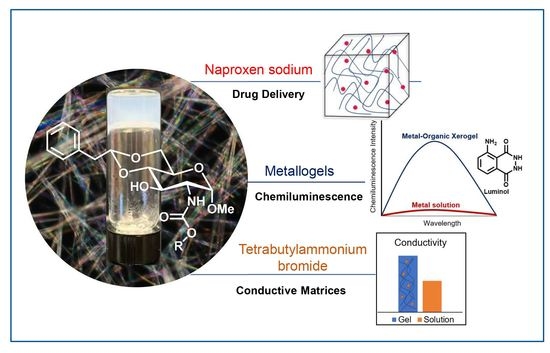
4,6-O-Phenylethylidene Acetal Protected D-Glucosamine Carbamate-Based Gelators and Their Applications for Multi-Component Gels
Abstract
:1. Introduction
2. Results and Discussion
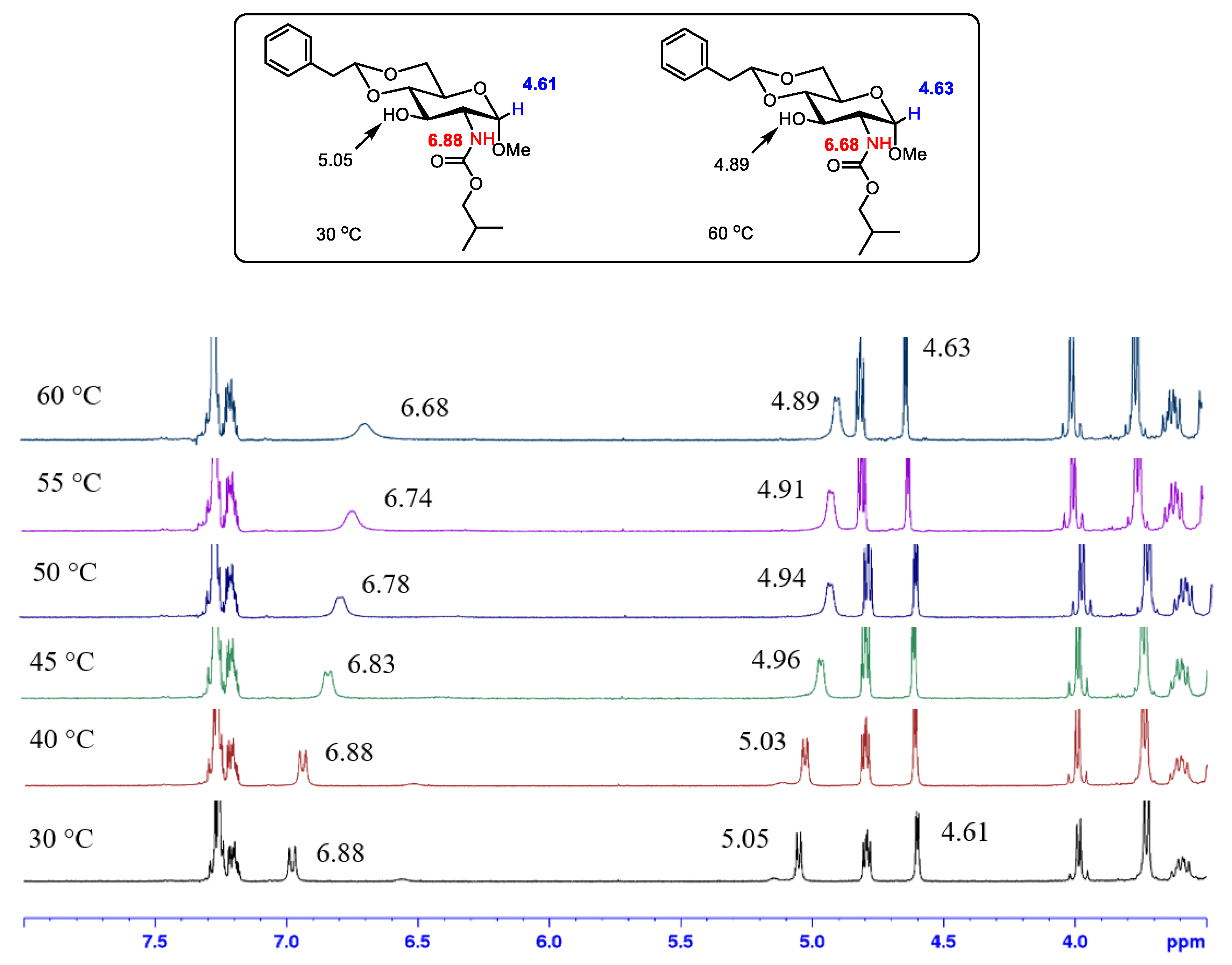
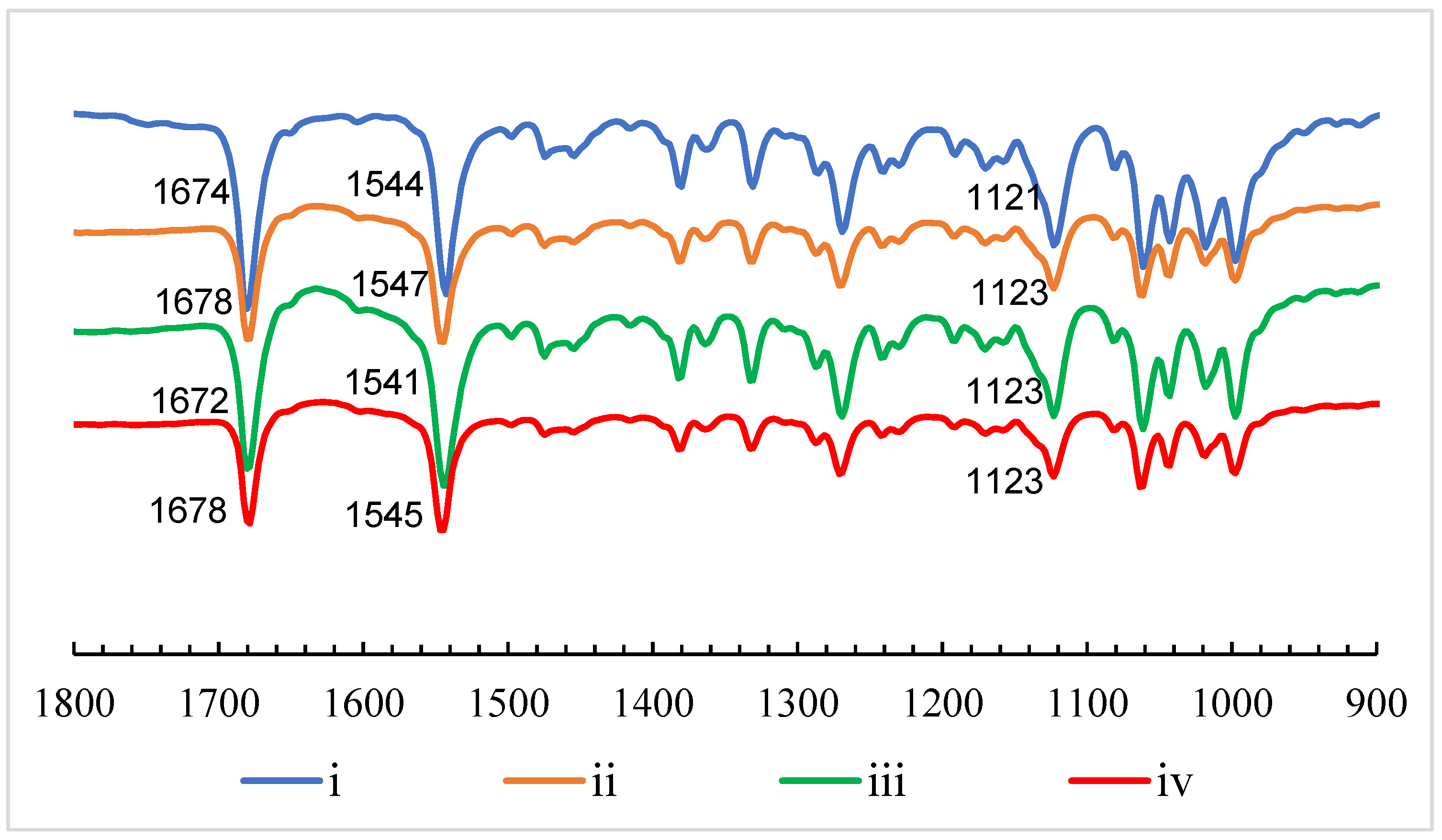
2.1. Gelation Mechanism by 1H NMR Studies
2.2. Metallogels Preparation and Analysis
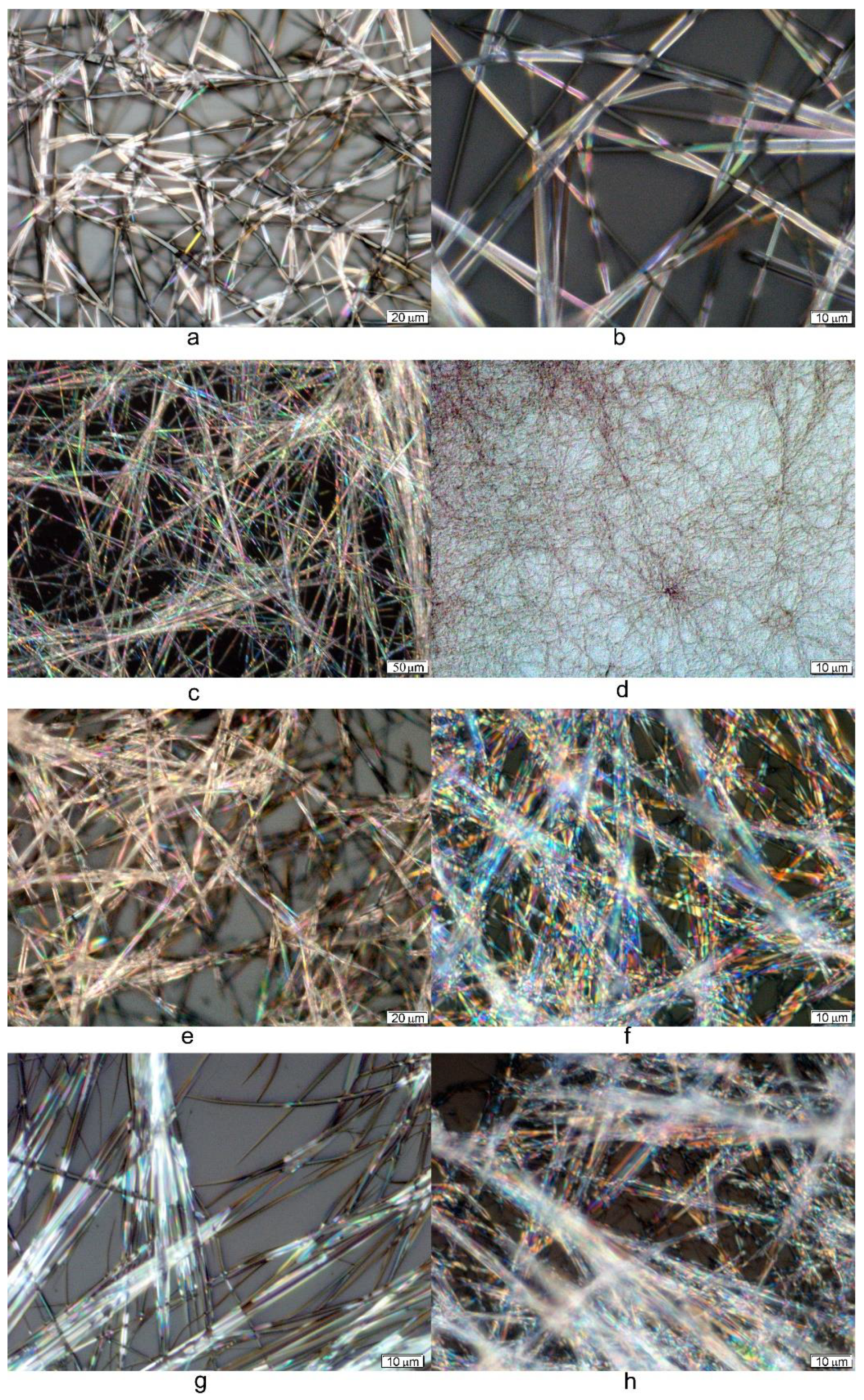
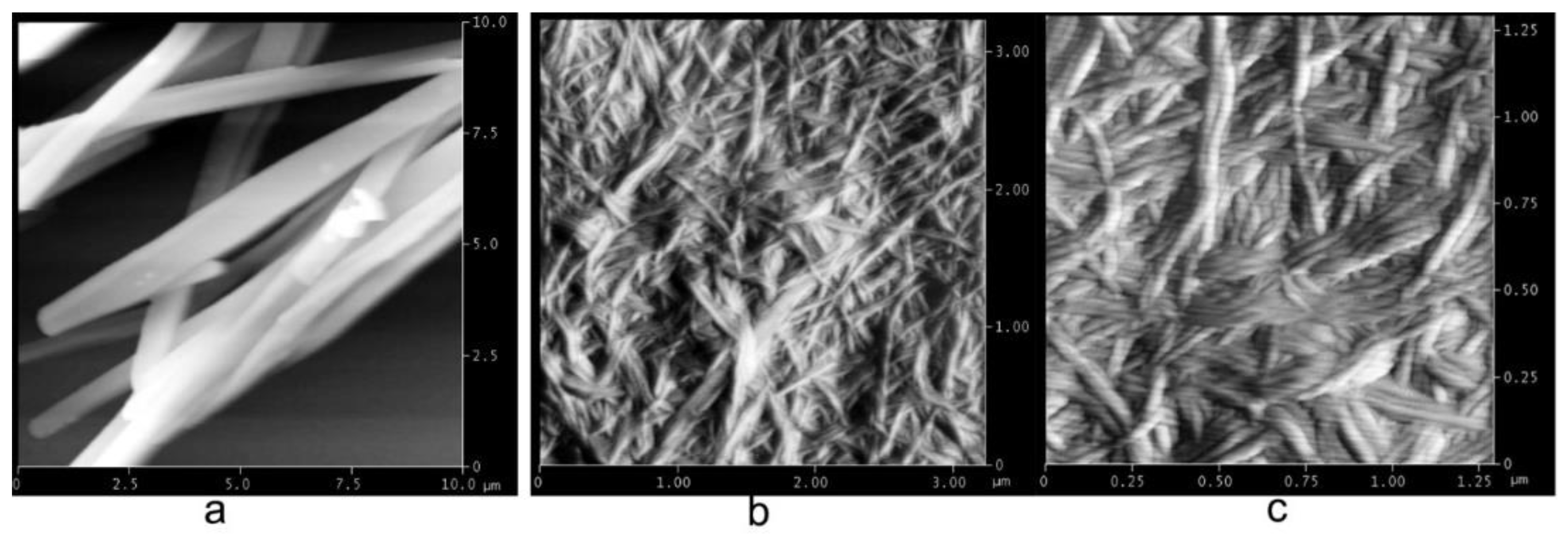
2.3. Gel Morphology Characterization
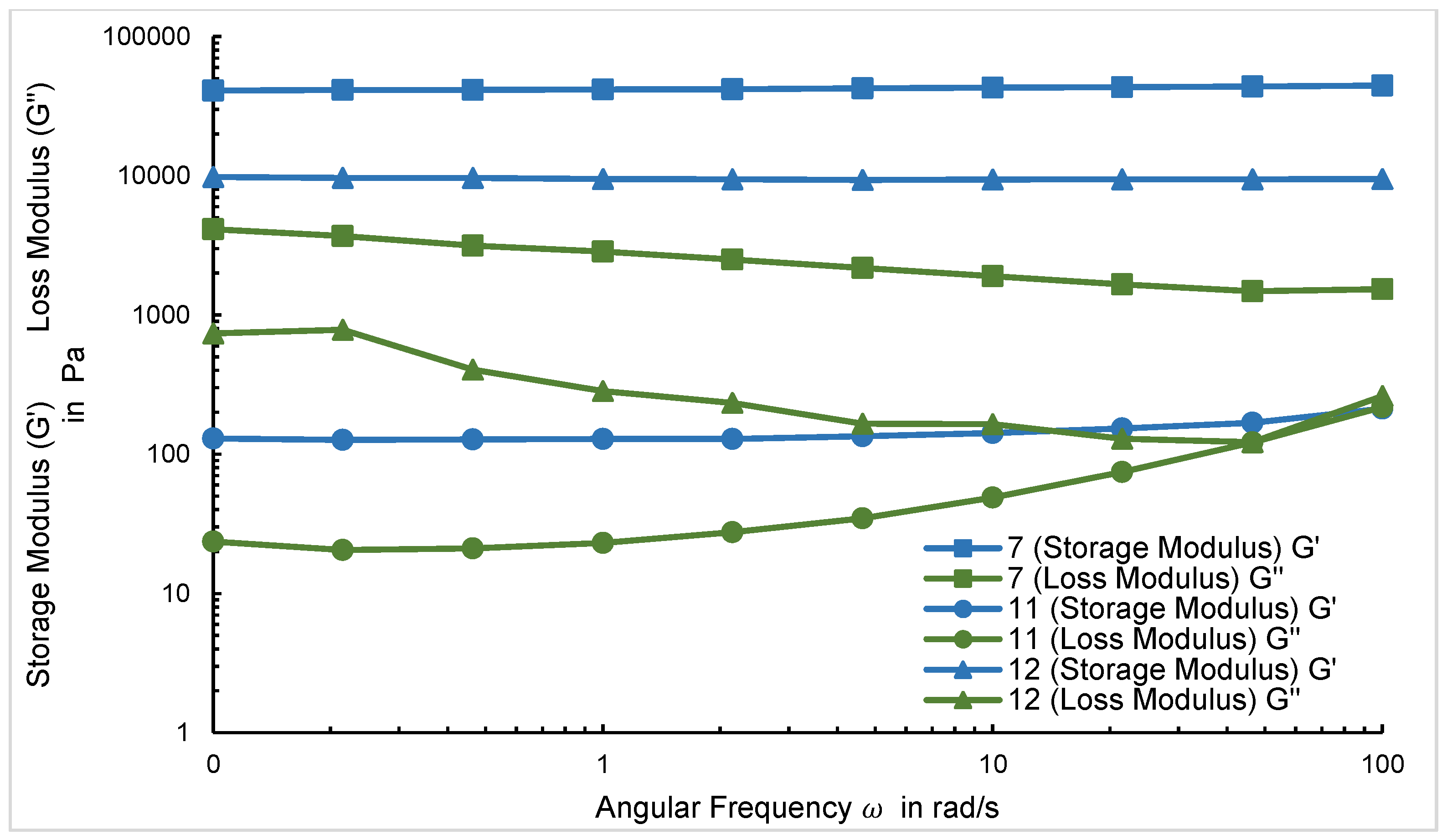
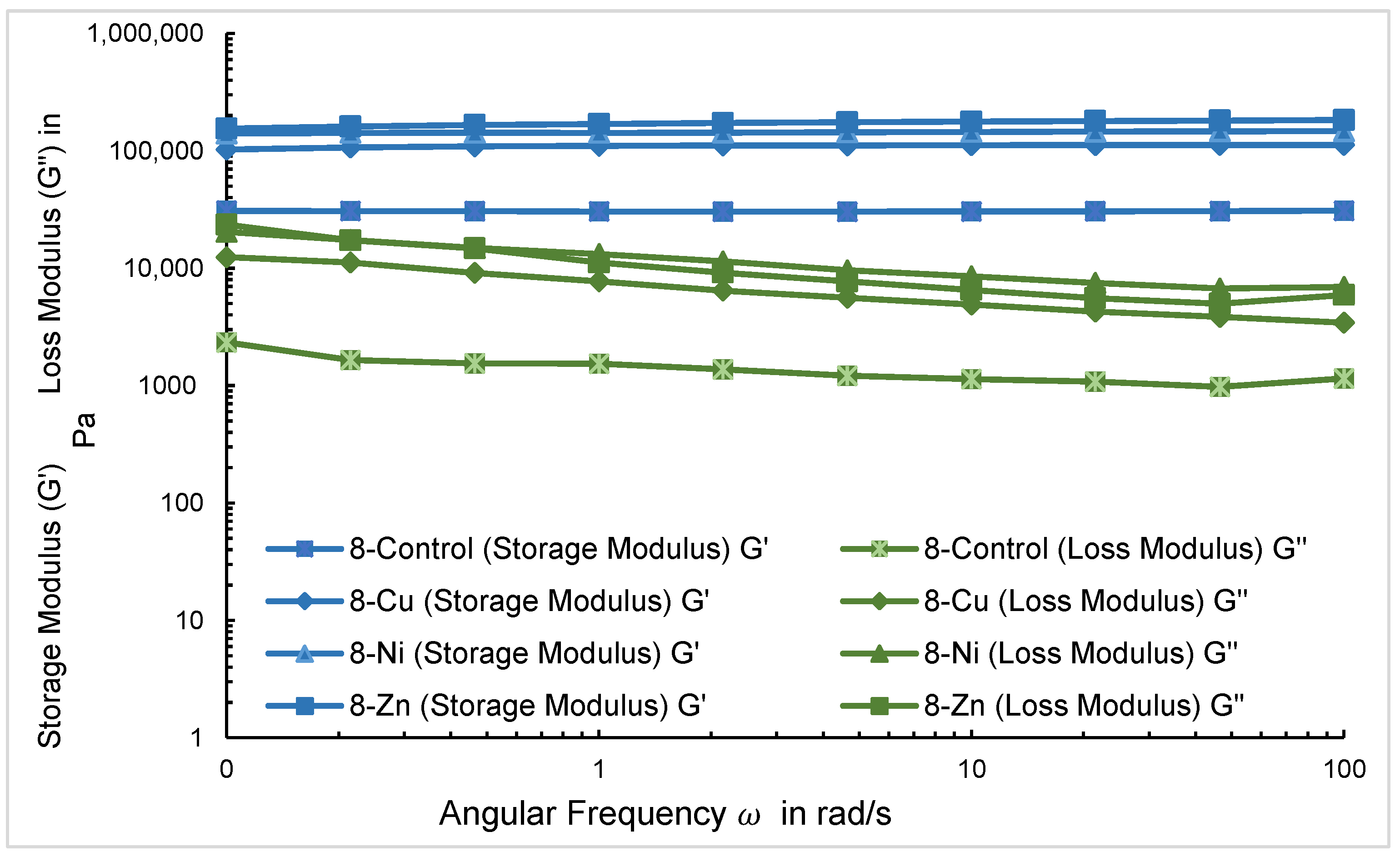
2.4. Rheological Analysis
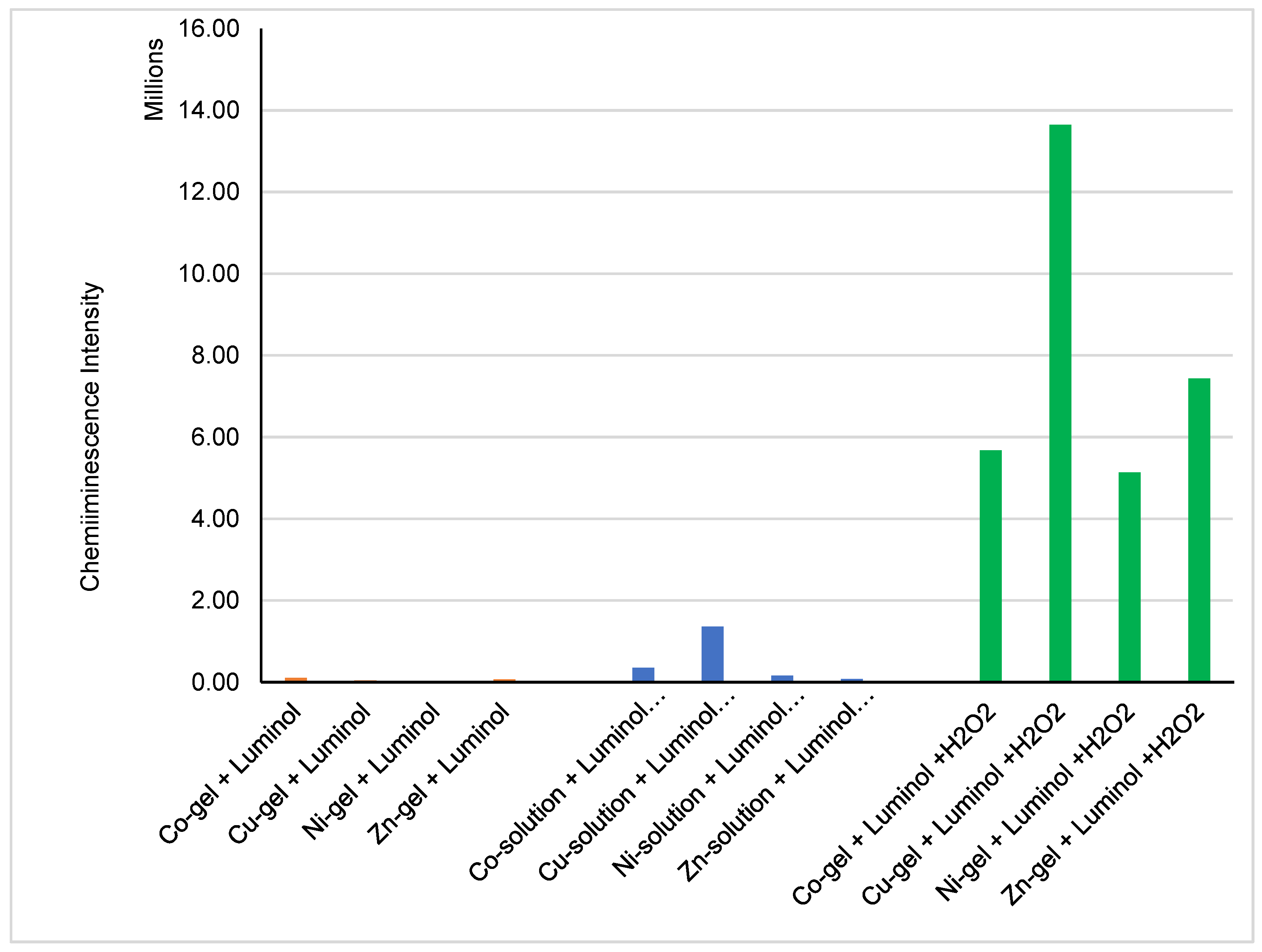
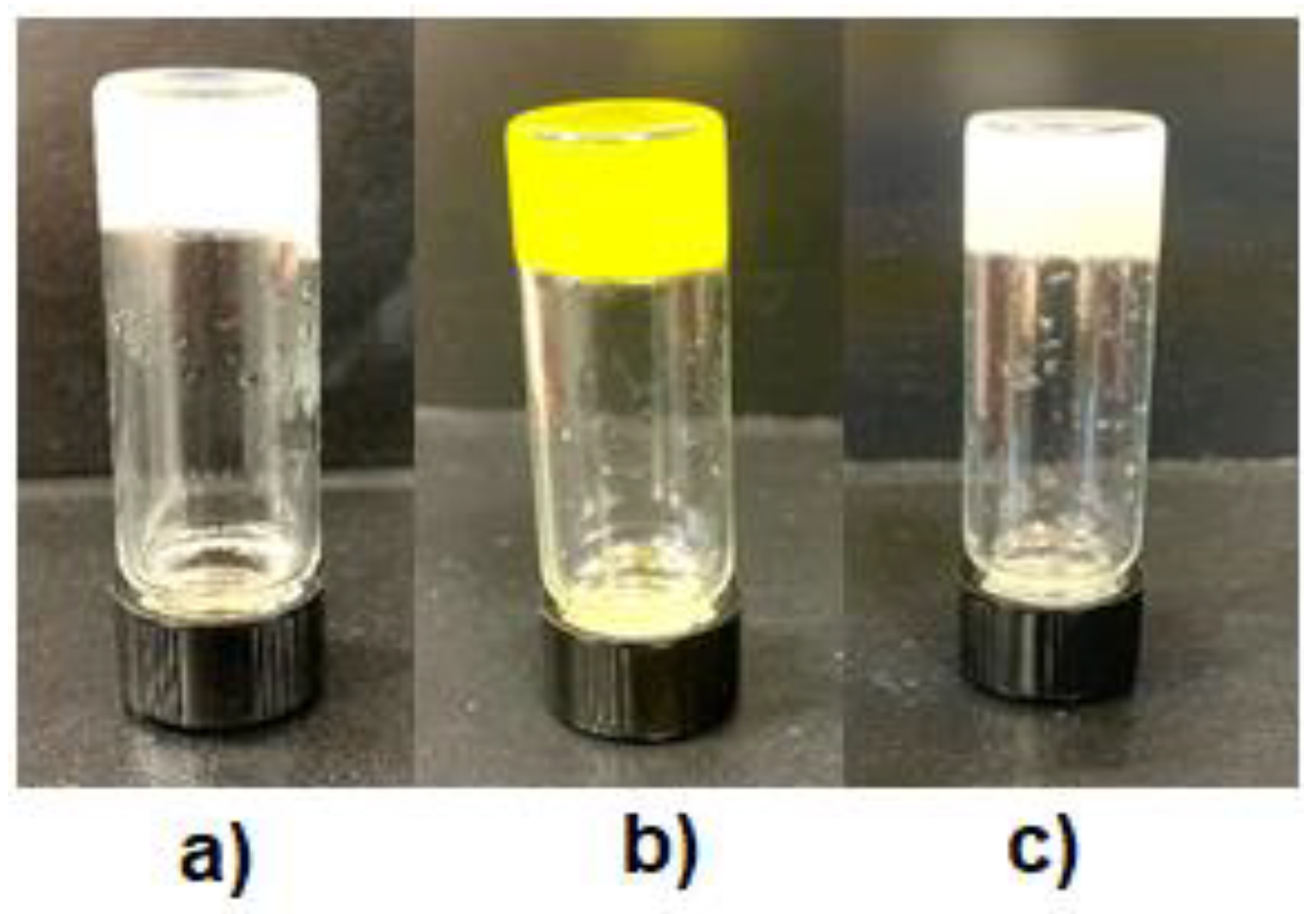
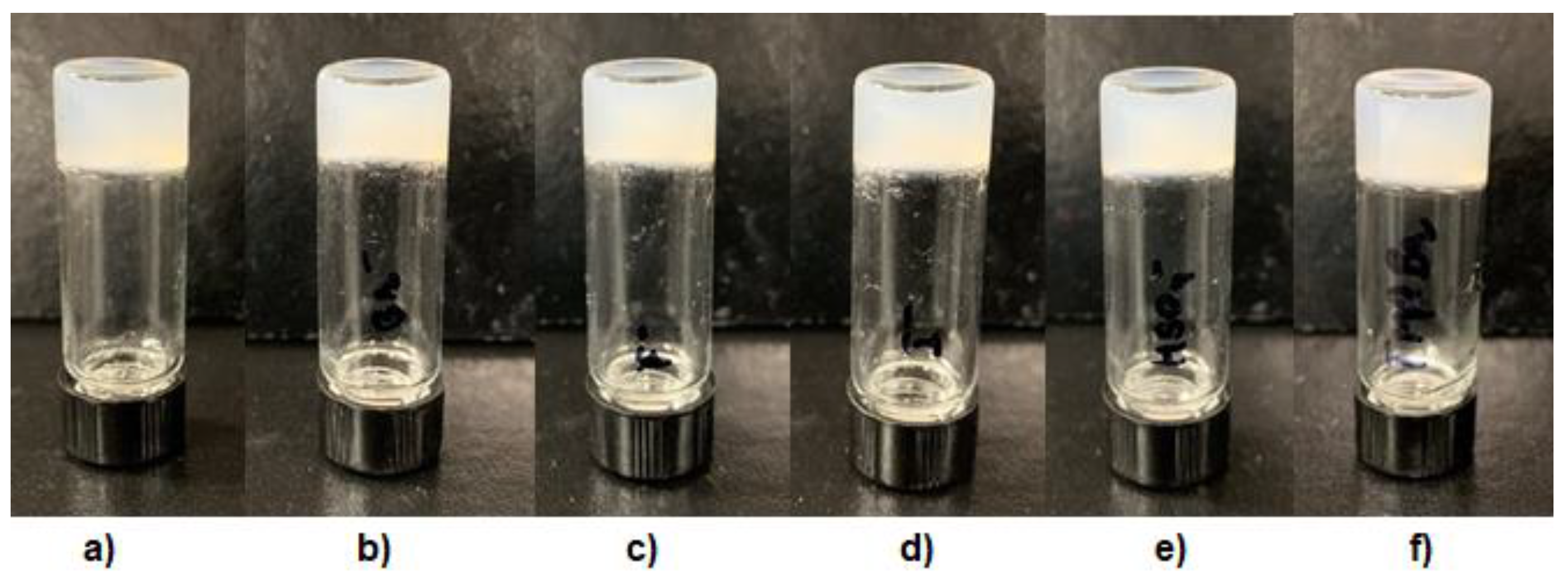
2.5. Chemiluminescence Properties of the Metal–Organic Xero Gels with Several Metal Ions
2.6. Drug loading and Their Sustained Release Studies
2.7. Co-Gels with Electrolytes
3. Conclusions
4. Materials and Methods
4.1. Optical Microscopy Studies
4.2. Atomic Force Microscopy Studies
4.3. Rheological Analyses
4.4. Preparation of Gels with Tetra-Alkyl Ammonium Salts and Conductivity Study
4.5. Metal–Organic Gels for Chemiluminescence Study
4.6. Naproxen Release Study
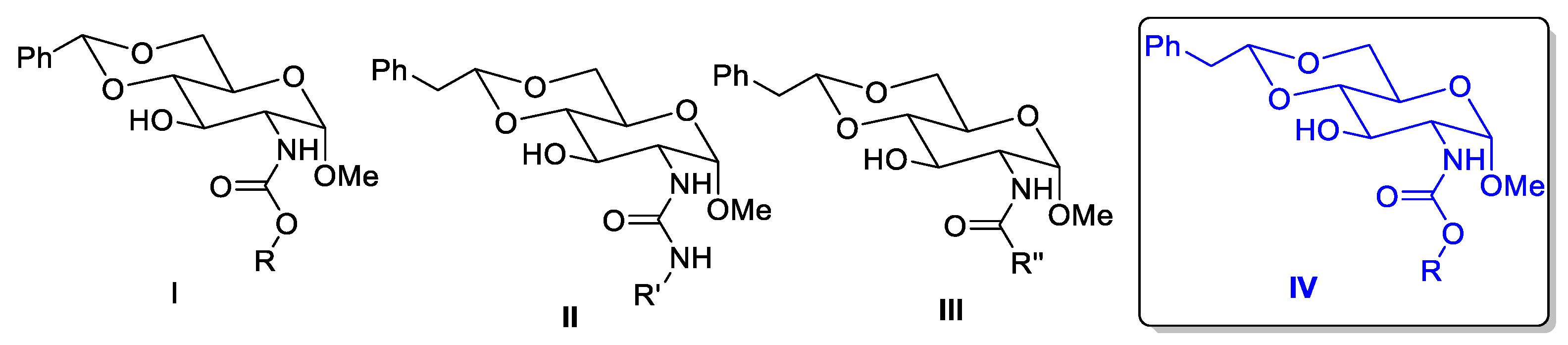
4.7. Synthesis of Carbamate Derivatives
4.8. General Procedure for the Preparation of Carbamate Compounds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, N.; Chakraborty, A.; Ghosh, R. Carbohydrate derived organogelators and the corresponding functional gels developed in recent time. Gels 2018, 4, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Z.J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Goh, S.S.; Liow, S.S.; Xue, K.; Loh, X.J. Molecular gel sorbent materials for environmental remediation and wastewater treatment. J. Mater. Chem. A 2019, 7, 18759–18791. [Google Scholar] [CrossRef]
