Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
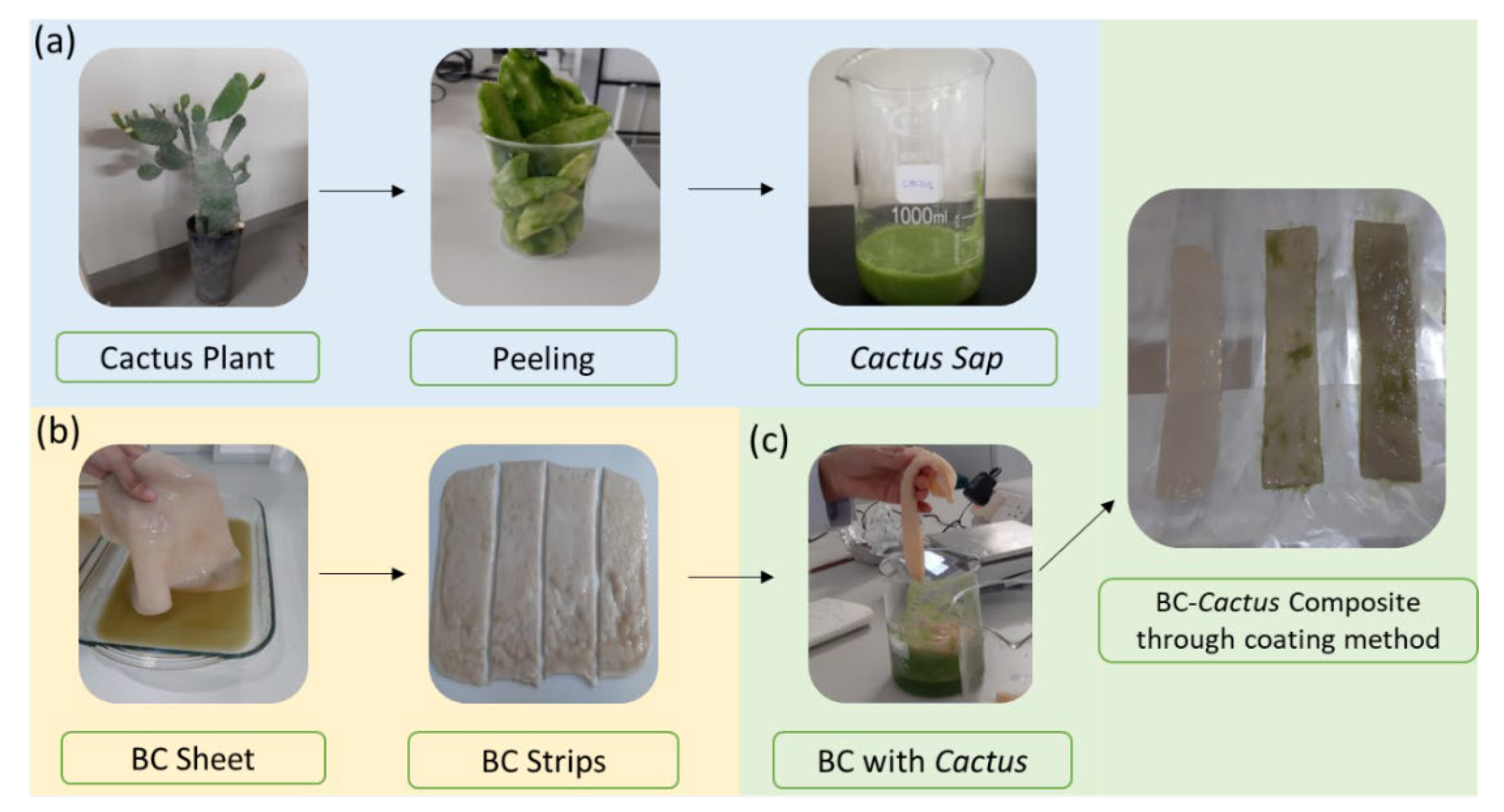
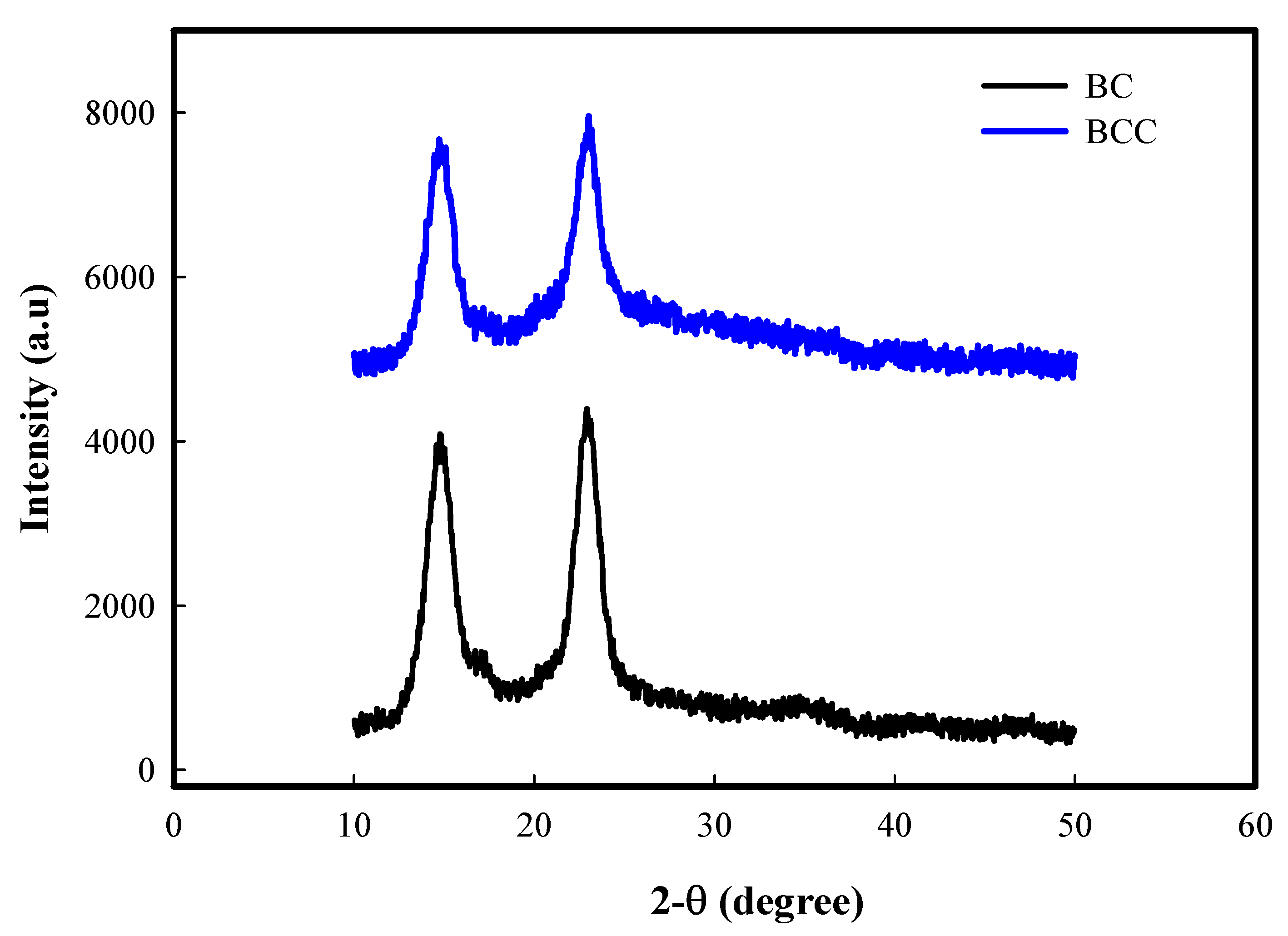
2.1. Composite Synthesis and Characterization
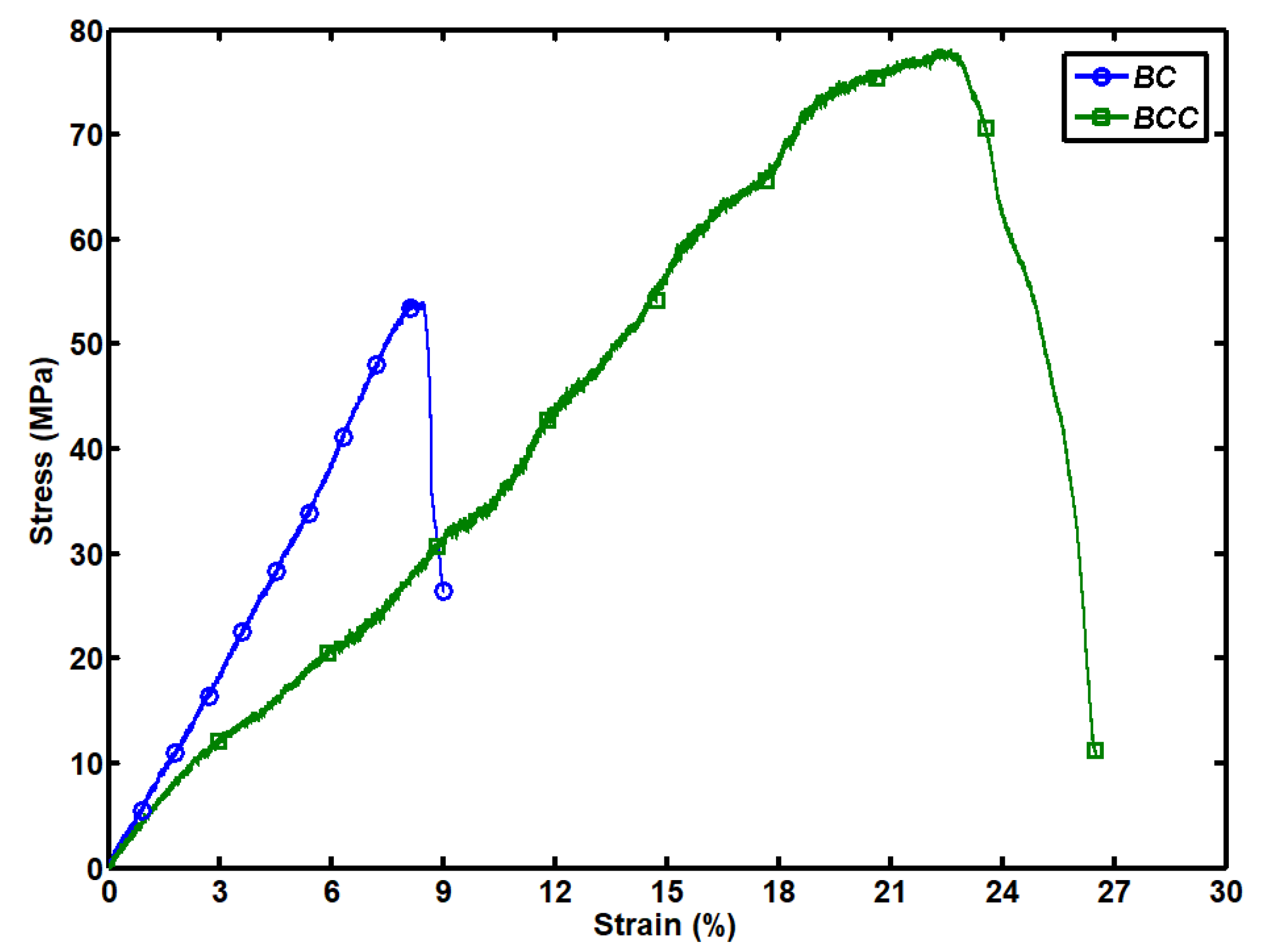
2.2. Mechanical Strength of BC and BCC Composite Hydrogel
2.3. Absorption Studies
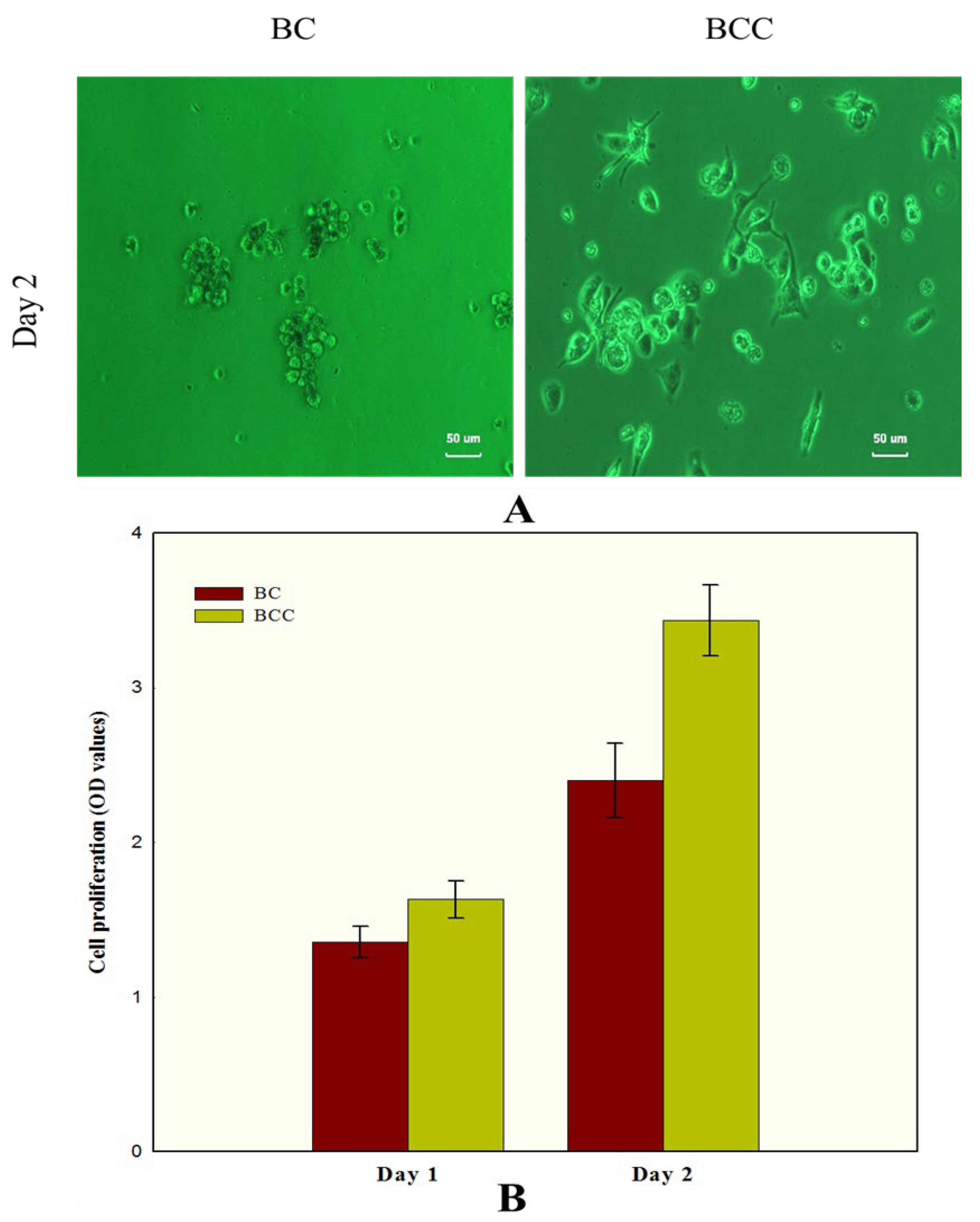
2.4. Biocompatibility of BCC Composite Hydrogel
3. Conclusions
4. Experimentation
4.1. Microbial Cell Culture and BC Production
4.2. Production of BCC
4.3. Characterization
4.4. Mechanical Testing
4.5. Water Holding and Release Experiments
4.6. Heavy Metal Absorption
4.7. Invitro-Biocompatibility Determination
4.8. Viability Assay of HaCaT
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, M.W.; Manan, S.; Kiprono, S.J.; Ul-Islam, M.; Yang, G. Synthesis, structure, and properties of bacterial cellulose. In Nanocellulose: From Fundamentals to Advanced Materials, 1st ed.; Huang, J., Dufresne, A., Lin, N., Eds.; Wiley VCH: Weinheim, Germany, 2019; pp. 81–113. [Google Scholar]
- Wajid Ullah, M.; Ul-Islam, M.; Khan, S.; Kim, Y.; Kon Park, J. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Ullah, M.W.; Islam, M.U.; Khan, S.; Shah, N.; Park, J.K. Recent advancements in bioreactions of cellular and cell-free systems: A study of bacterial cellulose as a model. Korean J. Chem. Eng. 2017, 34, 1591–1599. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Kamal, T.; Ul-Islam, S.; Shah, N.; Park, J.K. Recent advancement in cellulose based nanocomposite for addressing environmental challenges. Recent Pat. Nanotechnol. 2016, 10, 169–180. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: A review of recent advances for biomedical applications processing and properties of nanofibrous bacterial cellulose-containing polymer composites. Polym. Rev. 2019, 60, 144–170. [Google Scholar] [CrossRef]
- Li, S.; Jasim, A.; Zhao, W.; Fu, L.; Ullah, M.W.; Shi, Z.; Yang, G. Fabrication of pH-electroactive bacterial cellulose / polyaniline hydrogel for the development of a controlled drug release system. ES Mater. Manuf. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef]
- Bu, Y.; Cao, M.; Jiang, Y.; Gao, L.; Shi, Z.; Xiao, X.; Wang, M.; Yang, G.; Zhou, Y.; Shen, Y. Ultra-thin bacterial cellulose / poly (Ethylenedioxythiophene) nanofibers paper electrodes for all-solid-state flexible supercapacitors. Electrochim. Acta 2018, 271, 624–631. [Google Scholar] [CrossRef]
- Sáenz-Hernández, C.; Corrales-García, J.; Aquino-Pérez, G. Nopalitos, Mucilage, fiber, and cochineal. In Cacti: Biology and Uses; University of California Press: Berkeley, CA, USA, 2002. [Google Scholar]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia Spp. Mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Loro, J.F.; del Rio, I.; Perez-Santana, L. Preliminary studies of analgesic and anti-inflammatory properties of Opuntia dillenii aqueous extract. J. Ethnopharmacol. 1999, 67, 213–218. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Pichler, T.; Young, K.; Alcantar, N. Eliminating turbidity in drinking water using the mucilage of a common cactus. Water Supply 2012, 12, 179–186. [Google Scholar] [CrossRef]
- Chandra, S.; Eklund, L.; Villarreal, R.R. Use of cactus in mortars and concrete. Cem. Concr. Res. 1998, 28, 41–51. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ahmad, F.; Fatima, A.; Shah, N.; Yasir, S.; Ahmad, W.; Manan, S.; Ullah, M.W. Ex situ synthesis and characterization of high strength multipurpose bacterial cellulose-aloe vera hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 601988. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Gabr, M.H.; Elrahman, M.A.; Okubo, K.; Fujii, T. A study on mechanical properties of bacterial cellulose / epoxy reinforced by plain woven carbon fiber modified with liquid rubber. Compos. Part Appl. Sci. Manuf. 2010, 41, 1263–1271. [Google Scholar] [CrossRef]
- Indrarti, L.; Indriyati; Syampurwadi, A.; Pujiastuti, S. Physical and mechanical properties of modified bacterial cellulose composite films. AIP Conf. Proc. 2016, 1711, 050007. [Google Scholar] [CrossRef] [Green Version]
- Gindl-Altmutter, W.; Keckes, J. The structure and mechanical properties of spines from the cactus opuntia ficus-indica. BioResources 2012, 7, 1232–1237. [Google Scholar] [CrossRef]
- Kiangkitiwan, N.; Srikulkit, K. Preparation and properties of bacterial cellulose / graphene oxide composite films using dyeing method. Polym. Eng. Sci. 2021, 61, 1854–1863. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Kang, M.; Kim, S.M.; Khan, T.; Park, J.K. Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose 2013, 20, 253–263. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Corzo Salinas, D.R.; Sordelli, A.; Martínez, L.A.; Villoldo, G.; Bernal, C.; Pérez, M.S.; Cerrutti, P.; Foresti, M.L. Production of bacterial cellulose tubes for biomedical applications: Analysis of the effect of fermentation time on selected properties. Int. J. Biol. Macromol. 