Physical Properties of Peanut and Soy Protein-Based Emulsion Gels Induced by Various Coagulants
Abstract
:1. Introduction
2. Results and Discussion
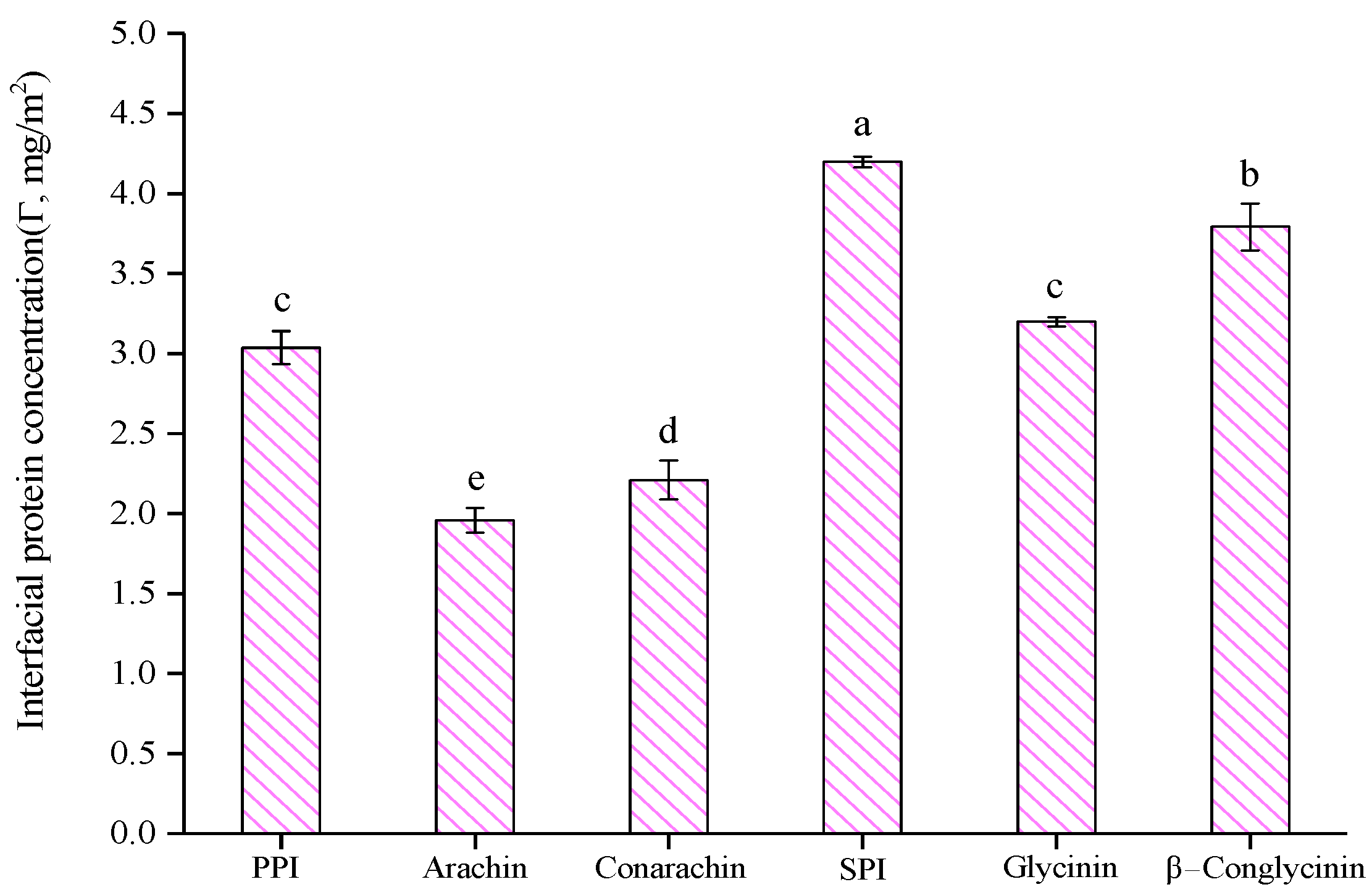
2.1. Emulsion Characteristics
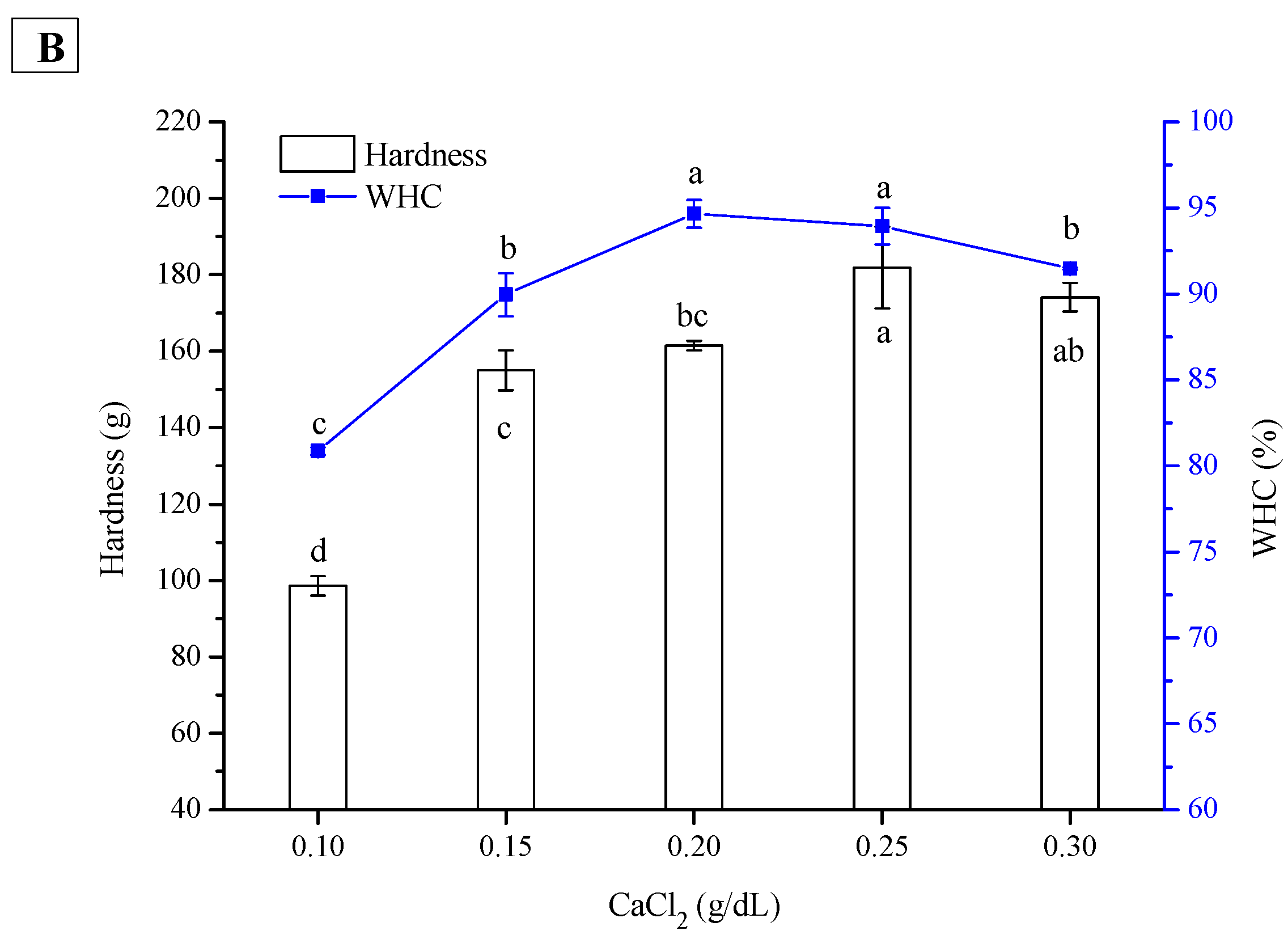
2.2. Effect of CaCl2 Concentration on Hardness and WHC of PPI and SPI Emulsion Gels
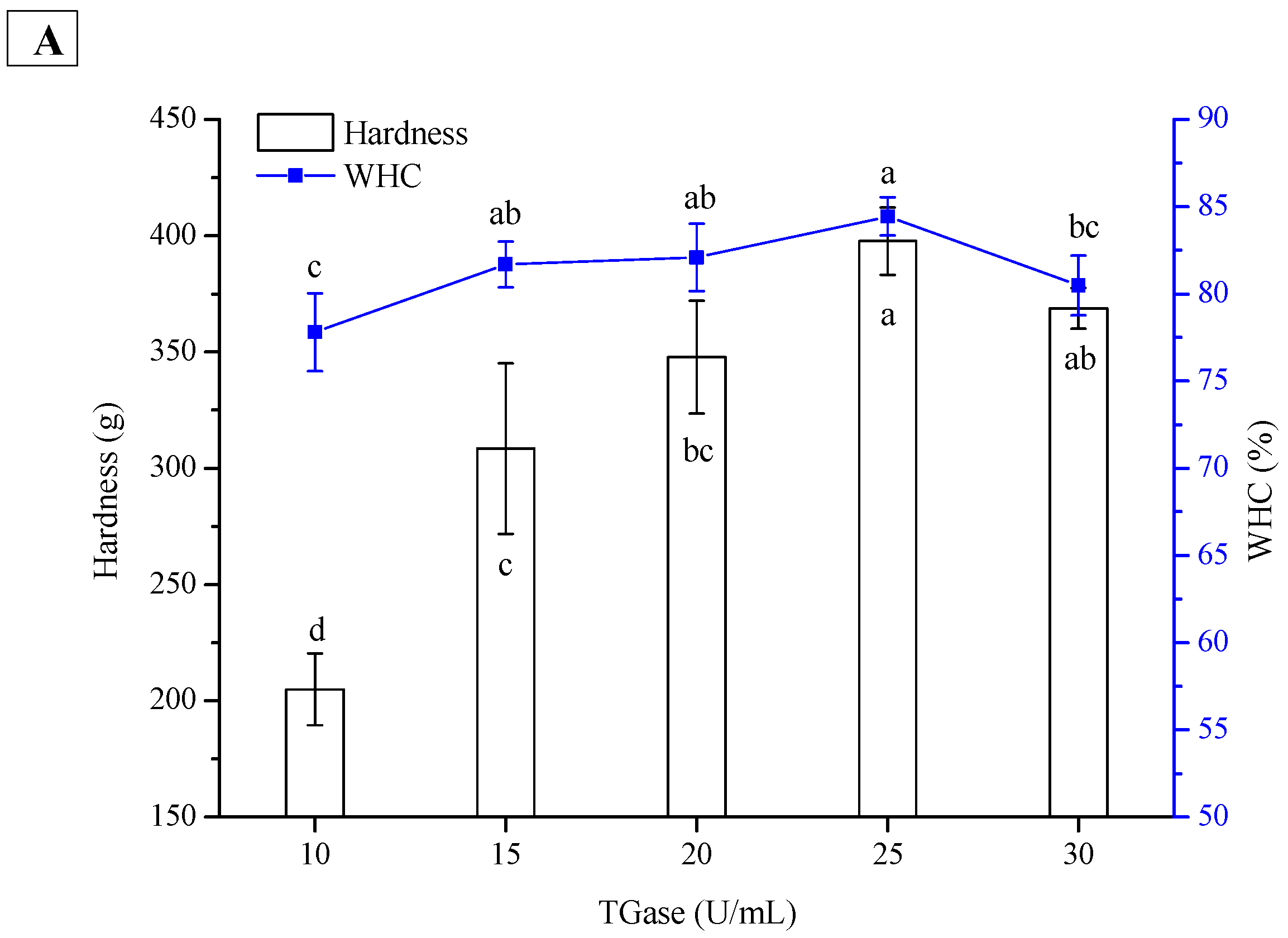
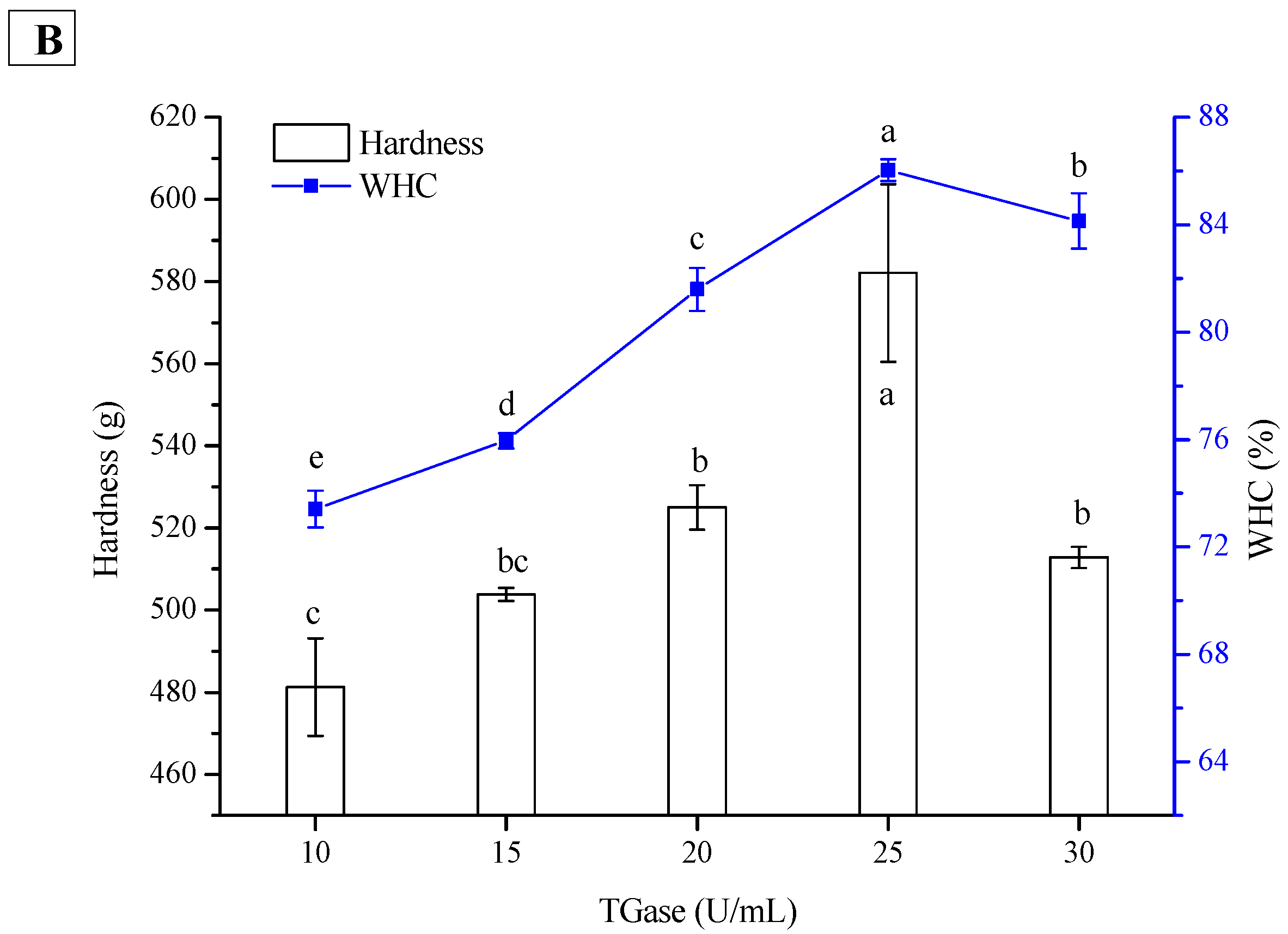
2.3. Effect of TGase Concentration on Hardness and WHC of PPI and SPI Emulsion Gels
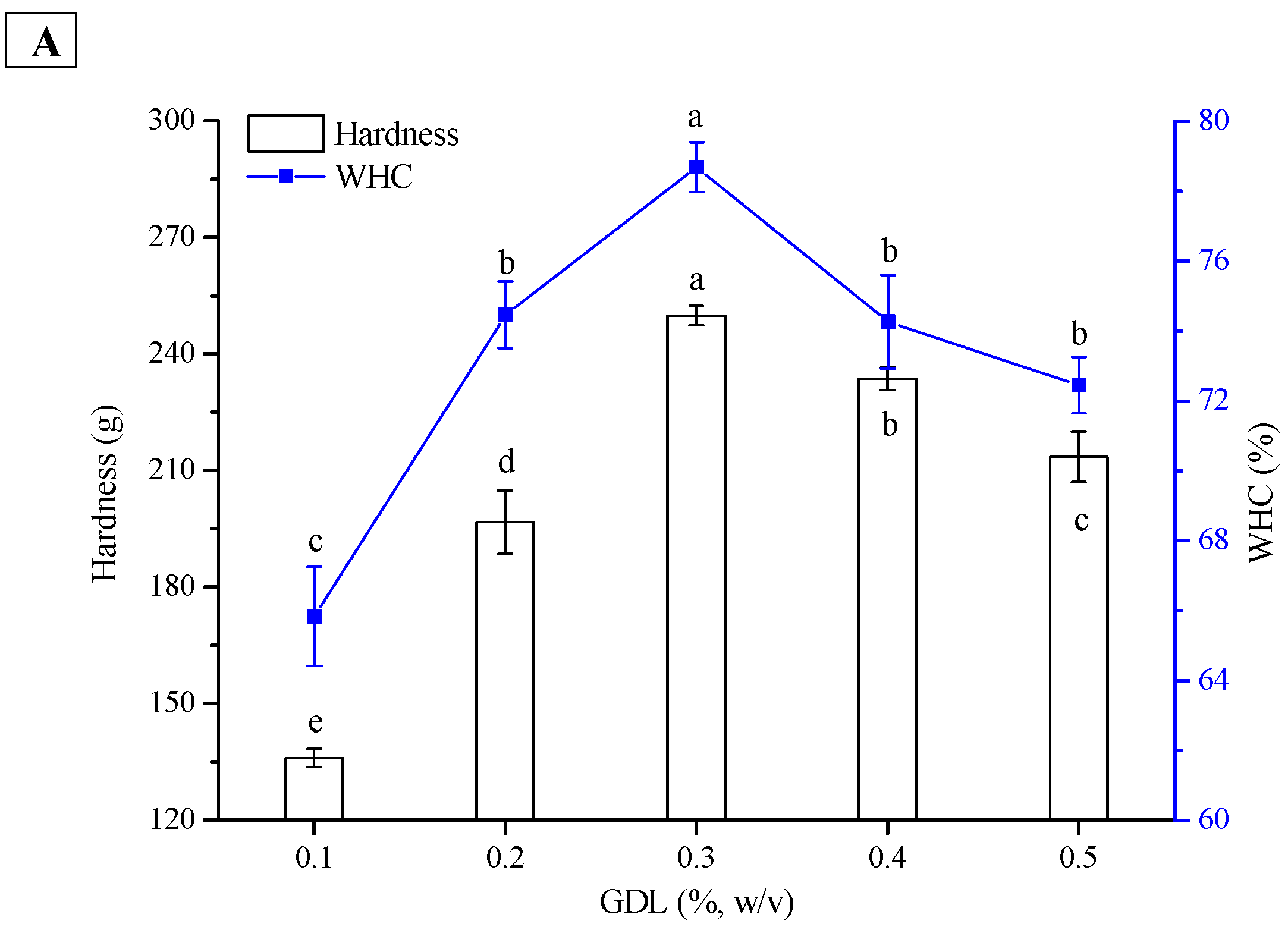
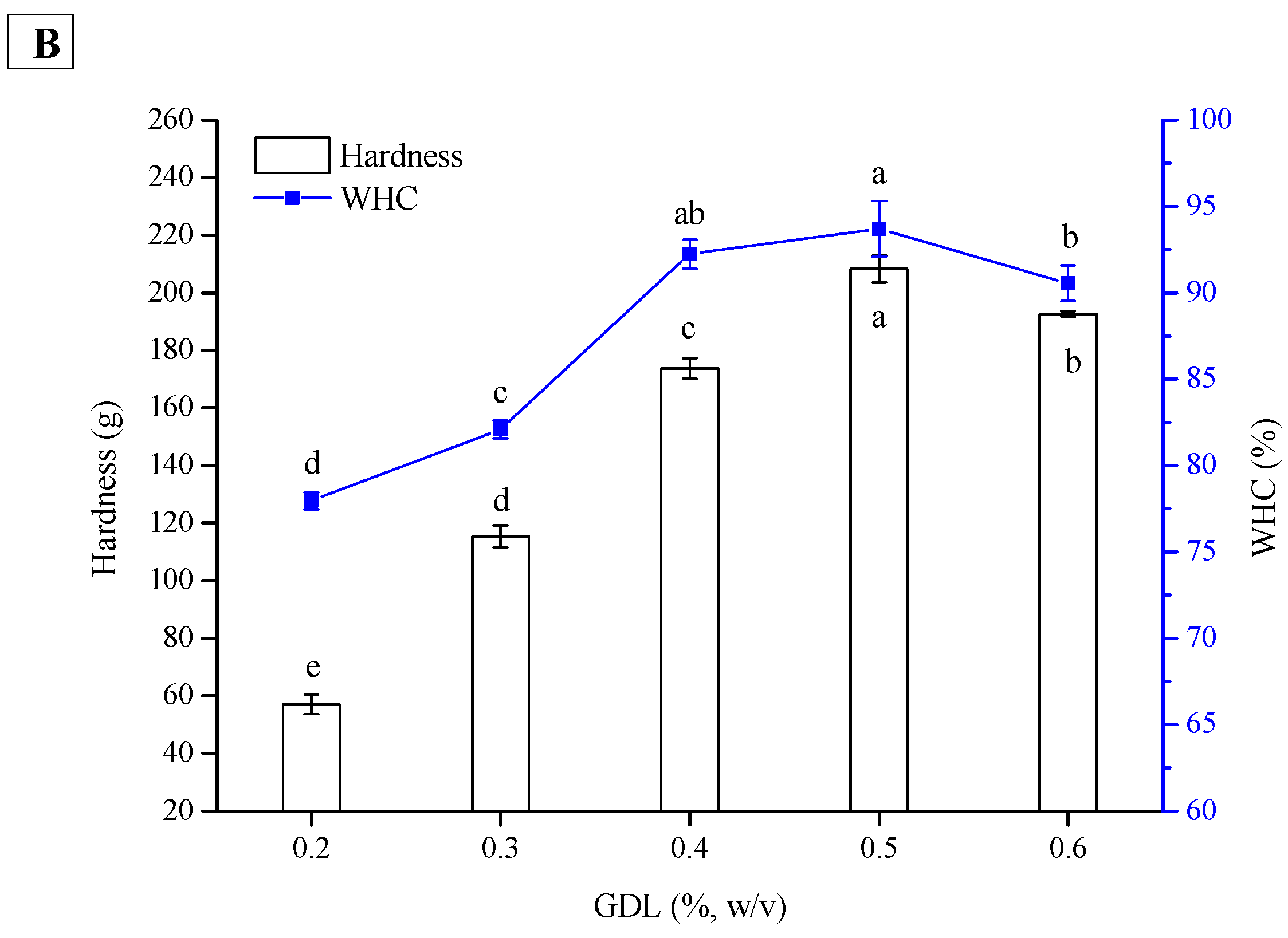
2.4. Effect of GDL Concentration on Hardness and WHC of PPI and SPI Emulsion Gels
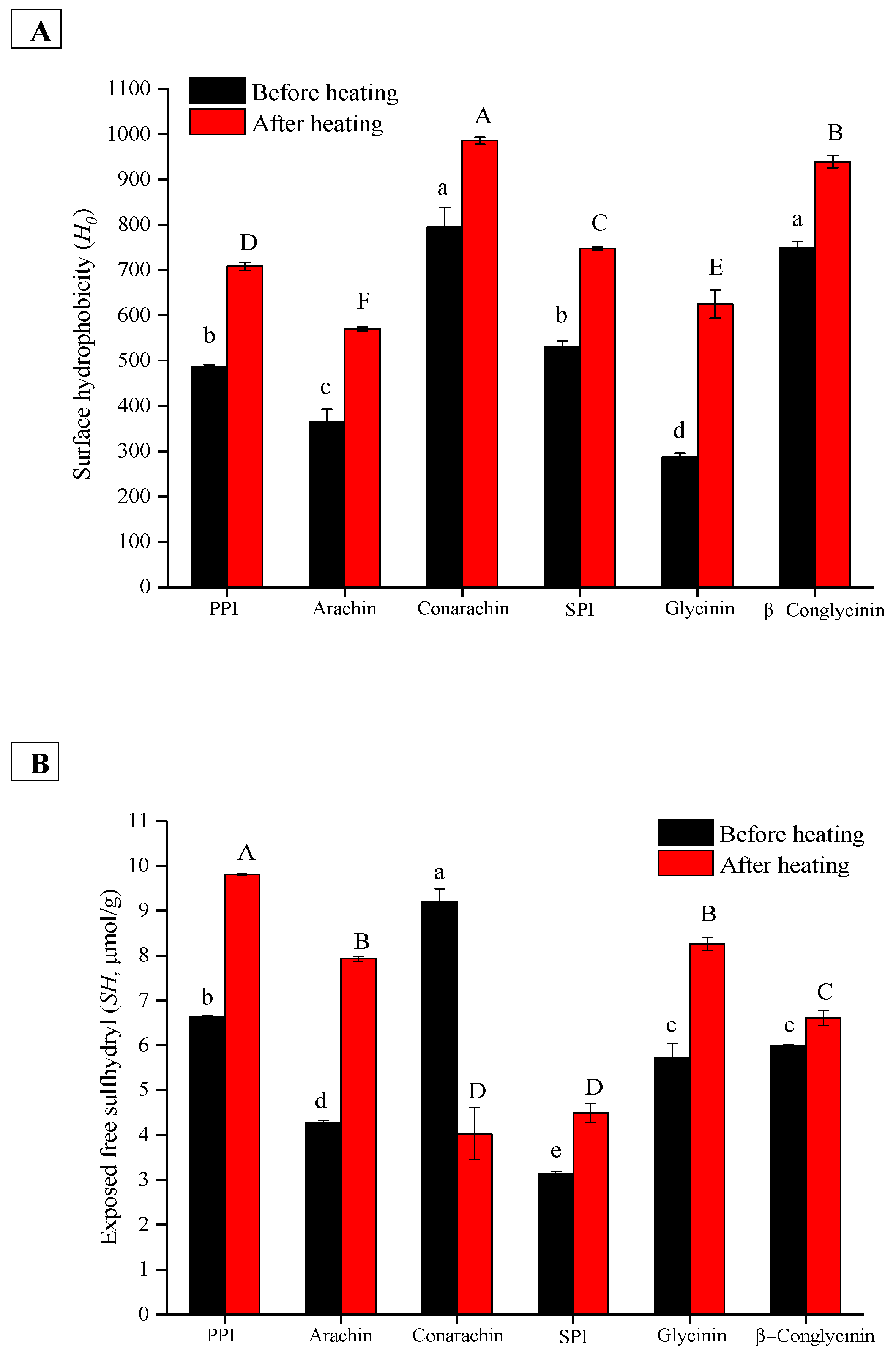
2.5. Textural Comparison of Protein-Stabilized Emulsion Gels Induced by Various Coagulants and Protein Structural Analysis
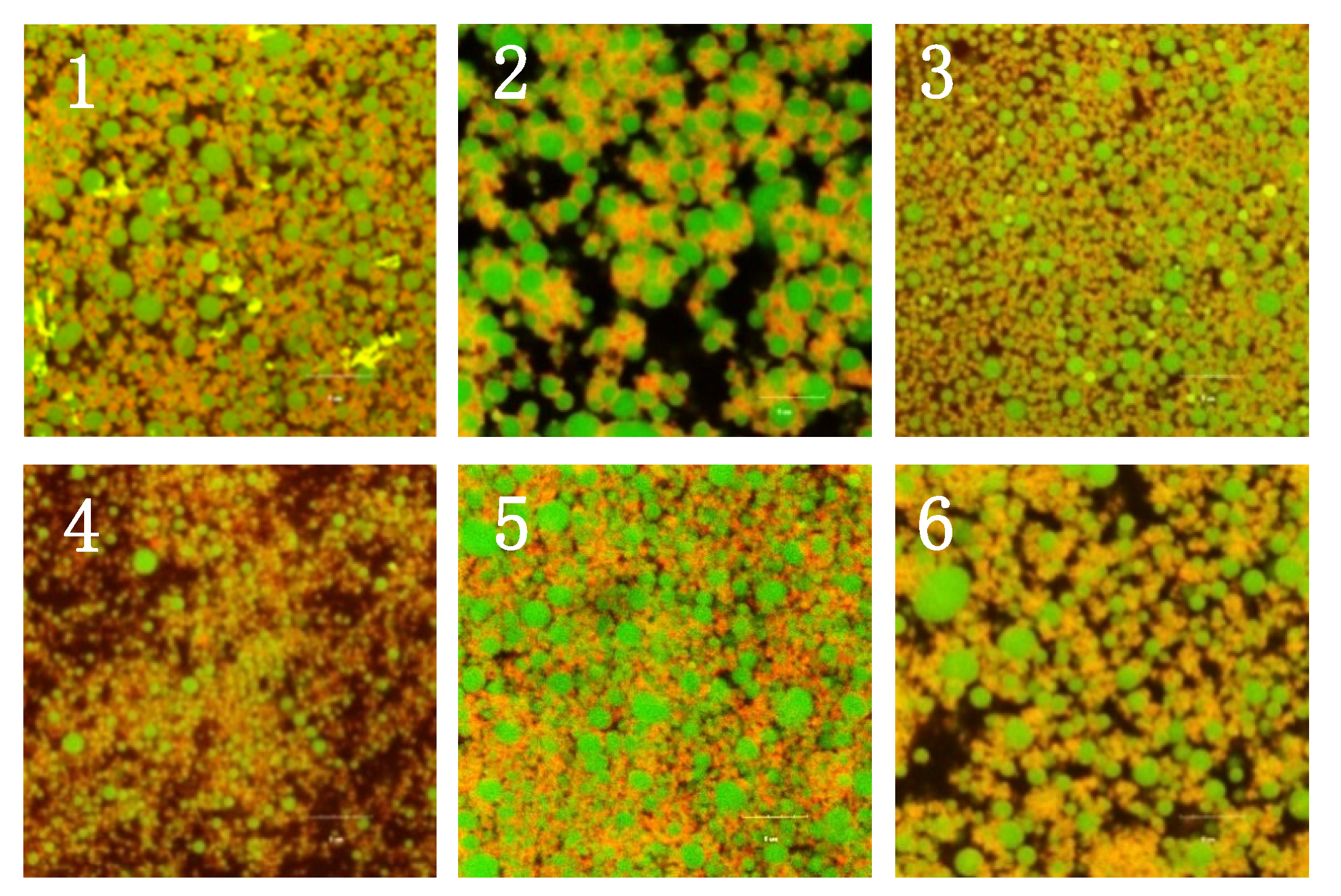
2.6. Microstructure of Different Protein-Stabilized Emulsion Gels Induced by GDL
2.7. Microstructure of Different Protein-Stabilized Emulsion Gels Induced by GDL
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Arachin and Conarachin from PPI
4.3. Extraction of Soy Protein Isolates (SPI), Glycinin and β−Conglycinin from Defatted Soybean Meal
4.4. Preparation of Different Protein-Stabilized Emulsions by Ultrasonic Method
4.5. Emulsion Characterization
4.6. Preparation of Emulsion Gels
