PESCs) composed of oppositely charged components. Curr. Opin. Colloid Interface Sci. 2018, 35, 124–141. [Google Scholar] [CrossRef]
- Pescosolido, L.; Feruglio, L.; Farra, R.; Fiorentino, S.; Colombo, I.; Coviello, T.; Matricardi, P.; Hennink, W.E.; Vermonden, T.; Grassi, M. Mesh size distribution determination of interpenetrating polymer network hydrogels. Soft Matter 2012, 8, 7708–7715. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Kalbáčová, M.H. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Pavithra, U.; Sivaranjani, V.; Balasubramanian, N.; Sakthivel, K.M.; Pruncu, C.I. A novel Ag/carrageenan–gelatin hybrid hydrogel nanocomposite and its biological applications: Preparation and characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104257. [Google Scholar] [CrossRef]
- Marmorat, C.; Arinstein, A.; Koifman, N.; Talmon, Y.; Zussman, E.; Rafailovich, M. Cryo-Imaging of Hydrogels Supermolecular Structure. Sci. Rep. 2016, 6, 25495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, S.D.; Kim, Y.-H.; Yi, G.; Ahn, Y.-S.; Yeo, J.-G.; Choi, Y.-T. Mesoporous SiO2 powders with high specific surface area by microwave drying of hydrogels: A facile synthesis. Microporous Mesoporous Mater. 2008, 108, 333–339. [Google Scholar] [CrossRef]
- Weber, J.; Bergström, L. Mesoporous Hydrogels: Revealing Reversible Porosity by Cryoporometry, X-ray Scattering, and Gas Adsorption. Langmuir 2010, 26, 10158–10164. [Google Scholar] [CrossRef]
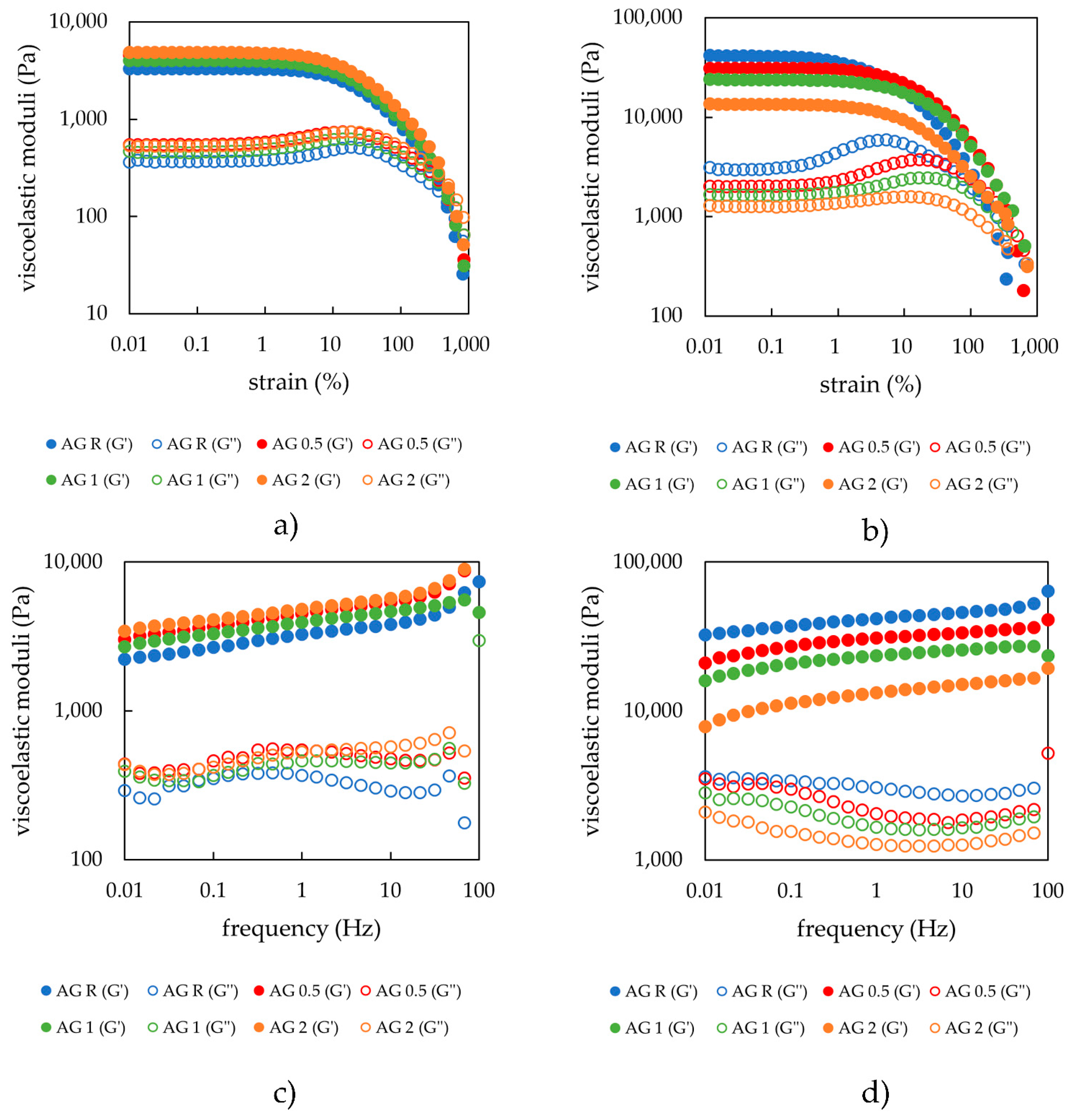








| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 157.5 ± 4.1 | 425.8 ± 2.2 | 3299 ± 277 | 366 ± 28 | 2.5 ± 1.0 | 13.3 ± 0.1 |
| 0.5 | 207.9 ± 2.1 | 414.1 ± 4.5 | 4576 ± 12 | 551 ± 15 | 1.8 ± 0.0 | 13.4 ± 0.4 |
| 1 | 194.9 ± 10.7 | 433.2 ± 10.2 | 4002 ± 81 | 461 ± 4 | 1.8 ± 0.0 | 12.7 ± 0.1 |
| 2 | 224.5 ± 0.0 | 468.0 ± 2.9 | 4880 ± 27 | 529 ± 8 | 1.8 ± 0.0 | 12.9 ± 0.3 |
| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 1814 ± 340.6 | 250.4 ± 131.7 | 41,386 ± 10,517 | 2977 ± 707 | 0.3 ± 0.1 | 7.6 ± 1.1 |
| 0.5 | 1005.7 ± 142.9 | 718.5 ± 129.3 | 31,216 ± 980 | 2010 ± 4 | 1.2 ± 0.5 | 7.6 ± 0.2 |