- Narayana, C.; Upadhyay, R.K.; Chaturvedi, R.; Sagar, R. A versatile carbohydrate based gelator for oil water separation, nanoparticle synthesis and dye removal. New J. Chem. 2017, 41, 2261–2267. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthelemy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2015, 54, 4517–4521. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Wu, J.; Liu, C.; Zhu, H.; Tu, T. Recent Advances in Supramolecular Gels and Catalysis. Chem. Asian J. 2018, 13, 712–729. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Saldias, C.; Diaz Diaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Cheuk, S.; Wang, G. Synthesis and characterization of d-glucosamine-derived low molecular weight gelators. Tetrahedron 2010, 66, 5962–5971. [Google Scholar] [CrossRef]
- Chen, A.; Adhikari, S.B.; Mays, K.; Wang, G. Synthesis and Study of Molecular Assemblies Formed by 4,6-O-(2-Phenylethylidene)-Functionalized D-Glucosamine Derivatives. Langmuir 2017, 33, 8076–8089. [Google Scholar] [CrossRef]
- Wang, G.; Cheuk, S.; Yang, H.; Goyal, N.; Reddy, P.V.N.; Hopkinson, B. Synthesis and Characterization of Monosaccharide-Derived Carbamates as Low-Molecular-Weight Gelators. Langmuir 2009, 25, 8696–8705. [Google Scholar] [CrossRef]
- Bietsch, J.; Olson, M.; Wang, G. Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption. Gels 2021, 7, 134. [Google Scholar] [CrossRef]