4.7. Emulsion Gel Characterization
4.7.1. Dynamic Viscoelastic Measurement
4.7.2. Emulsion Gel Textural Analysis
4.7.3. Emulsion Gel Water-Holding Capacity (WHC)
4.7.4. Confocal Laser Scanning Microscope (CLSM)
4.8. Protein Structural Properties
4.8.1. Surface Hydrophobicity
4.8.2. Exposed Free SH Content
4.8.3. Amino Acid Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Li, X.M.; Meng, R.; Xu, B.C.; Zhang, B. Investigation of the fabrication, characterization, protective effect and digestive mechanism of a novel Pickering emulsion gels. Food Hydrocoll. 2021, 117, 106708. [Google Scholar] [CrossRef]
- Lin, D.Q.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Jiang, Y.S.; Zhang, S.B.; Zhang, S.Y.; Peng, Y.X. Comparative study of high-intensity ultrasound and high-pressure homogenization on physicochemical properties of peanut protein-stabilized emulsions and emulsion gels. J. Food Process Eng. 2021, 44, e13710. [Google Scholar] [CrossRef]
- Hu, S.; Wu, J.; Zhu, B.; Du, M.; Wu, C.; Yu, C.; Song, L.; Xu, X. Low oil emulsion gel stabilized by defatted Antarctic krill (Euphausia superba) protein using high-intensity ultrasound. Ultrason. Sonochemistry 2021, 70, 105294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Donkor, P.O.; Ren, X.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocoll. 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Paglarini, C.S.; Martini, S.; Pollonio, M.A.R. Physical properties of emulsion gels formulated with sonicated soy protein isolate. Int. J. Food Sci. Technol. 2019, 54, 451–459. [Google Scholar] [CrossRef]