| 1 | 542.7 ± 0.0 | 1148.7 ± 0.0 | 23,829 ± 3 | 1642 ± 118 | 1.4 ± 0.3 | 8.2 ± 0.1 |
| 2 | 350.8 ± 35.5 | 1257.6 ± 12.2 | 13,506 ± 1217 | 1256 ± 122 | 0.9 ± 0.0 | 9.0 ± 0.3 |
| Concentration of Lecithin (wt.%) | Specific Surface Area (m2/g) |
|---|---|
| 0 (R) | 3.4 |
| 0.5 | 1.0 |
| 1 | 1.9 |
| 2 | 2.1 |
| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 150.4 ± 9.1 | 260.4 ± 18.6 | 1667 ± 192 | 165 ± 23 | 1.7 ± 0.0 | 10.9 ± 0.4 |
| 0.5 | 158.6 ± 12.5 | 275.3 ± 24.4 | 2138 ± 480 | 245 ± 66 | 1.3 ± 0.0 | 11.0 ± 0.7 |
| 1 | 110.8 ± 1.2 | 260.8 ± 5.4 | 1052 ± 1 | 104 ± 0 | 1.6 ± 0.3 | 13.8 ± 1.9 |
| 2 | 65.3 ± 17.9 | 278.2 ± 9.0 | 468 ± 15 | 41 ± 0 | 2.1 ± 0.4 | 17.3 ± 1.5 |
| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 479.2 ± 129.7 | 210.8 ± 119.7 | 26,342 ± 13,355 | 3191 ± 1346 | 1.6 ± 0.4 | 4.6 ± 1.4 |
| 0.5 | 894.9 ± 612.4 | 522.6 ± 51.8 | 68,513 ± 17,434 | 9861 ± 1533 | 0.6 ± 0.6 | 12.3 ± 2.1 |
| 1 | 1179.5 ± 106.7 | 209.1 ± 37.3 | 25,386 ± 741 | 2912 ± 45 | 1.2 ± 0.2 | 8.3 ± 1.8 |
| 2 | 553.5 ± 24.3 | 189.5 ± 17.4 | 4599 ± 500 | 1842 ± 1447 | 2.4 ± 0.0 | 7.6 ± 0.5 |
| Concentration of Lecithin (wt.%) | Specific Surface Area (m2/g) |
|---|---|
| 0 (R) | 9.1 |
| 0.5 | 6.3 |
| 1 | 5.9 |
| 2 | 4.7 |
| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 1665.3 ± 43.2 | 53.8 ± 8.2 | 8629 ± 304 | 398 ± 4 | 1.6 ± 0.3 | 13.6 ± 0.7 |
| 0.5 | 1005.5 ± 32.4 | 49.4 ± 18.4 | 6644 ± 1503 | 307 ± 44 | 1.2 ± 0.9 | 13.8 ± 0.6 |
| 1 | 666.6 ± 5.4 | 40.2 ± 3.2 | 4545 ± 129 | 377 ± 68 | 0.6 ± 0.1 | 12.7 ± 0.1 |
| 2 | 631.6 ± 24.7 | 39.1 ± 4.7 | 4398 ± 195 | 421 ± 5 | 0.7 ± 0.1 | 12.9 ± 0.1 |
| Cross-Over Point | Average Moduli Values in LVR | End of LVR | Mesh Size | |||
|---|---|---|---|---|---|---|
| Lecithin Concentration | G′ | Strain | G′ | G″ | Strain | Mesh |
| (wt.%) | (Pa) | (%) | (Pa) | (Pa) | (%) | (nm) |
| 0 (R) | 2470.0 ± 494.7 | 138.5 ± 13.5 | 14,514 ± 1413 | 532 ± 33 | 3.2 ± 0.0 | 11.6 ± 0.3 |
| 0.5 | 7122.4 ± 633.3 | 379.1 ± 233.0 | 62,099 ± 6505 | 1928 ± 65 | 5.0 ± 1.0 | 7.1 ± 0.1 |
| 1 | 4964.6 ± 275.8 | 502.2 ± 277.5 | 52,833 ± 10,153 | 2089 ± 246 | 3.0 ± 1.7 | 6.2 ± 1.3 |
