Comparison of the Physicochemical Properties of Carboxymethyl Agar Synthesized by Microwave-Assisted and Conventional Methods
Abstract
1. Introduction
2. Results and Discussion
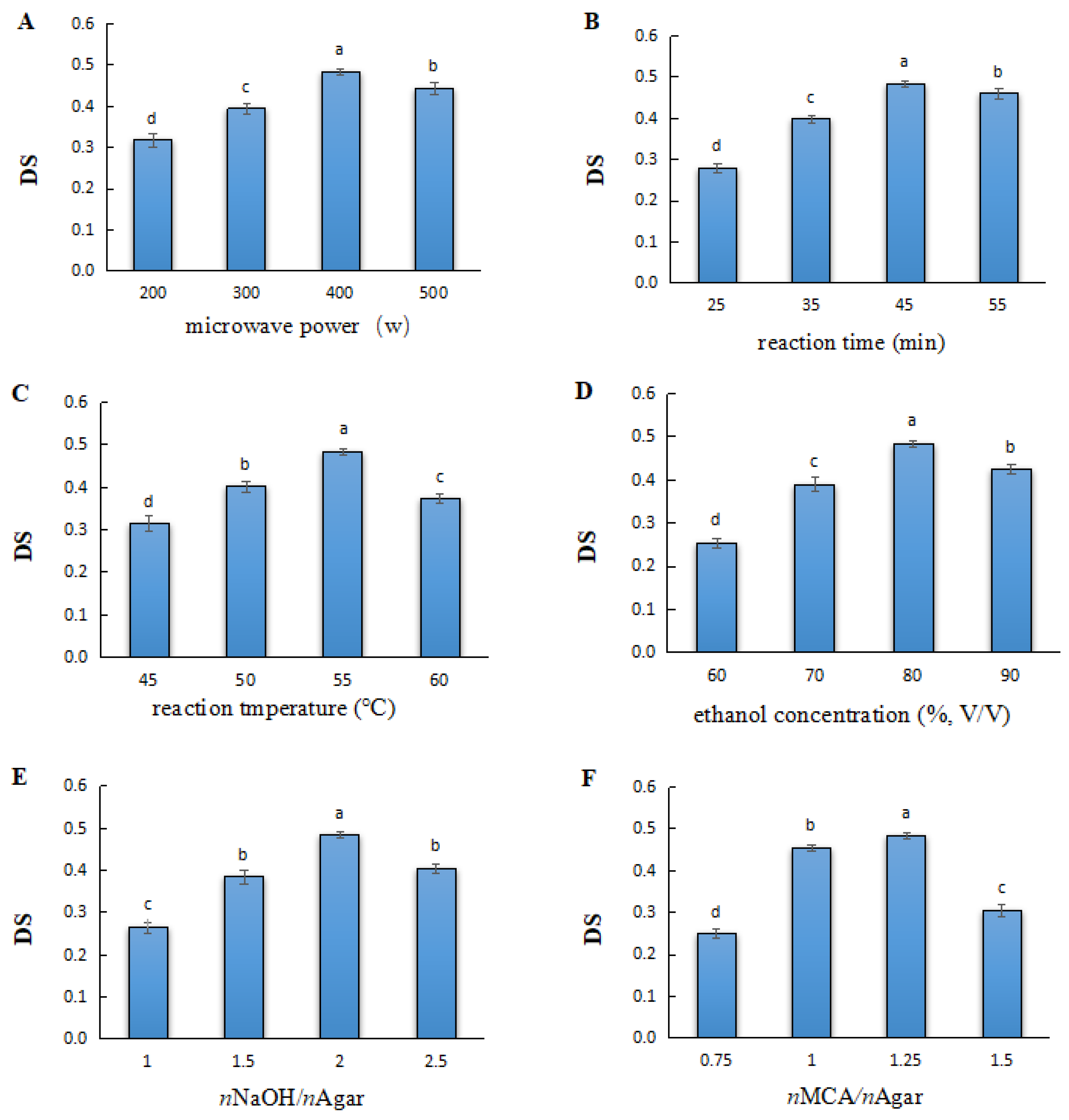
2.1. Effect of the Main Experimental Variables in the Microwave-Assisted Method on the DS of Carboxymethylated Agar
2.1.1. Microwave Power
2.1.2. Reaction Time
2.1.3. Reaction Temperature
2.1.4. Ethanol Concentration
2.1.5. Amount of NaOH
2.1.6. Amount of MCA
2.2. Effect of the Synthetic Method on the Solubility and Gel Properties of Carboxymethyl Agar Having Different DS Values
2.3. Effect of the Synthetic Method on the Color and Transparency of Carboxymethyl Agars with Different DS Values
2.4. Effect of the Synthetic Method on the Textural Properties of Carboxymethyl Agar with Different DS
2.5. Effect of the Synthetic Method on the Molecular Structure of Carboxymethyl Agar with Different DS Values
2.6. Effect of the Synthetic Method on the Zeta Potential and Particle Size of Carboxymethyl Agar with Different DS Values
2.7. Effect of the Synthetic Method on the Molecular Weight of Carboxymethyl Agar with Different DS Values
2.8. Effect of the Synthetic Method on the Surface Morphology of Carboxymethyl Agar Having Different DS Values
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Single-Factor Tests of the Microwave-Assisted Synthesis of Carboxymethyl Agar
4.3. Synthesis of Carboxymethyl Agar with Different DS Values Using Microwave-Assisted and Conventional Methods
4.4. Determination of DS
4.5. Determination of the Physicochemical Properties of Carboxymethyl Agar
4.6. Fourier Transform Infrared (FT-IR) Spectroscopy
4.7. Determination of the Zeta Potential and Particle Size
4.8. Molecular Weight Determination
4.9. Morphological Structure
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Armisén, R. Agar and agarose biotechnological applications. Hydrobiologia 1991, 221, 157–166. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Shen, Z.P.; Mu, H.M.; Lin, Y.; Zhang, J.L.; Jiang, X.L. Impact of alkali pretreatment on yield, physico-chemical and gelling properties of high quality agar from Gracilaria tenuistipitata. Food Hydrocoll. 2017, 70, 356–362. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.L.; Ye, J.; Zhao, P.; Xiao, M.T. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Xia, K.; Liu, X.; Zhao, J.K.; Zhang, X.D. The physicochemical property characterization of agar acetate. Carbohydr. Polym. 2014, 110, 32–37. [Google Scholar] [CrossRef]