- Tang, C.H.; Luo, L.J.; Liu, F.; Chen, Z. Transglutaminase-set soy globulin-stabilized emulsion gels: Influence of soy β−conglycinin/glycinin ratio on properties, microstructure and gelling mechanism. Food Res. Int. 2013, 51, 804–812. [Google Scholar] [CrossRef]
- Yan, C.; Fu, D.; McClements, D.J.; Xu, P.; Zou, L.; Zhu, Y.; Cheng, C.; Liu, W. Rheological and microstructural properties of cold-set emulsion gels fabricated from mixed proteins: Whey protein and lactoferrin. Food Res. Int. 2019, 119, 315–324. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.K.; Zheng, H.X.; Chen, H.Q.; Gao, Y.X. Characterization of beta-carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020, 102, 105600. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion gels with different proteins at the interface: Structures and delivery functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Q.; Liu, H.Z.; Wang, L.; Liu, L. Major protein fractions and subunit contents in peanut from different cultivars. Food Sci. 2013, 34, 42–46. (In Chinese) [Google Scholar]
- Yamada, T.; Aibara, S.; Morita, Y. Accumulation pattern of arachin and its subunits in maturation of groundnut seeds. Plant Cell Physiol. 1980, 21, 1217–1226. [Google Scholar] [CrossRef]