- Wang, D.; Chen, A.; Morris, J.; Wang, G. Stimuli-responsive gelators from carbamoyl sugar derivatives and their responses to metal ions and tetrabutylammonium salts. RSC Adv. 2020, 10, 40068–40083. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Lew, A.J.; Ortony, J.H. Beyond covalent crosslinks: Applications of supramolecular gels. Gels 2018, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Slavik, P.; Kurka, D.W.; Smith, D.K. Palladium-scavenging self-assembled hybrid hydrogels-reusable highly-active green catalysts for Suzuki-Miyaura cross-coupling reactions. Chem. Sci. 2018, 9, 8673–8681. [Google Scholar] [CrossRef] [Green Version]
- Piepenbrock, M.O.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Li, L.; Sun, R.; Zheng, R.; Huang, Y. Anions-responsive supramolecular gels: A review. Mater. Des. 2021, 205, 109759. [Google Scholar] [CrossRef]
- Bielejewski, M.; Nowicka, K.; Bielejewska, N.; Tritt-Goc, J. Ionic conductivity and thermal properties of a supramolecular ionogel made from a sugar-based low molecular weight gelator and a quaternary ammonium salt electrolyte solution. J. Electrochem. Soc. 2016, 163, G187–G195. [Google Scholar] [CrossRef]
- Guo, P.; Su, A.; Wei, Y.; Liu, X.; Li, Y.; Guo, F.; Li, J.; Hu, Z.; Sun, J. Healable, Highly Conductive, Flexible, and Nonflammable Supramolecular Ionogel Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19413–19420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, G.; Zhang, Y. Organometallic Hydrogels. ChemNanoMat 2016, 2, 364–375. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjoniksen, A.L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, Applicability, and Advanceability. Adv. Mater. 2019, 31, e1806204. [Google Scholar] [CrossRef]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem.-Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef]