- Belay, M.; Tyeb, S.; Rathore, K.; Kumar, M.; Verma, V. Synergistic effect of bacterial cellulose reinforcement and succinic acid crosslinking on the properties of agar. Int. J. Biol. Macromol. 2020, 165, 3115–3122. [Google Scholar] [CrossRef]
- Zhang, C.; An, D.; Xiao, Q.; Weng, H.F.; Zhang, Y.H.; Yang, Q.M.; Xiao, A. Preparation, characterization, and modification mechanism of agar treated with hydrogen peroxide at different temperatures. Food Hydrocoll. 2020, 101, 105527. [Google Scholar] [CrossRef]
- Xiao, Q.; An, D.; Zhang, C.; Weng, H.F.; Zhang, Y.H.; Chen, F.Q.; Xiao, A. Agar quality promotion prepared by desulfation with hydrogen peroxide. Int. J. Biol. Macromol. 2020, 145, 492–499. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.F.; Chen, G.; Xiao, A.F. Preparation and characterization of octenyl succinic anhydride modified agarose derivative. Food Chem. 2019, 279, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.Z.; Liu, X.; Luan, J.M.; Zhang, X.D. Characterization of physicochemical properties of carboxymethyl agar. Carbohydr. Polym. 2014, 111, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Yang, S.L.; Qi, B.; Yang, X.Q.; Li, C.S.; Ma, H.X.; Hu, X. Effect of three modification methods on physicochemical properties of agar. S. China Fish. Sci. 2021, 17, 97–103. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Nath, L.K.; Guha, M.; Kumar, R. Microwave assisted synthesis and evaluation of cross-linked carboxymethylated sago starch as superdisintegrant. Pharmacol. Pharm. 2011, 2, 42–46. [Google Scholar] [CrossRef][Green Version]
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of microwave treatment (low power and varying time) on potato starch: Microstructure, thermo-functional, pasting and rheological properties. Int. J. Biol. Macromol. 2020, 155, 27–35. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Tosun, N.G.; Özgür, A.; Tayhan, S.E.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641. [Google Scholar] [CrossRef]
- Hou, C.M.; Chen, Y.F.; Chen, W.N.; Li, W. Microwave-assisted methylation of cassava starch with dimethyl carbonate. Carbohydr. Res. 2011, 346, 1178–1181. [Google Scholar] [CrossRef]
- Liu, J.; Ming, J.; Li, W.J.; Zhao, G.H. Synthesis, characterisation and in vitro digestibility of carboxymethyl potato starch rapidly prepared with microwave-assistance. Food Chem. 2012, 133, 1196–1205. [Google Scholar] [CrossRef]
- Balsamo, V.; López-Carrasquero, F.; Laredo, E.; Conto, K.; Contreras, J.; Feijoo, J.L. Preparation and thermal stability of carboxymethyl starch/quaternary ammonium salts complexes. Carbohydr. Polym. 2011, 83, 1680–1689. [Google Scholar] [CrossRef]