- Monteiro, P.V.; Prakash, V. Effect of proteases on arachin, conarachin I, and conarachin II from peanut (Arachis hypogaea L.). J. Agric. Food Chem. 1994, 42, 268–273. [Google Scholar] [CrossRef]
- Monteiro, P.V.; Prakash, V. Functional properties of homogeneous protein fractions from peanut (Arachis hypogaea L.). J. Agric. Food Chem. 1994, 42, 274–278. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Su, X. Functional and confomational properties of arachin and conarachin. Mod. Food Sci. Technol. 2013, 29, 2095–2101. (In Chinese) [Google Scholar]
- Feng, X.L.; Liu, H.Z.; Shi, A.M.; Liu, L.; Wang, Q.; Adhikari, B. Effects of transglutaminase catalyzed crosslinking on physicochemical characteristics of arachin and conarachin-rich peanut protein fractions. Food Res. Int. 2014, 62, 84–90. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, W.; Zhang, L.; Tian, S.; Chen, F. Molecular and emulsifying properties of arachin and conarachin of peanut protein isolate from ultrasound-assisted extraction. LWT-Food Sci. Technol. 2020, 132, 109790. [Google Scholar] [CrossRef]
- Hu, X.; Amakye, W.K.; He, P.; Wang, M.; Ren, J. Effects of microfluidization and transglutaminase cross-linking on the conformations and functional properties of arachin and conarachin in peanut. LWT-Food Sci. Technol. 2021, 146, 111438. [Google Scholar] [CrossRef]
