Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants
Abstract
:1. Introduction
2. Results and Discussion
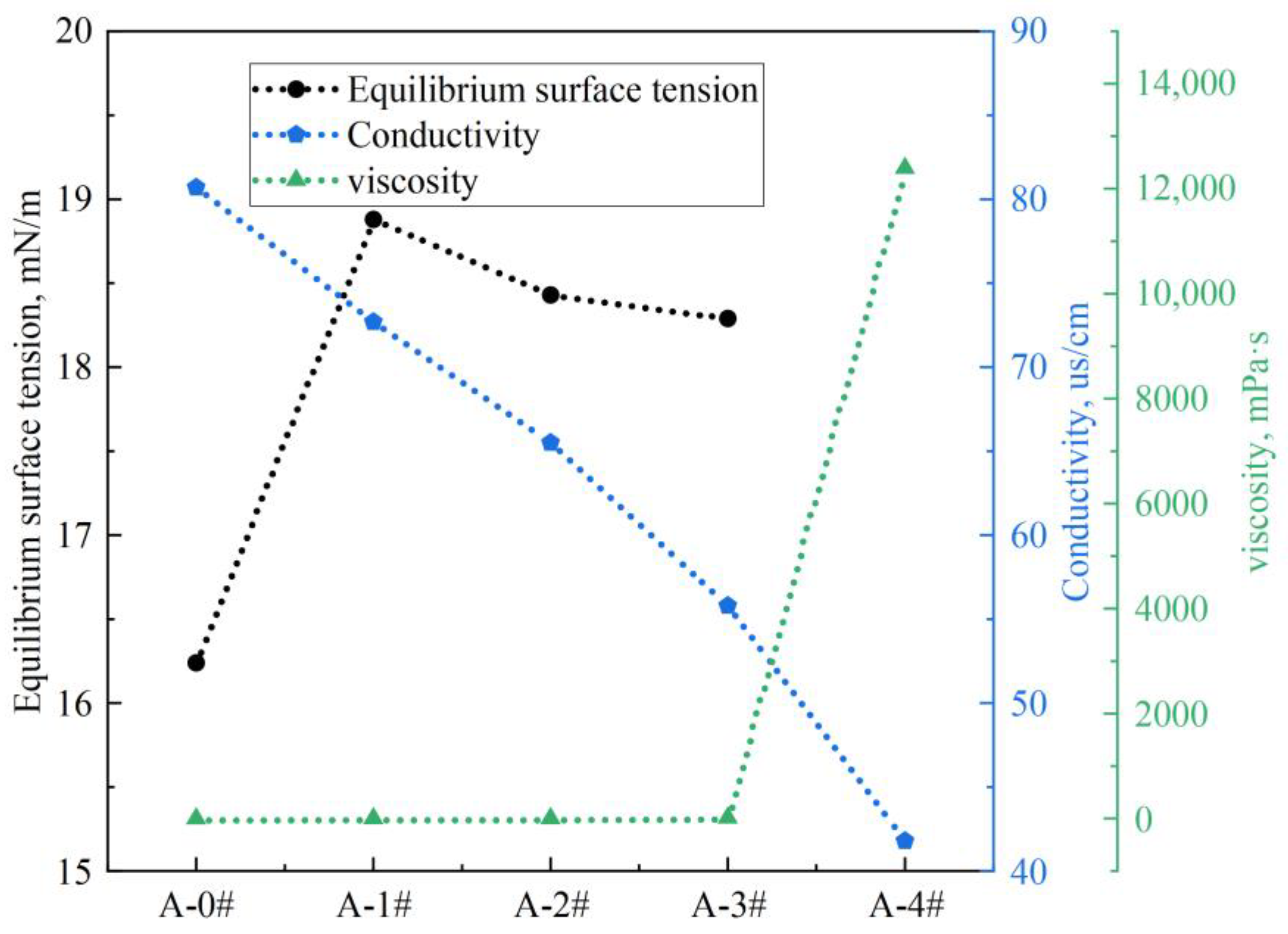
2.1. Basic Properties of Gel Dispersions
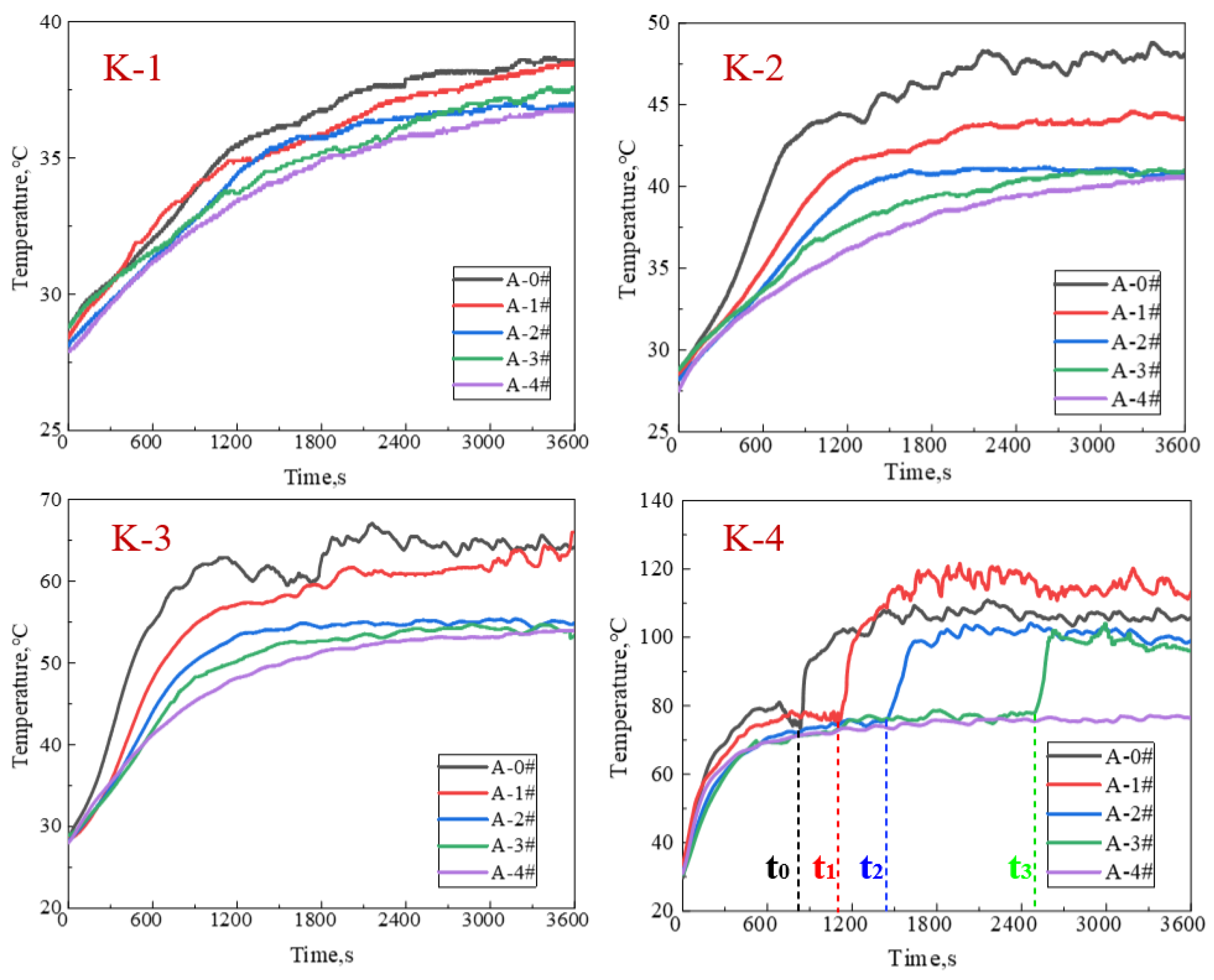
2.2. Foam Thermal Stability
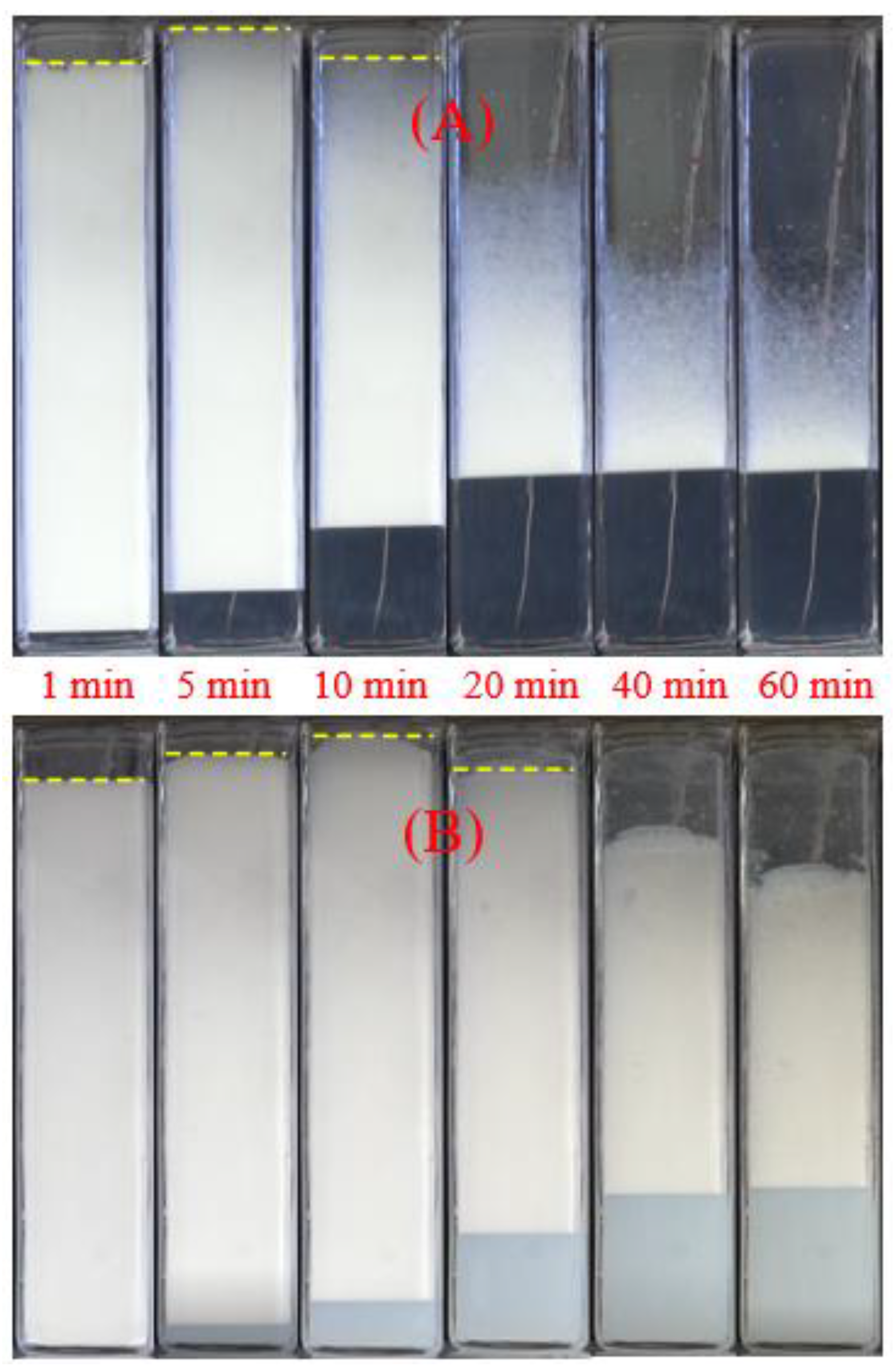
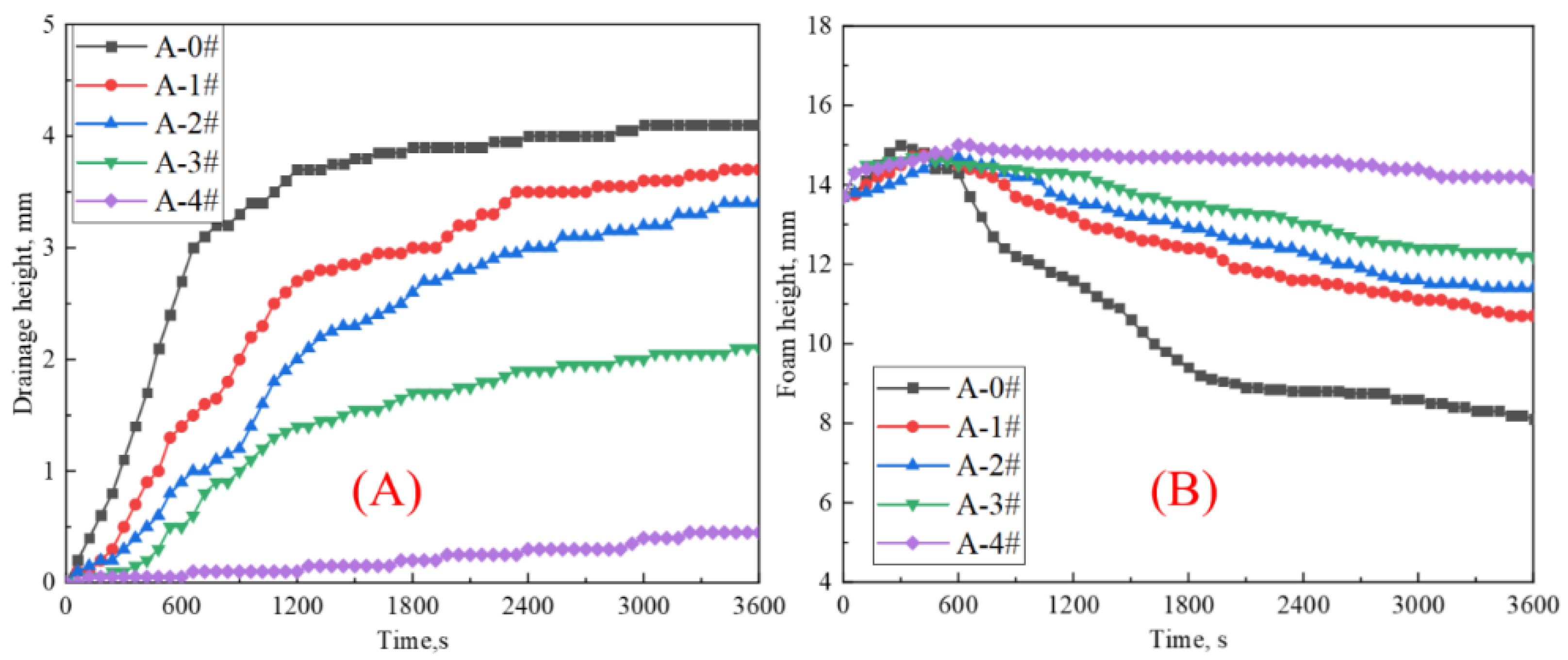
2.2.1. Foam Drainage and Decay
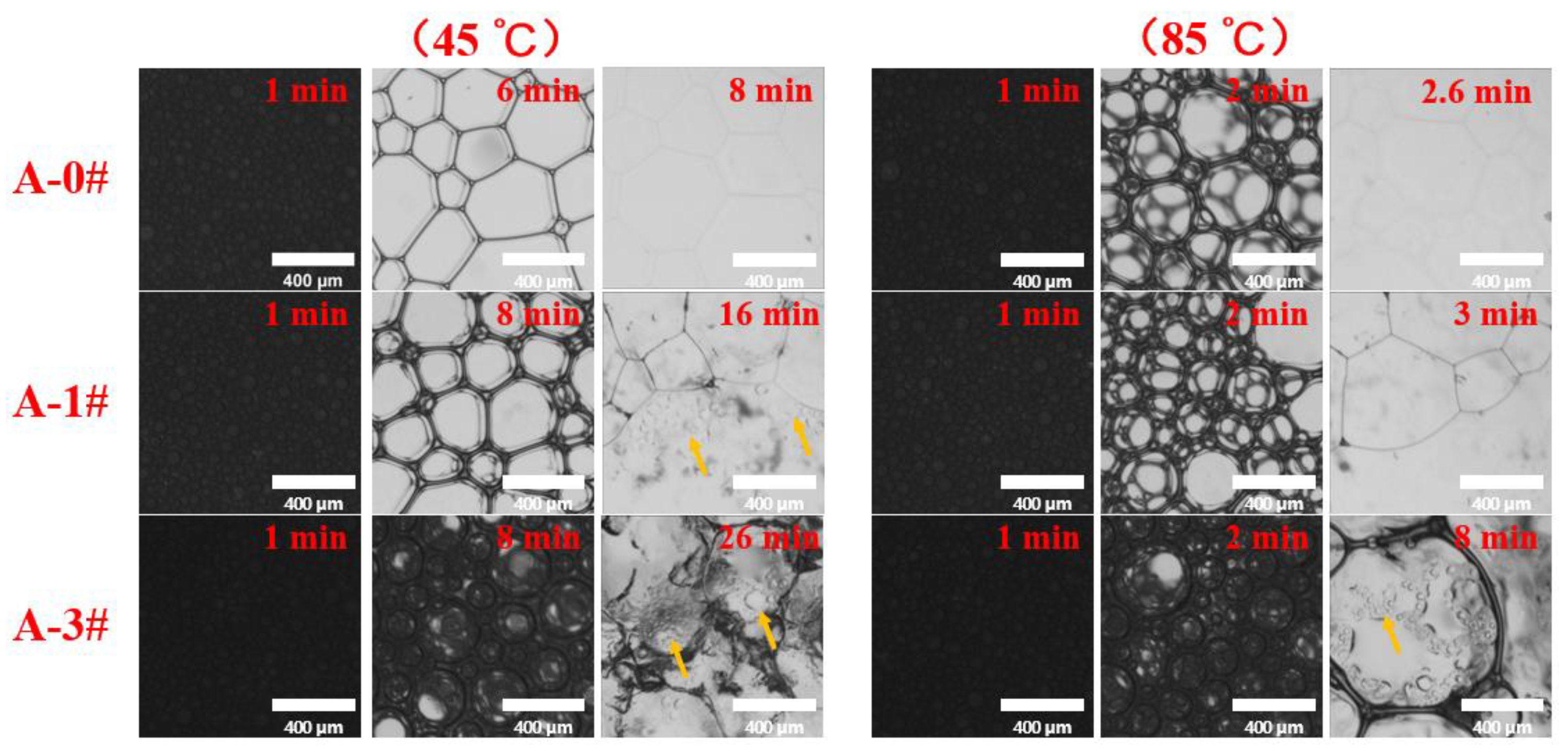
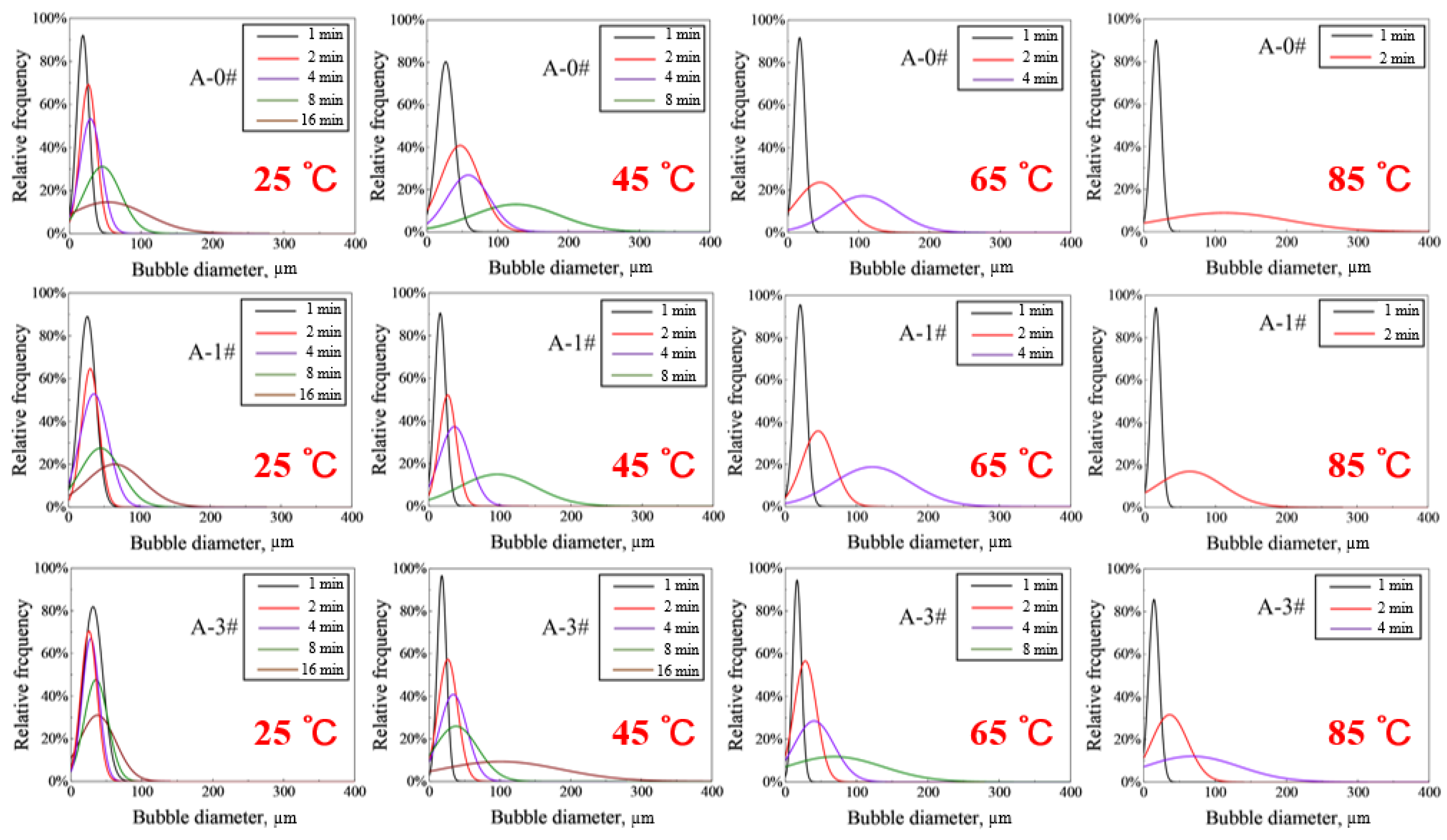
2.2.2. Foam Coarsening

3. Conclusions
4. Materials and Methods
4.1. Sample Preparation
4.2. Apparatus and Procedures
4.2.1. Testing for Dispersions Properties
4.2.2. Testing for Foaming Ability
4.2.3. Testing for Foam Thermal Stability
Testing for Foam Drainage and Foam Decay
Testing for Foam Coarsening
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debbabi, K.; Guittard, F.; Geribaldi, S. Novel highly fluorinated sulfamates: Synthesis and evaluation of their surfactant properties. J. Colloid Interface Sci. 2008, 326, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Littlejohn, K.A.; Hooley, P.; Cox, P.W. Formation and Stability of Food Foams and Aerated Emulsions: Hy-dro-phobins as Novel Functional Ingredients. Curr. Opin. Colloid Interface Sci. 2013, 18, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.; Okuda, T.; Kurose, K.; Tsai, T.-Y.; Nakai, S.; Nishijima, W.; Okada, M. Surface ozonation of polyvinyl chloride for its separation from waste plastic mixture by froth floatation. J. Mater. Cycles Waste Manag. 2010, 12, 326–331. [Google Scholar] [CrossRef]
