How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
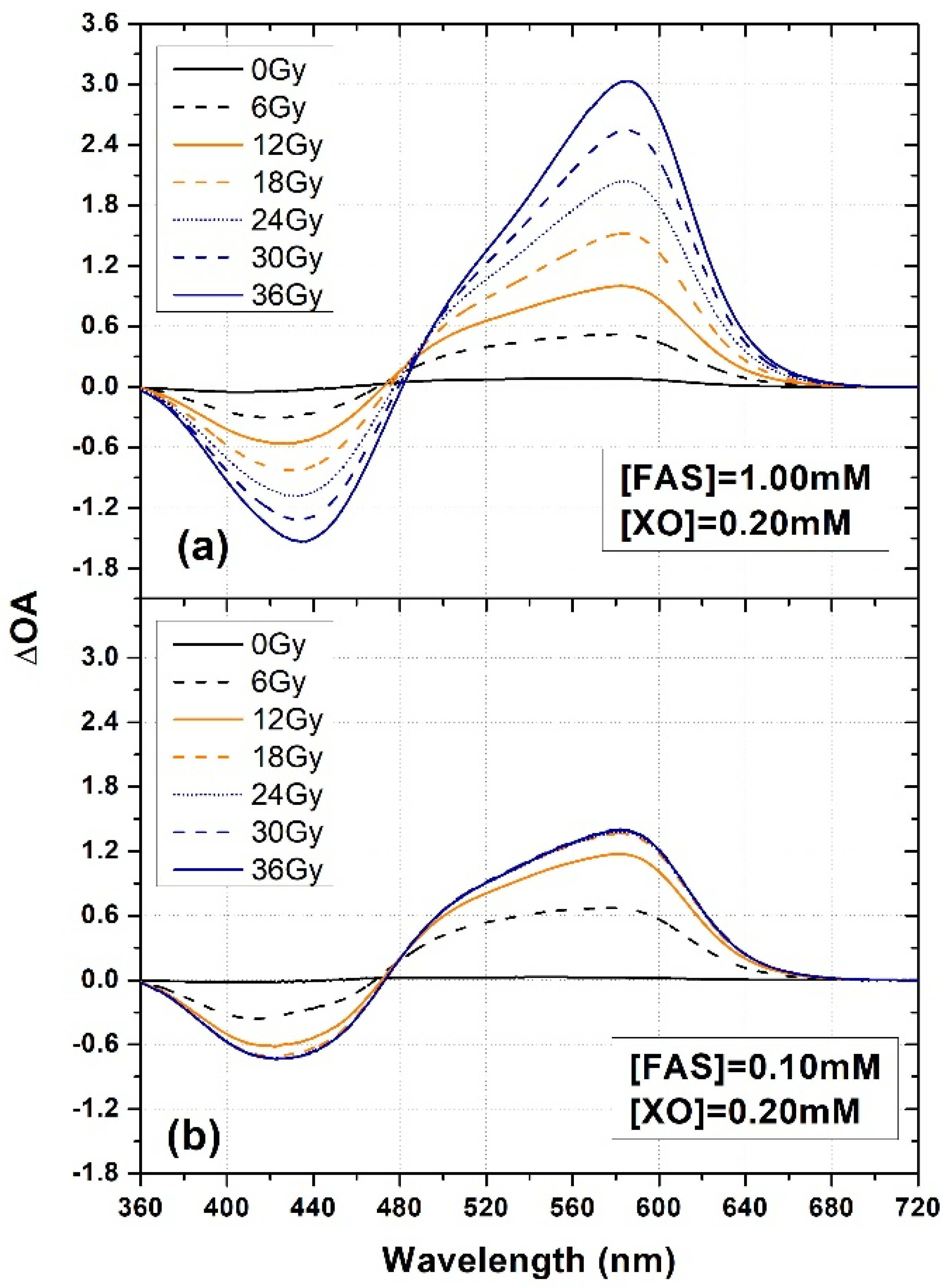
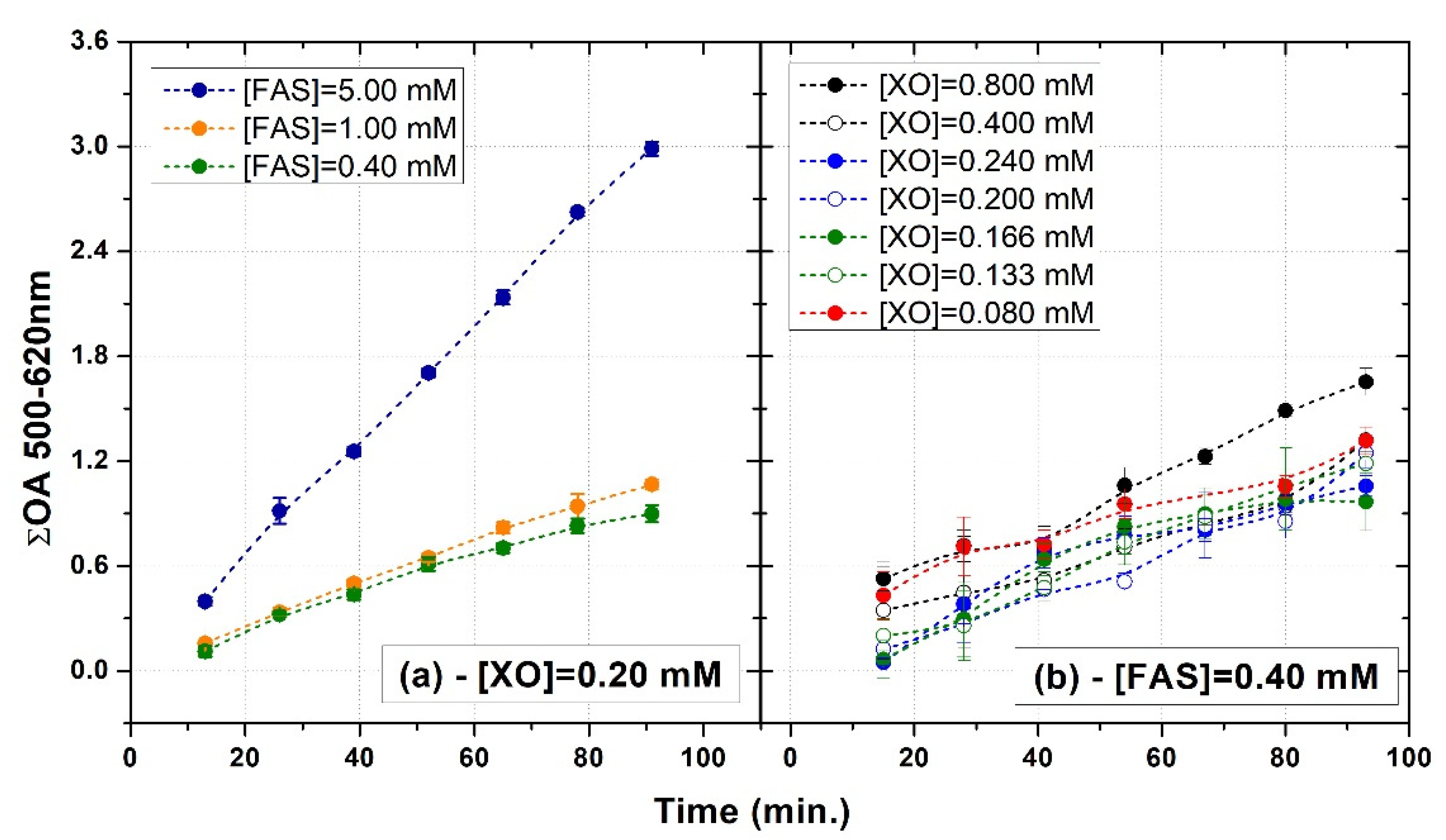
3.1. FAS Variation
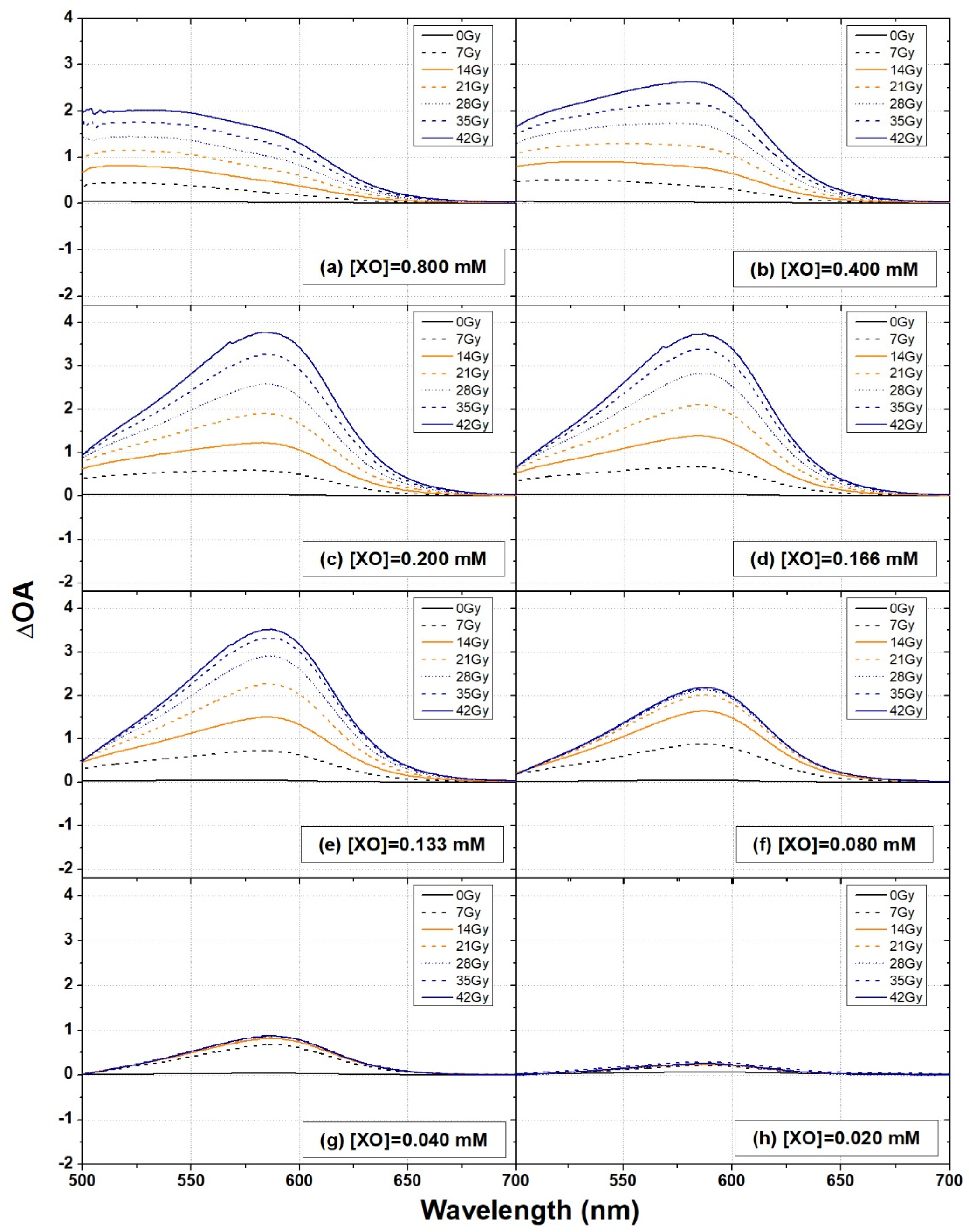
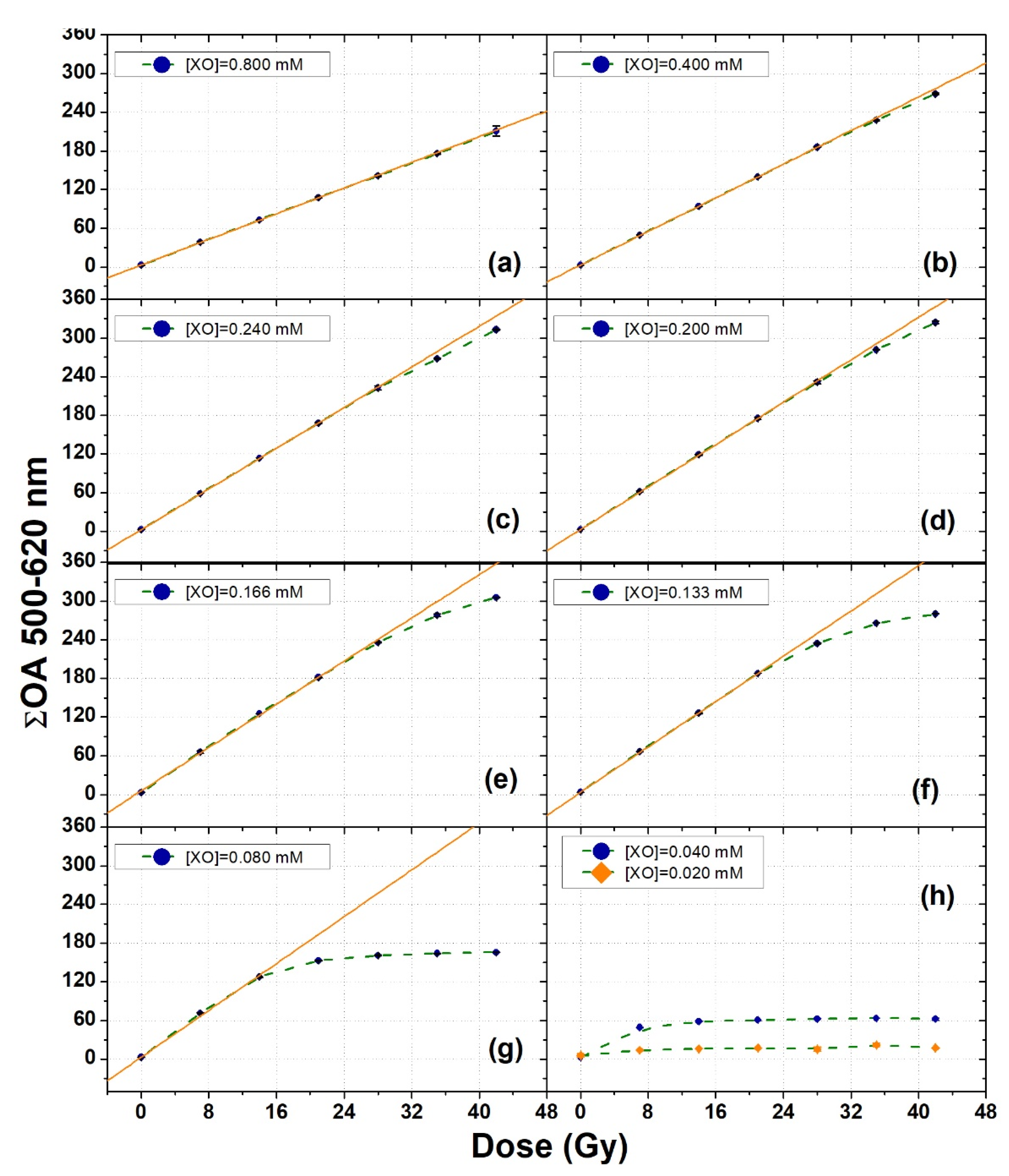
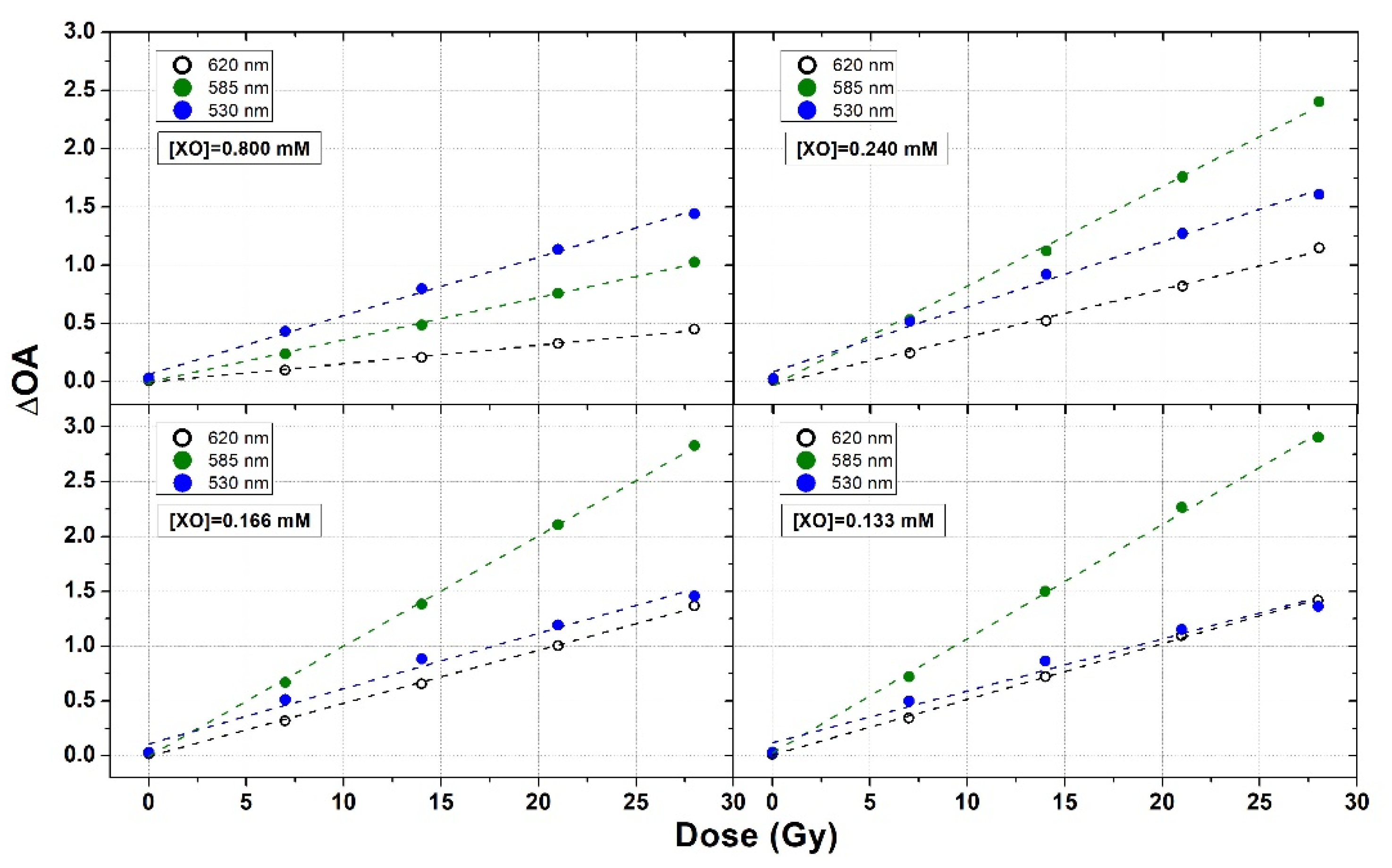
3.2. XO Variation
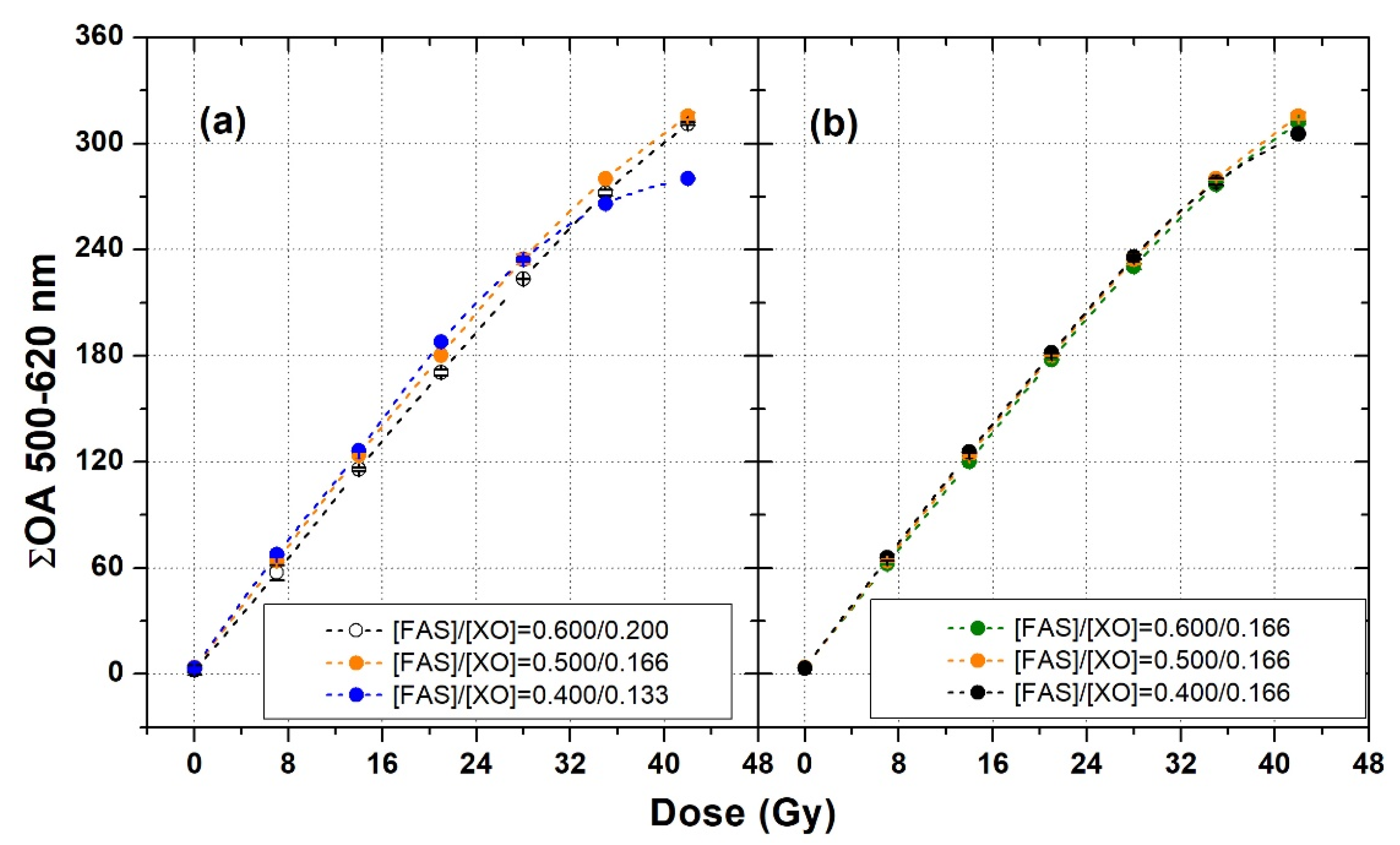
3.3. Fine Tuning of FAS and XO Concentrations
3.4. Self-Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fricke, H.; Morse, S. The chemical action of roentgen rays on diluted ferrous sulphate solutions as a measure of dose. Am. J. Roentgenol. 1927, 18, 426–430. [Google Scholar]
- Andreo, P.; Burns, D.T.; Nahum, A.E.; Seuntjens, J.; Attix, F.H. Primary Radiation Standards. In Fundamentals of Ionizing Radiation Dosimetry, 1st ed.; Wiley-VCH: Weinheim, Germany, 2017; ISBN 978-3-527-40921-1. [Google Scholar]
- Marrale, M.; d’Errico, F. Hydrogels for Three-Dimensional Ionizing-Radiation Dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef]
- Zahra, A.N.; Ghazale, G. A review study on application of gel dosimeters in low energy radiation dosimetry. Appl. Radiat. Isot. 2022, 179, 110015. [Google Scholar]
- Gay, C.; Collins, J.; Gebicki, J.M. Determination of Iron in Solutions with the Ferric–Xylenol Orange Complex. Anal. Biochem. 1999, 273, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.J.; Zahmatkesh, M.H.; Nitschke, K.N.; Baldock, C. Effect of saccharide additives on response of ferrous–agarose–Xylenol orange radiotherapy gel dosimeters. Med. Phys. 2003, 30, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Yang, L.; Chen, B.; Sheng, Z.; Luo, W.; Sui, G.; Lu, X.; Chen, J. Effect of D-(β)-Glucose on the Stability of Polyvinyl Alcohol Fricke Hydrogel Three-Dimensional Do-simeter for Radiotherapy. Nucl. Eng. Technol. 2016, 48, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Gallo, S.; Locarno, S. Influence of saccharides on the dosimetric properties of PVA-GTA Fricke gels. Il Nuovo Cimento C 2021, 43, 1–13. [Google Scholar]