| 2 | 4074.2 ± 182.3 | 900.1 ± 97.5 | 43,685 ± 3177 | 1761 ± 211 | 5.9 ± 2.3 | 8.1 ± 0.0 |
| Concentration of Lecithin (wt.%) | Specific Surface Area (m2/g) |
|---|---|
| 0 (R) | 2.9 |
| 0.5 | 0.8 |
| 1 | 1.2 |
| 2 | 1.6 |
| Physically Crosslinked Hydrogels | |||
| Sample | Agarose (wt.%) | Lecithin (wt.%) | |
| AG R | 1 | 0 | |
| AG 0.5 | 1 | 0.5 | |
| AG 1 | 1 | 1 | |
| AG 2 | 1 | 2 | |
| Ionically Crosslinked Hydrogels | |||
| Sample | Sodium Alginate (wt.%) | Calcium Chloride (mol·dm3) | Lecithin (wt.%) |
| ALG R | 2 | 0.1 | 0 |
| ALG 0.5 | 2 | 0.1 | 0.5 |
| ALG 1 | 2 | 0.1 | 1 |
| ALG 2 | 2 | 0.1 | 2 |
| Chemically Crosslinked Hydrogels | |||
| Sample | PVA (wt.%) | Chitosan (wt.%) | Lecithin (wt.%) |
| PVA R | 7.8 | 2.5 | 0 |
| PVA 0.5 | 7.8 | 2.5 | 0.5 |
| PVA 1 | 7.8 | 2.5 | 1 |
| PVA 2 | 7.8 | 2.5 | 2 |
| Conditioning Step | |||
| Temperature | 25 °C | ||
| Time | 180 s | ||
| Amplitude Sweep | Frequency Sweep | ||
| temperature | 25 °C | temperature | 25 °C |
| strain | 0.01–1000% | strain | 0.1% |
| points per decade | 8 | points per decade | 6 |
| frequency | 1 Hz | frequency | 0.01–100 Hz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heger, R.; Kadlec, M.; Trudicova, M.; Zinkovska, N.; Hajzler, J.; Pekar, M.; Smilek, J. Novel Hydrogel Material with Tailored Internal Architecture Modified by “Bio” Amphiphilic Components—Design and Analysis by a Physico-Chemical Approach. Gels 2022, 8, 115. https://doi.org/10.3390/gels8020115
Heger R, Kadlec M, Trudicova M, Zinkovska N, Hajzler J, Pekar M, Smilek J. Novel Hydrogel Material with Tailored Internal Architecture Modified by “Bio” Amphiphilic Components—Design and Analysis by a Physico-Chemical Approach. Gels. 2022; 8(2):115. https://doi.org/10.3390/gels8020115
Chicago/Turabian StyleHeger, Richard, Martin Kadlec, Monika Trudicova, Natalia Zinkovska, Jan Hajzler, Miloslav Pekar, and Jiri Smilek. 2022. "Novel Hydrogel Material with Tailored Internal Architecture Modified by “Bio” Amphiphilic Components—Design and Analysis by a Physico-Chemical Approach" Gels 8, no. 2: 115. https://doi.org/10.3390/gels8020115