- Sheng, Y.; Xue, M.; Zhang, S.; Wang, Y.; Zhai, X.; Zhao, Y.; Ma, L.; Liu, X. Role of nanoparticles in the performance of foam stabilized by a mixture of hydrocarbon and fluorocarbon surfactants. Chem. Eng. Sci. 2020, 228, 115977. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Li, C. Fluorinated and fluorine-free firefighting foams spread on heptane surface. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 1–8. [Google Scholar] [CrossRef]
- Fu, C.; Yu, J.; Liu, N. Nanoparticle-stabilized CO2 foam for waterflooded residual oil recovery. Fuel 2018, 234, 809–813. [Google Scholar] [CrossRef]
- Zhao, J.; Torabi, F.; Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 2020, 287, 119443. [Google Scholar] [CrossRef]
- Liu, Z.; Bode, V.; Hadayati, P. Understanding the stability mechanism of silica nanoparticles: The effect of cations and EOR chemicals. Fuel 2020, 280, 118650. [Google Scholar] [CrossRef]
- Nurudeen, Y.; Eswaran, P.; Kamal, I.A. A review of recent advances in foam-based fracturing fluid application in unconventional reservoirs. J. Ind. Eng. Chem. 2018, 66, 45–71. [Google Scholar]
- Wang, L.; Yao, B.; Cha, M.; Alqahtani, N.B.; Patterson, T.W.; Kneafsey, T.J. Waterless fracturing technologies for unconventional reservoirs-opportunities for liquid nitrogen. J. Nat. Gas Sci. Eng. 2016, 35, 160–174. [Google Scholar] [CrossRef] [Green Version]
- Wanniarachchi, W.A.M.; Ranjith, P.G.; Perera, M.S.A.; Lashin, A. Current opinions on foam-based hydro-fracturing in deep geological reservoirs. Geomech. Geophys. Geo. 2015, 1, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Magrabi, S.A.; Dlugogorski, B.Z.; Jameson, G.J. A comparative study of drainage characteristics in AFFF and FFFP compressed-air fire-fighting foams. Fire Safety J. 2002, 37, 21–52. [Google Scholar] [CrossRef]