- Penev, K.I.; Mequanint, K. Controlling sensitivity and stability of ferrous–xylenol orange–gelatin 3D gel dosime-ters by doping with phenanthroline-type ligands and glyoxal. Phys. Med. Biol. 2013, 58, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Vedelago, J.; Mattea, F.; Valente, M. Integration of Fricke gel dosimetry with Ag nanoparticles for experimental dose enhancement determination in theranostics. Appl. Radiat. Isot. 2018, 141, 182–186. [Google Scholar] [CrossRef]
- Babu, S.E.S.; Rafic, K.M.; Peace, B.T.; Raj, L.J.S.; Ravindran, B.P. Fricke xylenol glycine gel dosimeter for in vivo dosimetry at eùxtended treatment distance. Appl. Radiat. Isot. 2019, 145, 217–222. [Google Scholar] [CrossRef]
- Babu, S.; Peace, S.; Rafic, K.; Raj, E.; Christopher, S.; Ravindran, P. Escalation of optical transmittance and determination of diffusion co-efficient in low-bloom strength gelatin-based Fricke gel dosimeters. Radiat. Phys. Chem. 2019, 156, 300–306. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.; Furuta, T.; Fukasaku, K.; Takagi, S.; Noda, S.; Himeno, R.; Fukuda, S. A diffusion-free and linear-energy-transfer-independent nanocomposite Fricke gel dosimeter. Radiat. Phys. Chem. 2014, 96, 92–96. [Google Scholar] [CrossRef]
- Gallo, S.; Cremonesi, L.; Gambarini, G.; Ianni, L.; Lenardi, C.; Argentiere, S.; Bettega, D.; Gargano, M.; Ludwig, N.; Veronese, I. Study of the effect of laponite on Fricke xylenol orange gel dosimeter by optical techniques. Sensors Actuators B Chem. 2018, 272, 618–625. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, S.; Hu, X.; Chang, S.; Zhang, H. Influence of embedded boron nitride nanosheets on Fe3+ diffusion in Fricke gel dosimeter and its response to γ rays. J. Radioanal. Nucl. Chem. 2020, 324, 359–365. [Google Scholar] [CrossRef]
- Alves, A.V.S.; de Almeida, W.S.; Sussuchi, E.M.; Lazzeri, L.; D’Errico, F.; de Souza, S.O. Investigation of chelating agents/ligands for Fricke gel dosimeters. Radiat. Phys. Chem. 2018, 150, 151–156. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Liosi, G.; Mariani, M.; Dondi, D. An innovative Fe3+ selective ligand for Fricke-gel dosimeter. Radiat. Phys. Chem. 2020, 171, 108733. [Google Scholar] [CrossRef]