- Silva, D.A.; de Paula, R.C.M.; Feitosa, J.P.A.; de Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Fan, L.H.; Wang, L.B.; Gao, S.; Wu, P.H.; Li, M.J.; Xie, W.G.; Liu, S.H.; Wang, W. Synthesis, characterization and properties of carboxymethyl kappa carrageenan. Carbohydr. Polym. 2011, 86, 1167–1174. [Google Scholar] [CrossRef]
- Milotskyi, R.; Bliard, C.; Tusseau, D.; Benoit, C. Starch carboxymethylation by Reactive Extrusion: Reaction kinetics and Structure Analysis. Carbohydr. Polym. 2018, 194, 193–199. [Google Scholar] [CrossRef]
- Lawal, O.S.; Lechner, M.D.; Kulicke, W.M. Single and multi-step carboxymethylation of water yam (Dioscorea alata) starch: Synthesis and characterization. Int. J. Biol. Macromol. 2008, 42, 429–435. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Fiedorowicz, M.; Opalińska-Piskorz, J.; Tylingo, R. Rheology of potato starch chemically modified with microwave-assisted reactions. LWT–Food Sci. Technol. 2013, 53, 249–254. [Google Scholar] [CrossRef]
- An, D.; Xiao, Q.; Zhang, C.; Cai, M.H.; Zhang, Y.H.; Weng, H.F.; Chen, F.Q.; Xiao, A. Preparation, characterization, and application of high-whiteness agar bleached with hydrogen peroxide. Food Hydrocoll. 2021, 113, 106520. [Google Scholar] [CrossRef]
- Du, J.H.; Li, T.; Ni, H.; Jiang, Z.D.; Xiao, A.F.; Zhu, Y.B. Preparation of Biochemical Agar Using Gracilaria Agar as Raw Material (in Chinese). Chin. J. Process Eng. 2018, 18, 434–440. [Google Scholar] [CrossRef]
- Kim, H.; Ryoo, S.W. Exploitation of Culture Medium for Mycobacterium tuberculosis. J. Bacteriol. Virol. 2011, 41, 237–244. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-forming and self-healing agarose-based hydrogels for tissue adhesives and potential wound dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Soto-Reyes, N.; Temis-Pérez, A.L.; López-Malo, A.; Rojas-Laguna, R.; Sosa-Morales, M.E. Effects of shape and size of agar gels on heating uniformity during pulsed microwave treatment. J. Food Sci. 2015, 80, E1021–E1025. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Maurya, S. Microwave synthesis, characterization, and zinc uptake studies of starch-graft-poly (ethyl acrylate). Int. J. Biol. Macromol. 2010, 47, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, K.Y.; Hu, X.T.; Jin, Z.Y.; Miao, M. Structure, properties and potential applications of phytoglycogen and waxy starch subjected to carboxymethylation. Carbohydr. Polym. 2020, 234, 115908. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Pan, S.Y.; Hu, H.; Miao, W.H.; Xu, X.Y. Synthesis and properties of carboxymethyl kudzu root starch. Carbohydr. Polym. 2010, 80, 174–179. [Google Scholar] [CrossRef]