- Hagenaars, A.; Meyer, I.J.; Herzke, D. The search for alternative aqueous film forming foams (AFFF) with a low environmental impact: Physiological and transcriptomic effects of two Forafac (R) fluorosurfactants in turbot. Aquat. Toxicol. 2011, 104, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rotander, A. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF)—ScienceDirect. Environ. Int. 2015, 82, 28–34. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Wang, Q.; Zhao, Y.; Liu, X. Study of environmental-friendly firefighting foam based on the mixture of hydrocarbon and silicone surfactants. Fire Technol. 2020, 56, 1059–1075. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Sun, X. Experimental Study on Effect of Foam Stabilizers on Aqueous Film-Forming Foam. Fire Technol. 2017, 54, 211–228. [Google Scholar] [CrossRef]
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs); Secretariat of the Stockholm Convention: Geneva, Switzerland, 2009. [Google Scholar]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid(PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Giles, S.L.; Snow, A.W.; Farley, J.P.; Fleming, J.W.; Ananth, R. An analytically defined fire-suppressing foam formulation for evaluation of fluorosurfactant replacement. J. Surfactants Deterg. 2018, 21, 711–722. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Kuprin, D.; Abduragimov, I. Silica foams for fire prevention and firefighting. ACS Appl. Mater. Inter. 2016, 8, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, N.; Miao, X. Comparative Studies on Foam Stability, Oil-film Interaction and Fire Extinguishing Performance for Fluorine-free and Fluorinated Foams. Process Saf. Environ. Prot. 2019, 133, 201–215. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticlestabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef]