- de Almeida, W.D.S.; Alves, A.V.S.; Oliveira, W.F.; da Silveira, M.A.L.; de Souza, S.O.; d’Errico, F.; Sussuchi, E.M. Radiochromic Fricke gels with eriochrome cyanine R for radiotherapy dosimetry. Radiat. Phys. Chem. 2021, 191, 109830. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Hailat, T.F.; Al-Shorman, M.Y.; Aldweri, F.M.; Kanan, H.M.; Awad, S.I. Investigation of a novel chemically cross-linked fricke-Methylthymol blue-synthetic polymer gel dosimeter with glutaraldehyde cross-linker. Radiat. Meas. 2018, 118, 77–85. [Google Scholar] [CrossRef]
- Rabaeh, K.; Eyadeh, M.M.; Hailat, T.F.; Aldweri, F.M.; Alheet, S.M.; Eid, R.M. Characterization of ferrous-methylthymol blue-polyvinyl alcohol gel dosimeters using nuclear magnetic resonance and optical techniques. Radiat. Phys. Chem. 2018, 148, 25–32. [Google Scholar] [CrossRef]
- Parwaie, W.; Geraily, G.; Shirazi, A.; Shakeri, A.; Massumi, H.; Farzin, M. Analysis of the ferrous benzoic methylthy-mol-blue gel dosimeter in low-dose-level measurements. Radiat. Phys. Chem. 2020, 173, 108943. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Chen, J.; Chen, B.; Luo, W.; Sui, G.; Lu, X.; Chen, J. Preparation and characterization of novel Sulfosalicylic acid-Ferrous-PVA hydrogel as a 3D dosimeter. J. Radioanal. Nucl. Chem. Artic. 2015, 304, 481–487. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Hailat, T.F.; Eyadeh, M.M.; Al-Shorman, M.Y.; Aldweri, F.M.; Alheet, S.M.; Madas, B.G.; Awad, S.I. Dosimetric properties of sulfosalicylic acid-ferrous-polyvinyl alcohol glutaraldehyde hydrogel do-simeters using magnetic and optical techniques. Radiat. Phys. Chem. 2020, 177, 109106. [Google Scholar] [CrossRef]
- Chu, K.C.; Jordan, K.J.; Battista, J.J.; Van Dyk, J.; Rutt, B.K. Polyvinyl alcohol–Fricke hydrogel and cryogel: Two new gel dosimetry systems with low Fe3+ diffusion. Phys. Med. Biol. 2000, 45, 955–969. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, G. Novel GTA-PVA Fricke gels for three-dimensional dose mapping in radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Collura, G.; Gallo, S.; Tranchina, L.; Abbate, B.F.; Bartolotta, A.; D’Errico, F.; Marrale, M. Analysis of the response of PVA-GTA Fricke-gel dosimeters with clinical magnetic resonance imaging. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 414, 146–153. [Google Scholar] [CrossRef]