- Karan, C.K.; Sau, M.C.; Bhattacharjee, M. A copper(II) metal-organic hydrogel as a multifunctional precatalyst for CuAAC reactions and chemical fixation of CO2 under solvent free conditions. Chem. Commun. 2017, 53, 1526–1529. [Google Scholar] [CrossRef]
- Anh, H.T.P.; Huang, C.-M.; Huang, C.-J. Intelligent Metal-Phenolic Metallogels as Dressings for Infected Wounds. Sci. Rep. 2019, 9, 11562. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Shi, W.; Huang, J.; Yan, Y. Adaptive soft molecular self-assemblies. Soft Matter 2016, 12, 337–357. [Google Scholar] [CrossRef]
- Karan, C.K.; Bhattacharjee, M. A Copper Metal-Organic Hydrogel as a Catalyst for SO2 and CO2 Fixation under Ambient Conditions. Eur. J. Inorg. Chem. 2019, 2019, 3605–3611. [Google Scholar] [CrossRef]
- Li, B.; Xiao, D.; Gai, X.; Yan, B.; Ye, H.; Tang, L.; Zhou, Q. A multi-responsive organogel and colloid based on the self-assembly of a Ag(I)-azopyridine coordination polymer. Soft Matter 2021, 17, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.W.; Zhao, T.T.; Li, Y.F.; Huang, C.Z. Dimension conversion: From a 1D metal-organic gel into a 3D metal-organic porous network with high-efficiency multiple enzyme-like activities for cascade reactions. Nanoscale Horiz. 2020, 5, 119–123. [Google Scholar] [CrossRef]