2021, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible pure ZnO nanoparticles-3D bacterial cellulose biointerfaces with antibacterial properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Sun, Y.; Zheng, Y.-D.; He, W.; Yang, Y.-Y.; Xie, Y.-J.; Feng, Z.-X.; Qiao, K. A Biocompatible bacterial cellulose / tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, T.; Ul-Islam, M.; Khan, S.B.; Bakhsh, E.M.; Chani, M.T.S. Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels. Gels 2022, 8, 88. https://doi.org/10.3390/gels8020088
Kamal T, Ul-Islam M, Khan SB, Bakhsh EM, Chani MTS. Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels. Gels. 2022; 8(2):88. https://doi.org/10.3390/gels8020088
Chicago/Turabian StyleKamal, Tahseen, Mazhar Ul-Islam, Sher Bahadar Khan, Esraa M. Bakhsh, and Muhammad Tariq Saeed Chani. 2022. "Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels" Gels 8, no. 2: 88. https://doi.org/10.3390/gels8020088
APA StyleKamal, T., Ul-Islam, M., Khan, S. B., Bakhsh, E. M., & Chani, M. T. S. (2022). Preparation, Characterization, and Biological Features of Cactus Coated Bacterial Cellulose Hydrogels. Gels, 8(2), 88. https://doi.org/10.3390/gels8020088