- Taño, J.E.; Hayashi, S.I.; Hirota, S.; Gonzales, C.A.B.; Yasuda, H. Effect of the glucono-δ-lactone concentration on the sensitivity and stability of PVA-GTA-I radio-chromic gel dosimeter. Radiat. Meas. 2020, 134, 106311. [Google Scholar] [CrossRef]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic diffusion of Fe ions in Fricke-XO-Pluronic F-127 and Fricke-XO-gelatine 3D radiotherapy dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Kadłubowski, S.; Kozicki, M. Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter. Materials 2022, 15, 984. [Google Scholar] [CrossRef]
- Farajzadeh, E.; Sina, S. Developing a radiochromic dosimeter for dosimetry in blood irradiation chambers. Radiat. Phys. Chem. 2021, 188, 109637. [Google Scholar] [CrossRef]
- Pérez, P.; Torres, P.R.; Bruna, A.; Brunetto, M.; Aon, E.; Franco, D.; Mattea, F.; Figueroa, R.; Santibáñez, M.; Valente, M. Fricke gel xylenol orange dosimeter layers for stereotactic radiosurgery: A preliminary approach. Appl. Radiat. Isot. 2021, 178, 109936. [Google Scholar] [CrossRef]
- Gallo, S.; Pasquale, S.; Lenardi, C.; Veronese, I.; Gueli, A.M. Effect of ionizing radiation on the colorimetric properties of PVA-GTA Xylenol Orange Fricke gel dosimeters. Dye. Pigment. 2021, 187, 109141. [Google Scholar] [CrossRef]
- Smith, S.T.; Boase, N.; Masters, K.S.; Hosokawa, K.; Asena, A.; Crowe, S.B.; Kairn, T.; Trapp, J.V. A very low diffusion Fricke gel dosimeter with functionalised xylenol orange-PVA (XOPVA). Phys. Med. Biol. 2019, 64, 205017. [Google Scholar] [CrossRef] [PubMed]
- Vedelago, J.; Quiroga, A.; Triviño, S.; Mattea, F.; Valente, M. Parameter estimation and mathematical modeling of the diffusion process of a benzoic acid infused Fricke gel dosimeter. Appl. Radiat. Isot. 2019, 151, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Marini, A.; Cascone, M.G.; D’Errico, F. Dosimetric and chemical characteristics of Fricke gels based on PVA matrices cross-linked with glutaraldehyde. Phys. Med. Biol. 2019, 64, 085015. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, S.; Liosi, G.; D’Agostino, G.; Marconi, R.; Mariani, M.; Buttafava, A.; Dondi, D. The role of hydrogels in the radical production of the Fricke-gel-dosimeter. Radiat. Phys. Chem. 2018, 142, 137–140. [Google Scholar] [CrossRef]