- Guo, M.X.; Li, Y.F. Cu (II)-based metal-organic xerogels as a novel nanozyme for colorimetric detection of dopamine. Spectrochim. Acta Part A 2019, 207, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Jiang, Z.W.; Zhen, S.J.; Huang, C.Z.; Li, Y.F. A copper(II)/cobalt(II) organic gel with enhanced peroxidase-like activity for fluorometric determination of hydrogen peroxide and glucose. Microchim. Acta 2019, 186, 168. [Google Scholar] [CrossRef]
- Bailey, T.S.; Pluth, M.D. Chemiluminescent Detection of Enzymatically Produced Hydrogen Sulfide: Substrate Hydrogen Bonding Influences Selectivity for H2S over Biological Thiols. J. Am. Chem. Soc. 2013, 135, 16697–16704. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Peng, Z.W.; Jiang, Z.W.; Tang, X.Q.; Huang, C.Z.; Li, Y.F. Novel Iron(III)-Based Metal-Organic Gels with Superior Catalytic Performance toward Luminol Chemiluminescence. ACS Appl. Mater. Interfaces 2017, 9, 31834–31840. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y.; Lv, C.; Liu, W.; Zhang, Z.; Peng, X. Copper-based metal-organic xerogels on paper for chemiluminescence detection of dopamine. Anal. Methods 2020, 12, 4191–4198. [Google Scholar] [CrossRef]
- Sun, X.; Lei, J.; Jin, Y.; Li, B. Long-Lasting and Intense Chemiluminescence of Luminol Triggered by Oxidized g-C3N4 Nanosheets. Anal. Chem. 2020, 92, 11860–11868. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, L.; Yan, M.; Xiao, T.; Fan, L.; Xue, Y.; Huang, J.; Yang, X. An intensive and glow-type chemiluminescence of luminol-embedded, guanosine-derived hydrogel. Talanta 2021, 230, 122351. [Google Scholar] [CrossRef]