- Verma, A.; Chauhan, G.; Baruah, P.P.; Ojha, K. Morphology, Rheology, and Kinetics of Nanosilica Stabilized Gelled Foam Fluid for Hydraulic Fracturing Application. Ind. Eng. Chem. Res. 2018, 57, 13449–13462. [Google Scholar] [CrossRef]
- Verma, A.; Chauhan, G.; Ojha, K.; Padmanabhan, E. Characterization of Nano-Fe2O3-Stabilized Polymer-Free Foam Fracturing Fluids for Unconventional Gas Reservoirs. Energy Fuels 2019, 33, 10570–10582. [Google Scholar] [CrossRef]
- Fei, Y.; Johnson, R.L.; Gonzalez, M.; Haghighi, M.; Pokalai, K. Experimental and numerical investigation into nano-stabilized foams in low permeability reservoir hydraulic fracturing applications. Fuel 2018, 213, 133–143. [Google Scholar] [CrossRef]
- Fei, Y.; Pokalai, K.; Johnson, R.; Gonzalez, M.; Haghighi, M. Experimental and simulation study of foam stability and the effects on hydraulic fracture proppant placement. J. Nat. Gas Sci. Eng. 2017, 46, 544–554. [Google Scholar] [CrossRef]
- Fei, Y.; Zhu, J.; Xu, B.; Li, X.; Gonzalez, M.; Haghighi, M. Experimental investigation of nanotechnology on worm-like micelles for high-temperature foam stimulation. J. Ind. Eng. Chem. 2017, 50, 190–198. [Google Scholar] [CrossRef]
- Rognmo, A.U.; Heldal, S.; Fern, M.A. Silica nanoparticles to stabilize CO2-foam for improved CO2 utilization: Enhanced CO2 storage and oil recovery from mature oil reservoirs. Fuel 2018, 216, 621–626. [Google Scholar] [CrossRef]
- Guan, M.P.; Pilus, R.M.; Mustaffa, A. Relationship between fly ash nanoparticle-stabilized-foam and oil production in core displacement and simulation studies. Fuel 2020, 266, 117033. [Google Scholar]