- Welch, M.L.; Jaffray, D.A. The correction of time and temperature effects in MR-based 3D Fricke Xylenol orange dosimetry. Phys. Med. Biol. 2017, 62, 3221–3236. [Google Scholar] [CrossRef] [PubMed]
- Marrale, M.; Collura, G.; Gallo, S.; Nici, S.; Tranchina, L.; Abbate, B.; Marineo, S.; Caracappa, S.; d’Errico, F. Analysis of spatial diffusion of ferric ions in PVA-GTA gel dosimeters analyzed via magnetic resonance imaging. Nucl. Instr. Meth. B 2017, 396, 50–55. [Google Scholar] [CrossRef]
- Marini, A.; Lazzeri, L.; Cascone, M.; Ciolini, R.; Tana, L.; D’Errico, F. Fricke gel dosimeters with low-diffusion and high-sensitivity based on a chemically cross-linked PVA matrix. Radiat. Meas. 2017, 106, 618–621. [Google Scholar] [CrossRef]
- Soliman, Y.S.; El Gohary, M.I.; Gawad, M.A.; Amin, E.A.; Desouky, O.S. Fricke gel dosimeter as a tool in quality assurance of the radiotherapy treatment plans. Appl. Radiat. Isot. 2017, 120, 126–132. [Google Scholar] [CrossRef]
- Gambarini, G.; Veronese, I.; Bettinelli, L.; Felisi, M.; Gargano, M.; Ludwig, N.; Lenardi, C.; Carrara, M.; Collura, G.; Gallo, S.; et al. Study of optical absorbance and MR relaxation of Fricke xylenol orange gel dosimeters. Radiat. Meas. 2017, 106, 622–627. [Google Scholar] [CrossRef]
- Del Lama, L.S.; Petchevist, P.C.D.; de Almeida, A. Fricke Xylenol Gel characterization at megavoltage radiation energy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 394, 89–96. [Google Scholar] [CrossRef]
- El Gohary, M.; Soliman, Y.; Amin, E.; Gawad, M.A.; Desouky, O. Effect of perchloric acid on the performance of the Fricke xylenol gel dosimeter. Appl. Radiat. Isot. 2016, 113, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Marrale, M.; Brai, M.; Gagliardo, C.; Gallo, S.; Longo, A.; Tranchina, L.; Abbate, B.; Collura, G.; Gallias, K.; Caputo, V.; et al. Correlation between ferrous ammonium sulfate concentration, sensitivity and stability of Fricke gel dosimeters exposed to clinical X-ray beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 335, 54–60. [Google Scholar] [CrossRef]