- Yang, C.P.; He, L.; Huang, C.Z.; Li, Y.F.; Zhen, S.J. Continuous singlet oxygen generation for persistent chemiluminescence in Cu-MOFs-based catalytic system. Talanta 2021, 221, 121498. [Google Scholar] [CrossRef]
- Zong, L.-P.; Ruan, L.-Y.; Li, J.; Marks, R.S.; Wang, J.-S.; Cosnier, S.; Zhang, X.-J.; Shan, D. Fe-MOGs-based enzyme mimetic and its mediated electrochemiluminescence for in situ detection of H2O2 released from Hela cells. Biosens. Bioelectron. 2021, 184, 113216. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, M.; Wang, H.; Li, Y. Novel manganese(II)-based metal-organic gels: Synthesis, characterization and application to chemiluminescent sensing of hydrogen peroxide and glucose. Microchim. Acta 2019, 186, 696. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Xiao, B.W.; Li, C.M.; Wang, D.M.; Huang, C.Z.; Li, Y.F. Co-metal-organic-frameworks with pure uniform crystal morphology prepared via Co2 + exchange-mediated transformation from Zn-metallogels for luminol catalysed chemiluminescence. Spectrochim. Acta Part A 2017, 175, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huo, Z.P.; Ding, Y.; Wang, L.; Zhu, J.; Zhang, C.N.; Pan, X.; Nazeeruddin, M.K.; Dai, S.Y.; Gratzel, M. Gel electrolyte materials formed from a series of novel low molecular mass organogelators for stable quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 15921–15930. [Google Scholar] [CrossRef]
- Bielejewski, M.; Lapinski, A.; Demchuk, O. Molecular interactions in high conductive gel electrolytes based on low molecular weight gelator. J. Colloid Interface Sci. 2017, 490, 279–286. [Google Scholar] [CrossRef]