- Puppo, M.C.; Speroni, F.; Chapleau, N.; de Lamballerie, M.; Anon, M.C.; Anton, M. Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocoll. 2005, 19, 289–296. [Google Scholar] [CrossRef]
- Sok Line, V.L.; Remondetto, G.E.; Subirade, M. Cold gelation of β-lactoglobulin oil-in-water emulsions. Food Hydrocoll. 2005, 19, 269–278. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef]
- Joseph, D.; Lanier, T.C.; Hamann, D.D. Temperature and pH affect transglutaminase-catalyzed setting of crude fish actomyosin. J. Food Sci. 1994, 59, 1018–1023. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Salami, M.; Mohammadian, M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020, 153, 523–532. [Google Scholar] [CrossRef]
- Ringgenberg, E.; Alexander, M.; Corredig, M. Effect of concentration and incubation temperature on the acid induced aggregation of soymilk. Food Hydrocoll. 2013, 30, 463–469. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Rheological studies on the gelation process of soybean 7S and 1 1S proteins in the presence of glucono-delta-lactone. J. Agric. Food Chem. 1993, 41, 8–14. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and water-holding properties and microstructure of soy protein isolate emulsion gels induced by CaCl2, glucono-d-lactone (GDL) and transglutaminase: Influence of thermal treatments before and/or after emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.S.; Sanchez, C.; Mellema, M.; Banon, S. Study of calcium−soy protein interactions by isothermal titration calorimetry and pH Cycle. J. Agric. Food Chem. 2009, 57, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Remondetto, G.E.; Gonzalez, R.; Subirade, M. Formation of soy protein isolate cold-set gels: Protein and salt effects. J. Food Sci. 2005, 70, C67–C73. [Google Scholar] [CrossRef]
- Dicksinson, E.; Yamamoto, Y. Rheology of milk protein gels and protein-stabilized emulsion gels cross-linked with transglutaminase. J. Agric. Food Chem. 1996, 44, 1371–1377. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological characteristics and gelation mechanism of tofu (soybean curd). J. Agric. Food Chem. 1995, 43, 1808–1812. [Google Scholar] [CrossRef]
- Nagano, T.; Hirotsuka, M.; Mori, H. Dynamic viscoelastic study on the gelatin of 7S globulin from soybeans. J. Agric. Food Chem. 1992, 40, 941–944. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lu, Q.Y. Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds. Food Hydrocoll. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Zhang, S.B.; Yan, D.Q.; Jiang, Y.S.; Ding, C.H. Competitive displacement of interfacial soy proteins by Tween 20 and its effect on the physical stability of emulsions. Food Hydrocoll. 2021, 113, 106515. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]