- Cavinato, C.C.; Sakuraba, R.K.; Cruz, J.C.; Campos, L.L. Optical response of the FXG solution to different phantom materials. Radiat. Meas. 2011, 46, 1928–1931. [Google Scholar] [CrossRef]
- Babic, S.; McNiven, A.; Battista, J.; Jordan, K. Three-dimensional dosimetry of small megavoltage radiation fields using radiochromic gels and optical CT scanning. Phys. Med. Biol. 2009, 54, 2463–2481. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. An apparent threshold dose response in ferrous xylenol-orange gel dosimeters when scanned with a yellow light source. Phys. Med. Biol. 2008, 53, 1637–1650. [Google Scholar] [CrossRef]
- Galante, A.; Cervantes, H.; Cavinato, C.; Campos, L.; Rabbani, S. MRI study of radiation effect on Fricke gel solutions. Radiat. Meas. 2008, 43, 550–553. [Google Scholar] [CrossRef]
- Davies, J.; Baldock, C. Sensitivity and stability of the Fricke–gelatin–xylenol orange gel dosimeter. Radiat. Phys. Chem. 2008, 77, 690–696. [Google Scholar] [CrossRef]
- Hill, B.; Bäck, S.Å.J.; Lepage, M.; Simpson, J.; Healy, B.; Baldock, C. Investigation and analysis of ferrous sulfate polyvinylalcohol (PVA) gel dosimeter. Phys. Med. Biol. 2002, 47, 4233–4246. [Google Scholar] [CrossRef]
- Pedersen, T.V.; Olsen, D.R.; Skretting, A. Measurement of the ferric diffusion coefficient in agarose and Gelatin gels by utilization of the evolution of a radiation induced edge as reflected in relaxation rate images. Phys. Med. Biol. 1997, 42, 1575–1585. [Google Scholar] [CrossRef]
- Kron, T.; Jonas, D.; Pope, J.M. Fast T1 imaging of dual gel samples for diffusion measurements in NMR dosimetry gels. Magn. Reson. Imaging 1997, 15, 211–221. [Google Scholar] [CrossRef]
- Rae, W.I.D.; Willemse, C.A.; Lötter, M.G.; Engelbrecht, J.S.; Swarts, J.C. Chelator effect on ion diffusion in ferrous-sulfate-doped gelatin gel dosimeters as analyzed by MRI. Med Phys. 1996, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tarte, B.J.; Jardine, P.A.; Van Doorn, T. Laser-scanned agarose gel sections for radiation field mapping. Int. J. Radiat. Oncol. 1996, 36, 175–179. [Google Scholar] [CrossRef]