- Rza, B.; Xl, B.; Xu, Z.C. Thermal stability and insulation characteristics of three-phase fire-fighting foam exposed to radiant heating—ScienceDirect. Process Saf. Environ. 2021, 146, 360–368. [Google Scholar]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Íñiguez-Palomares, R.; Rioa, E.; Langevina, D. On the long-term stability of foams stabilised by mixtures of NPs and oppositely charged short chain surfactants. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- AlYousef, Z.A.; Almobarky, M.A.; Schechter, D.S. The effect of NP aggregation on surfactant foam stability. J. Colloid Interf. Sci. 2018, 511, 365–373. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K. Synergy between NPs and surfactants in stabilizing foams for oil recovery. Energy Fuel 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Sheng, Y.; Peng, Y.; Yan, C.; Li, Y. Influence of nanoparticles on rheological properties and foam properties of mixed solutions of fluorocarbon and hydrocarbon surfactants. Powder Technol. 2021, 398, 117067. [Google Scholar] [CrossRef]
- Wang, J.; Xue, G.; Tian, B.; Li, S.; Chen, K.; Wang, D.; Li, Z. Interaction between surfactants and SiO2 NPs in multiphase foam and its plugging ability. Energy Fuel 2017, 31, 408–417. [Google Scholar] [CrossRef]
- Yuan, Z.; Menghua, Q.; Chen, X. Phase behavior, rheological property, and transmutation of vesicles in fluorocarbon and hydrocarbon surfactant mixtures. Langmuir 2012, 28, 9355–9364. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic Effect of Mixed Surfactant Systems on Foam Behavior and Surface Tension. J. Surfactants Deterg. 2013, 16, 621–630. [Google Scholar] [CrossRef]