- Sharma, P.; Chen, A.; Wang, D.; Wang, G. Synthesis and Self-Assembling Properties of Peracetylated β-1-Triazolyl Alkyl D-Glucosides and D-Galactosides. Chemistry 2021, 3, 935–958. [Google Scholar] [CrossRef]


| R: | Toluene | Pump Oil | Glycerol | Et-Glycol | EtOH:H2O (1:1) | EtOH:H2O (1:2) | DMSO:H2O (1:1) | DMSO:H2O (1:2) | H2O | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 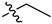 | P | UG 20.0O | G 6.7T | UG 20.0T | G 20.0T | G 20.0T | S | G 6.7T | U G 6.7O |
| 3 | 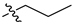 | P | G 4.0O | G 6.7T | G 20.0O | G 10.0O | P | G 20.0O | G 10.0O | I |
| 4 |  | G 20.0O | G 3.3O | G 5.0O | P | P | P | G 20.0O | G 6.7O | I |
| 5 |  | P | G 6.7O | G 4.0O | G 20.0O | G 10.0O | G 6.7O | G 20.0O | G 4.0O | P |
| 6 | 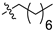 | S | G 6.7O | G 6.7T | G 4.0O | G 2.8O | P | G 2.8O | G 6.7O | P |
| 7 | 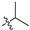 | S | S | G 5.0C | G 20.0C | G 20.0O | G 2.8T | G 6.7T | G 3.3C | G 2.0T |
| 8 | 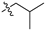 | S | G 6.7T | G 3.3C | P | S | G 1.7 O | G 4.0T | G 4.0O | G 10.0O |
| 9 | 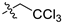 | S | S | G 6.7T | S | G 10.0O | P | P | I | I |
| 10 | 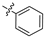 | P | UG 6.7T | G10.0C | S | P | P | P | P | I |
| 11 | 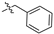 | S | G 3.6T | G 1.3T | G 5.0C | G 10.0T | G 3.3O | G 6.7T | G 6.7O | G 4.0O |
| 12 | 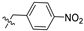 | S | G 20.0O | G 6.7T | G 10.0C | G 3.3O | G 2.8O | G 2.5O | G 1.8O | I |
| 13 | 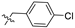 | S | S | G 6.7C | G 10.0T | P | S | G 20.0T | P | I |