- Lee, W.K.; Namasivayam, P.; Ho, C. Effects of sulfate starvation on agar polysaccharides of Gracilaria species (Gracilariaceae, Rhodophyta) from Morib, Malaysia. J. Appl. Phycol. 2014, 26, 1791–1799. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.F.; Ni, H.; Hong, Q.L.; Lin, K.H.; Xiao, A.F. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019, 87, 260–305. [Google Scholar] [CrossRef]
- Gope, S.; Samyor, D.; Paul, A.K.; Das, A.B. Effect of alcohol-acid modification on physicochemical, rheological and morphological properties of glutinous rice starch. Int. J. Biol. Macromol. 2016, 93, 860–867. [Google Scholar] [CrossRef]
- Crudden, A.; Afoufa-Bastien, D.; Fox, P.F.; Brisson, G.; Kelly, A.L. Effect of hydrolysis of casein by plasmin on the heat stability of milk. Int. Dairy J. 2005, 15, 1017–1025. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Sindhu, R.V.; Sukanya, S.; Aiswarya, N.; Ponnusami, V. Statistical studies on high molecular weight pullulan production in solid state fermentation using jack fruit seed. Carbohydr. Polym. 2013, 98, 854–860. [Google Scholar] [CrossRef]




| Samples | Dissolving Temperature (°C) | Gelling Temperature (°C) | Gel Melting Temperature (°C) | Gel Strength (g cm−2) |
|---|---|---|---|---|
| Original agar | 97.6 ± 1.3 a | 38.7 ± 0.7 a | 94.7 ± 0.8 a | 1145 ± 28 a |
| CAg1 | 77.3 ± 1.8 b | 36.4 ± 0.5 b | 68.2 ± 0.4 b | 446 ± 23 c |
| CAg2 | 64.5 ± 0.9 c | 30.7 ± 0.5 c | 59.6 ± 0.5 d | 128 ± 8 e |
| MAg1 | 78.3 ± 1.1 b | 37.1 ± 0.4 b | 69.5 ± 0.7 b | 545 ± 19 b |
| MAg2 | 65.7 ± 0.6 c | 31.3 ± 0.7 c | 60.3 ± 1.1 d | 152 ± 5 d |
| Samples | L* | a* | b* | WH | T (%) |
|---|---|---|---|---|---|
| Original agar | 87.98 ± 0.23 a | 0.72 ± 0.03 e | 16.45 ± 0.11 e | 79.61 ± 0.7 a | 35.6 ± 0.9 c |
| CAg1 | 86.23 ± 0.42 b | 1.65 ± 0.06 c | 17.63 ± 0.23 d | 77.56 ± 0.9 b | 82.1 ± 1.4 b |
| CAg2 | 84.11 ± 0.33 c | 2.71 ± 0.08 a | 19.16 ± 0.14 b | 74.96 ± 0.4 c | 93.3 ± 1.9 a |
| MAg1 | 86.68 ± 0.16 b | 1.48 ± 0.10 d | 17.95 ± 0.07 c | 77.60 ± 0.4 b | 81.5 ± 0.5 b |
| MAg2 | 84.65 ± 0.21 c | 2.53 ± 0.06 b | 19.68 ± 0.17 a | 74.91 ± 0.6 c | 94.1 ± 1.3 a |
| Samples | Hardness (g) | Cohesiveness | Springiness (mm) | Chewiness (mJ) |
|---|---|---|---|---|
| Original agar | 1532.67 ± 34.05 a | 0.59 ± 0.05 d | 3.81 ± 0.08 c | 34.94 ± 1.87 a |
| CAg1 | 1143.25 ± 55.51 b | 0.69 ± 0.02 c | 4.11 ± 0.11 b | 31.77 ± 3.39 c |
| CAg2 | 282.50 ± 10.21 d | 0.67 ± 0.08 c | 4.32 ± 0.07 a | 9.73 ± 0.25 d |
| MAg1 | 1000.67 ± 23.03 c | 0.80 ± 0.05 b | 4.32 ± 0.08 a | 32.46 ± 4.48 b |
| MAg2 | 263.83 ± 33.13 d | 0.89 ± 0.01 a | 4.35 ± 0.1 a | 9.99 ± 1.27 d |
| Samples | Mw (g·mol−1) | Mn (g·mol−1) | Mw/Mn |