- Appleby, A.; Leghrouz, A. Imaging of radiation dose by visible color development in fer-rous-agarose-xylenol-orange-gels. Med. Phys. 1991, 18, 309–312. [Google Scholar] [CrossRef]
- Appleby, A.; Christman, E.A.; Leghrouz, A. Imaging of spatial radiation dose distribution in agarose gels using magnetic resonance. Med. Phys. 1987, 14, 382–384. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef]
- Gallo, S.; Gambarini, G.; Veronese, I.; Argentiere, S.; Gargano, M.; Ianni, L.; Lenardi, C.; Ludwig, N.; Pignoli, E.; D’Errico, F. Does the gelation temperature or the sulfuric acid concentration influence the dosimetric properties of radiochromic PVA-GTA Xylenol Orange Fricke gels? Radiat. Phys. Chem. 2019, 160, 35–40. [Google Scholar] [CrossRef]
- Gallo, S.; Artuso, E.; Brambilla, M.; Gambarini, G.; Lenardi, C.; Monti, A.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of radiochromic PVA-GTA Fricke gels for dosimetry in X-rays external radiation therapy. J. Phys. D: Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Gallo, S.; Collura, G.; Longo, A.; Bartolotta, A.; Tranchina, L.; Iacoviello, G.; d’Errico, F.; Marrale, M. Preliminary MR relaxometric analysis of Fricke-gel dosimeters produced with Poly-vinyl alcohol and glutaraldehyde. Nucl. Technol. Radiat. Prot. 2017, 32, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Gallo, S.; Lizio, D.; Monti, A.F.; Veronese, I.; Brambilla, M.G.; Lenardi, C.; Torresin, A.; Gambarini, G. Temperature be-havior of radiochromic poly(vinyl-alcohol)–glutaraldehyde Fricke gel dosimeters in practice. J. Phys. D: Appl. Phys. 2020, 53, 365003. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Atsumi, H.; Hashimoto, K.; Shimada, Y.; Kudo, Y.; Endo, M.; Yokota, F.; Shida, J.; Yotsuyanagi, T. Highly sensitive colour change system within slight differences in metal ion concentrations based on homo–binuclear complex formation equilibrium for visual threshold detection of trace metal ions. Anal. Chim. Acta 2004, 527, 131–138. [Google Scholar] [CrossRef]