| 14 | 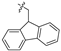 | P | G 4.0O | G 6.7T | G 6.7T | UG 6.7O | UG 6.7O | G 4.0O | G 10.0T | I |
 | ||||||||
| R | I | EtOH:H2O (1:2) | DMSO:H2O (1:2) | H2O | IV | EtOH:H2O (1:2) | DMSO: H2O (1:2) | H2O |
 | I-a | G 10.0 | G 10.0 | I | 5, IV-a | G 6.7 | G 4.0 | P |
 | I-b | G 2.2 | G 1.7 | G 4.0 | 8, IV-b | G 1.7 | G 4.0 | G 10.0 |
 | I-c | G 10.0 | G 20.0 | I | 11, IV-c | G 3.3 | G 6.7 | G 4.0 |
| Metal Salt (1.5 Eq.) | Concentration of Compound 8 |
|---|---|
| Cu(OAc)2⋅H2O | G 3.0O |
| Cu(SO4)⋅5H2O | G 3.0O |
| CuBr2 | G 3.0O |
| Hg(OAc)2 | P |
| Zn(OAc)2⋅2H2O | G 2.5O |
| NiCl2⋅6H2O | G 2.5O |
| Pb(OAc)4 | G 3.8O |
| FeCl2 | G 3.0O |
| FeCl3⋅6H2O | G 3.0O |
| AgNO3 | G 3.8O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Wang, G. 4,6-O-Phenylethylidene Acetal Protected D-Glucosamine Carbamate-Based Gelators and Their Applications for Multi-Component Gels. Gels 2022, 8, 191. https://doi.org/10.3390/gels8030191
Sharma P, Wang G. 4,6-O-Phenylethylidene Acetal Protected D-Glucosamine Carbamate-Based Gelators and Their Applications for Multi-Component Gels. Gels. 2022; 8(3):191. https://doi.org/10.3390/gels8030191
Chicago/Turabian StyleSharma, Pooja, and Guijun Wang. 2022. "4,6-O-Phenylethylidene Acetal Protected D-Glucosamine Carbamate-Based Gelators and Their Applications for Multi-Component Gels" Gels 8, no. 3: 191. https://doi.org/10.3390/gels8030191
APA StyleSharma, P., & Wang, G. (2022). 4,6-O-Phenylethylidene Acetal Protected D-Glucosamine Carbamate-Based Gelators and Their Applications for Multi-Component Gels. Gels, 8(3), 191. https://doi.org/10.3390/gels8030191