|---|---|---|---|
| Original agar | 4.498 × 104 | 1.735 × 104 | 2.593 |
| CAg1 | 9.561 × 103 | 5.837 × 103 | 1.638 |
| CAg2 | 2.506 × 103 | 2.104 × 103 | 1.191 |
| MAg1 | 1.542 × 104 | 1.236 × 104 | 1.248 |
| MAg2 | 7.463 × 103 | 5.193 × 103 | 1.437 |
| No. | Microwave Power (W) | Temperature (°C) | Time (min) | Ethanol Concentration (%) | nNaOH/nAgar | nMCA/nAgar |
|---|---|---|---|---|---|---|
| 1 | 500 | 55 | 45 | 80 | 2.0 | 1.00 |
| 2 | 400 | 55 | 45 | 80 | 2.0 | 1.00 |
| 3 | 300 | 55 | 45 | 80 | 2.0 | 1.00 |
| 4 | 200 | 55 | 45 | 80 | 2.0 | 1.00 |
| 5 | 400 | 60 | 45 | 80 | 2.0 | 1.00 |
| 6 | 400 | 50 | 45 | 80 | 2.0 | 1.00 |
| 7 | 400 | 45 | 45 | 80 | 2.0 | 1.00 |
| 8 | 400 | 55 | 55 | 80 | 2.0 | 1.00 |
| 9 | 400 | 55 | 35 | 80 | 2.0 | 1.00 |
| 10 | 400 | 55 | 25 | 80 | 2.0 | 1.00 |
| 11 | 400 | 55 | 45 | 60 | 2.0 | 1.00 |
| 12 | 400 | 55 | 45 | 70 | 2.0 | 1.00 |
| 13 | 400 | 55 | 45 | 90 | 2.0 | 1.00 |
| 14 | 400 | 55 | 45 | 80 | 2.5 | 1.00 |
| 15 | 400 | 55 | 45 | 80 | 1.5 | 1.00 |
| 16 | 400 | 55 | 45 | 80 | 1.0 | 1.00 |
| 17 | 400 | 55 | 45 | 80 | 2.0 | 1.5 |
| 18 | 400 | 55 | 45 | 80 | 2.0 | 1.25 |
| 19 | 400 | 55 | 45 | 80 | 2.0 | 0.75 |
| Sample | Microwave Power (W) | Time (min) | Temperature (°C) | DS |
|---|---|---|---|---|
| CAg1 | − | 45 | 55 | 0.25 |
| CAg2 | − | 240 | 55 | 0.47 |
| MAg1 | 400 | 25 | 55 | 0.25 |
| MAg2 | 400 | 45 | 55 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, B.; Yang, S.; Zhao, Y.; Wang, Y.; Yang, X.; Chen, S.; Wu, Y.; Pan, C.; Hu, X.; Li, C.; et al. Comparison of the Physicochemical Properties of Carboxymethyl Agar Synthesized by Microwave-Assisted and Conventional Methods. Gels 2022, 8, 162. https://doi.org/10.3390/gels8030162
Qi B, Yang S, Zhao Y, Wang Y, Yang X, Chen S, Wu Y, Pan C, Hu X, Li C, et al. Comparison of the Physicochemical Properties of Carboxymethyl Agar Synthesized by Microwave-Assisted and Conventional Methods. Gels. 2022; 8(3):162. https://doi.org/10.3390/gels8030162
Chicago/Turabian StyleQi, Bo, Shaoling Yang, Yongqiang Zhao, Yueqi Wang, Xianqing Yang, Shengjun Chen, Yanyan Wu, Chuang Pan, Xiao Hu, Chunsheng Li, and et al. 2022. "Comparison of the Physicochemical Properties of Carboxymethyl Agar Synthesized by Microwave-Assisted and Conventional Methods" Gels 8, no. 3: 162. https://doi.org/10.3390/gels8030162
APA StyleQi, B., Yang, S., Zhao, Y., Wang, Y., Yang, X., Chen, S., Wu, Y., Pan, C., Hu, X., Li, C., & Wang, L. (2022). Comparison of the Physicochemical Properties of Carboxymethyl Agar Synthesized by Microwave-Assisted and Conventional Methods. Gels, 8(3), 162. https://doi.org/10.3390/gels8030162