| PPI | Arachin | Conarachin | SPI | Glycinin | β−Conglycinin | |
|---|---|---|---|---|---|---|
| Asp | 12.55 ± 0.003 b | 13.12 ± 0.39 a | 12.56 ± 0.33 b | 12.36 ± 0.82 bc | 12.23 ± 0.13 cd | 12.10 ± 0.72 d |
| Thr | 2.49 ± 0.04 d | 2.18 ± 0.01 e | 2.19 ± 0.002 e | 3.25 ± 0.09 b | 3.58 ± 0.15 a | 2.8 ± 0.02 c |
| Ser | 4.77 ± 0.01 c | 4.71 ± 0.09 c | 4.68 ± 0.02 c | 5.01 ± 0.01 b | 4.99 ± 0.13 b | 5.23 ± 0.001 a |
| Glu | 21.61 ± 0.10 d | 22.64 ± 0.06 c | 23.48 ± 0.09 b | 21.81 ± 0.09 cd | 22.59 ± 0.55 c | 24.53 ± 0.03 a |
| Gly | 4.53 ± 0.04 a | 4.12 ± 0.01 c | 4.212 ± 0.01 bc | 4.12 ± 0.01 c | 4.34 ± 0.09 b | 3.31 ± 0.08 d |
| Ala | 4.02 ± 0.02 a | 4.01 ± 0.01 a | 3.14 ± 0.003 c | 3.66 ± 0.01 b | 3.70 ± 0.08 b | 3.26 ± 0.09 c |
| Cys | 0.50 ± 0.001 ab | 0.45 ± 0.05 b | 0.80 ± 0.01 a | 0.56 ± 0.11 ab | 0.57 ± 0.17 ab | 0.45 ± 0.05 b |
| Val | 4.53 ± 0.05 bc | 4.34 ± 0.01 c | 4.33 ± 0.04 c | 4.69 ± 0.03 b | 4.97 ± 0.14 a | 4.10 ± 0.02 d |
| Met | 1.27 ± 0.002 b | 0.77 ± 0.004 d | 2.47 ± 0.02 a | 1.14 ± 0.03 c | 1.2 ± 0.02 b | 0.81 ± 0.04 d |
| Ile | 3.74 ± 0.03 c | 3.57 ± 0.02 d | 3.81 ± 0.02 c | 4.83 ± 0.04 a | 4.53 ± 0.09 b | 4.75 ± 0.04 c |
| Leu | 6.89 ± 0.03 c | 6.86 ± 0.04 c | 5.95 ± 0.01 d | 7.40 ± 0.06 b | 7.47 ± 0.03 b | 7.68 ± 0.05 a |
| Tyr | 4.45 ± 0.001 b | 5.08 ± 0.01 a | 1.92 ± 0.01 e | 3.77 ± 0.01 c | 3.70 ± 0.06 c | 3.50 ± 0.002 d |