- Rosen, M.J.; Hua, X.Y. Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interf. Sci. 1982, 86, 164–172. [Google Scholar] [CrossRef]
- Kennedy, J.R.M.; Kent, K.E.; Brown, J.R. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mat. Sci. Eng. C Mater. 2015, 48, 347–353. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N. Sol-gel synthesis at neutral pH in W/O microemulsion: A method for enzyme nanoencapsulation in silica gel nanoparticles. Colloid Surf. A 2006, 288, 52–61. [Google Scholar] [CrossRef]
- Vatanparast, H.; Samiee, A.; Bahramian, A.; Javadi, A. Surface behavior of hydrophilic silica NP-SDS surfactant solutions: I. Effect of NP concentration on foamability and foam stability. Colloid Surf. A 2017, 513, 430–441. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z.; Miao, Q.; Deng, Z.; Zhu, Y. Foams stabilized by in situ-modified NPs and anionic surfactants for enhanced oil recovery. Energy Fuel 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Biswas, S.C.; Chattoraj, D.K. Kinetics of adsorption of cationic surfactants at silica-water interface. J. Colloid Interf. Sci. 1998, 205, 12–20. [Google Scholar] [CrossRef]
- Chorro, M.; Chorro, C.; Dolladille, O.; Partyka, S.; Zana, R. Adsorption mechanism of conventional and dimeric cationic surfactants on silica surface: Effect of the state of the surface. J. Colloid Interf. Sci. 1999, 210, 134–143. [Google Scholar] [CrossRef]
- Carn, F.; Colin, A.; Pitois, O.; Vignes-Adler, M.; Backov, R. Foam drainage in the presence of NP- surfactant mixtures. Langmuir 2009, 25, 7847–7856. [Google Scholar] [CrossRef]
- Binks, B.P.; Campbell, S.; Mashinchi, S.; Piatko, M.P. Dispersion behavior and aqueous foams in mixtures of a vesicle-forming surfactant and edible NPs. Langmuir 2015, 31, 2967–2978. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Sun, Q.; Wang, P.; Wang, S.; Liu, W. CO2 foam properties and the stabilizing mechanism of sodium bis (2-ethylhexyl) sulfosuccinate and hydrophobic NP mixtures. Soft Matter 2016, 12, 946–956. [Google Scholar] [CrossRef]
- Hu, N.; Li, Y.; Wu, Z. Foams stabilization by silica nanoparticle with cationic and anionic surfactants in column flotation: Effects of particle size. J. Taiwan Inst. Chem. E 2018, 88, 62–69. [Google Scholar] [CrossRef]
- Sheng, Y.; Xue, M.; Zhang, S. Effect of xanthan gum and silica nanoparticles on improving foam properties of mixed solutions of short-chain fluorocarbon and hydrocarbon surfactants. Chem. Eng. Sci. 2021, 245, 116952. [Google Scholar] [CrossRef]
- Denkov, N.D.; Ivanov, I.B.; Kralchevsky, P.A.; Wasan, D.T. A possible mechanism of stabilization of emulsions by solid particles. J. Colloid Interf. Sci. 1992, 150, 589–593. [Google Scholar] [CrossRef]
- Sheng, Y.; Xue, M.; Wang, Y.; Zhai, X. Aggregation behavior and foam properties of the mixture of hydrocarbon and fluorocarbon surfactants with addition of nanoparticles. J. Mol. Liq. 2020, 323, 115070. [Google Scholar] [CrossRef]
- Tang, B.; Wu, Z.; Chen, W.H. Effect of nanosilica on foam and thermal stability of a foam extinguishing agent. Nanomater. Energy 2017, 6, 67–73. [Google Scholar] [CrossRef]
- Wu, J.; Mei, P.; Chen, W.; Li, Z.; Mei, Q. Surface Properties and Solubility Enhancement of Anionic/Nonionic Surfactant Mixtures Based on Sulfonate Gemini Surfactants. J. Surfactants Deterg. 2019, 22, 1331–1342. [Google Scholar] [CrossRef]