| Phe | 5.44 ± 0.01 b | 5.88 ± 0.02 a | 4.38 ± 0.02 d | 5.43 ± 0.03 b | 5.19 ± 0.10 c | 5.82 ± 0.03 a |
| His | 3.00 ± 0.01 b | 2.91 ± 0.02 b | 3.21 ± 0.03 a | 3.31 ± 0.0001 a | 2.96 ± 0.10 b | 2.91 ± 0.03 b |
| Lys | 3.32 ± 0.06 d | 2.29 ± 0.01 e | 5.02 ± 0.02 c | 5.98 ± 0.05 b | 5.00 ± 0.12 c | 6.26 ± 0.05 a |
| Arg | 12.57 ± 0.03 c | 13.05 ± 0.05 b | 13.98 ± 0.04 a | 7.71 ± 0.03 e | 7.60 ± 0.06 e | 8.09 ± 0.04 d |
| Pro | 4.34 ± 0.18 bc | 4.00 ± 0.32 c | 3.85 ± 0.021 c | 4.97 ± 0.022 ab | 5.33 ± 0.08 a | 4.31 ± 0.08 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Jiang, Y.; Zhang, S.; Chen, L. Physical Properties of Peanut and Soy Protein-Based Emulsion Gels Induced by Various Coagulants. Gels 2022, 8, 79. https://doi.org/10.3390/gels8020079
Zhang S, Jiang Y, Zhang S, Chen L. Physical Properties of Peanut and Soy Protein-Based Emulsion Gels Induced by Various Coagulants. Gels. 2022; 8(2):79. https://doi.org/10.3390/gels8020079
Chicago/Turabian StyleZhang, Shaobing, Yushan Jiang, Shuyan Zhang, and Lin Chen. 2022. "Physical Properties of Peanut and Soy Protein-Based Emulsion Gels Induced by Various Coagulants" Gels 8, no. 2: 79. https://doi.org/10.3390/gels8020079
APA StyleZhang, S., Jiang, Y., Zhang, S., & Chen, L. (2022). Physical Properties of Peanut and Soy Protein-Based Emulsion Gels Induced by Various Coagulants. Gels, 8(2), 79. https://doi.org/10.3390/gels8020079