- Jiang, N.; Sheng, Y.; Li, C.; Lu, S. Surface activity, foam properties and aggregation behavior of mixtures of short-chain fluorocarbon and hydrocarbon surfactants. J. Mol. Liq. 2018, 268, 249–255. [Google Scholar] [CrossRef]
- Ross, J.; Miles, G.D. Standard Test Method for Foaming Properties of Surface-Active Agents. ASTM Standard Method D 1173-53, Reapproved; KRÜSS Scientific: Hamburg, Germany, 2001. [Google Scholar] [CrossRef]
- Sheng, Y.; Wu, X.; Lu, S.; Li, C. Experimental study on foam properties of mixed systems of silicone and hydrocarbon surfactants. J. Surfactants Deterg. 2016, 19, 823–831. [Google Scholar] [CrossRef]
- Sheng, Y.; Can, B.; Yang, L.; Yun, P. Thermal Stability of Gel Foams Stabilized by Xanthan Gum, Silica Nanoparticles. Gels 2021, 7, 179. [Google Scholar] [CrossRef]


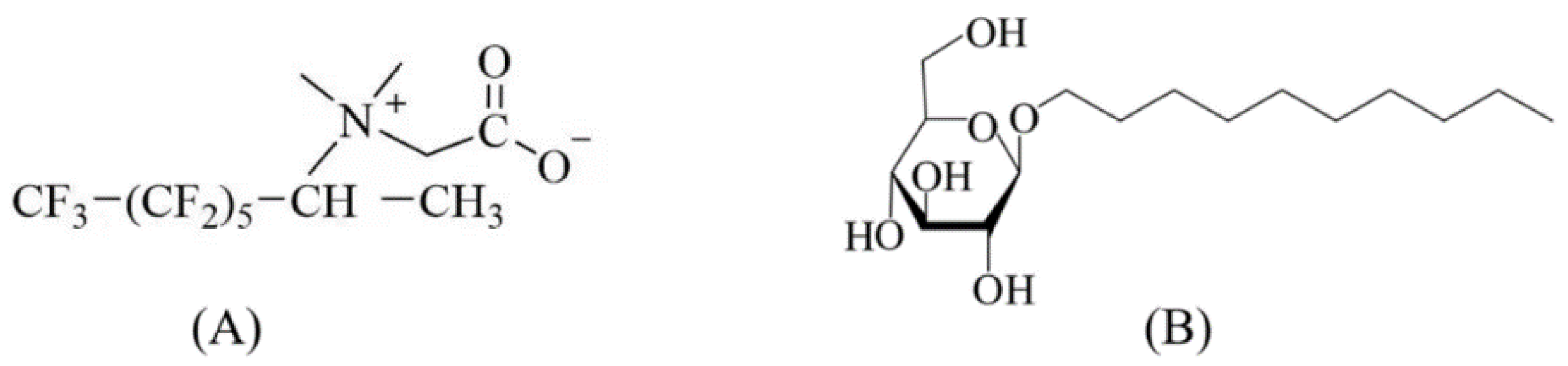
| Samples | APG0810 (%) | FS-50 (%) | NaCl (mM) | NPs (%) |
|---|---|---|---|---|
| A-0# | 0.5 | 0.25 | 1 | 0 |
| A-1# | 0.5 | 0.25 | 1 | 1 |
| A-2# | 0.5 | 0.25 | 1 | 3 |
| A-3# | 0.5 | 0.25 | 1 | 5 |
| A-4# | 0.5 | 0.25 | 1 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Peng, Y.; Zhang, S.; Guo, Y.; Ma, L.; Wang, Q.; Zhang, H. Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants. Gels 2022, 8, 123. https://doi.org/10.3390/gels8020123
Sheng Y, Peng Y, Zhang S, Guo Y, Ma L, Wang Q, Zhang H. Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants. Gels. 2022; 8(2):123. https://doi.org/10.3390/gels8020123
Chicago/Turabian StyleSheng, Youjie, Yunchuan Peng, Shanwen Zhang, Ying Guo, Li Ma, Qiuhong Wang, and Hanling Zhang. 2022. "Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants" Gels 8, no. 2: 123. https://doi.org/10.3390/gels8020123