- Liosi, G.; Dondi, D.; Vander Griend, G.; Lazzaroni, S.; d’Agostino, G.; Mariani, M. Fricke-gel dosimeter: Overview of Xylenol Orange chemical behavior. Radiat. Phys. Chem. 2017, 140, 74–77. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Yotsuyanag, T. Visual Threshold Detection of Trace Metal Ions Using a Bi-Functional Metal-lochromic Reagent. Proceedings of IUPAC International Congress on Analytical Sciences 2001 (ICAS 2001), Tokyo, Japan, 6–10 August 2001. [Google Scholar]




| Year | Author | Gel Agent (GA) | GA (%) | FAS (mM) | XO (mM) |
|---|---|---|---|---|---|
| 2022 | Piotrowski et al. [29] | Pluronic F-127 | 25.0 | 0.01–5.00 | 0.03–0.50 |
| 2021 | Dudek et al. [28] | Pluronic F-127 | 25.0 | 1.00 | 0.165 |
| 2021 | Farajzadeh & Sina [30] | Gelatin | 0–220 mM | 0.02–2.50 | 0.02–0.20 |
| 2021 | Pérez et al. [31] | Gelatin | 3.0 | 1.0 | 0.165 |
| 2021 | Gallo et al. [32] | PVA + GTA | 8.0 | 0.5 | 0.165 |
| 2019 | Smith et al. [33] | PVA | 10.0–20.0 | 0.4 | 0.20–0.40 |
| Gelatin | 10.0 | 0.1–0.4 | 0.10–0.40 | ||
| 2019 | Vedelago et al. [34] | Gelatin | 4.0 | 0.3–0.6 | 0.10–0.20 |
| 2019 | Babu et al. [11] | Gelatin | 5.0 | 0.3 | 0.050 |
| 2019 | Lazzeri et al. [35] | PVA + GTA | 10.0–12.5 | 0.5 | 0.165 |
| 2018 | Lazzaroni et al. [36] | PVA + GTA | 10.0 | 0.5 | 0.165 |
| Gelatin | 3.0 | 0.5 | 0.165 | ||
| 2017 | Welch et al. [37] | Gelatin | 6.0 | 0.3 | 0.050 |
| 2017 | Marini et al. [38] | PVA + GTA | 9.1 | 0.5 | 0.165 |
| Gelatin | 2.9 | 0.5 | 0.165 | ||
| 2017 | Marrale et al. [39] | PVA + GTA | 10.0 | 1.5 | 0.165 |
| Agarose | 3.0 | 1.5 | 0.165 | ||
| 2017 | Soliman et al. [40] | Gelatin | 4.0 | 1.0 | 0.100 |
| 2017 | Gambarini et al. [41] | Gelatin | 3.0 | 1.0 | 0.165 |
| Agarose | 1.5 | 1.0 | 0.165 | ||
| 2017 | Del Lama et al. [42] | Gelatin | 0–250 mM | 0.3–5.0 | 0.05–0.25 |
| 2016 | El Gohary et al. [43] | Gelatin | 4.0 | 1.0 | 0.10 |
| 2014 | Marrale et al. [44] | Agarose | 3.0 | 0.5–5.0 | 0.165 |
| 2010 | Cavinato et al. [45] | Gelatin | 5.0 | 1.0 | 0.1 |
| 2009 | Babic et al. [46] | Gelatin | 6.0 | 1.0 | 0.05 |
| 2008 | Babic et al. [47] | Gelatin | 4.0 | 0.1–0.9 | 0.025–0.100 |
| 2008 | Davies et al. [48] | Gelatin | 3.85 | 1.0 | 0.10 |
| 2008 | Galante et al. [49] | Gelatin | 1.0, 5.0, 10.0 | 1.0 | 0.10 |
| 2003 | Healy et al. [6] | Agarose | 1.0 | 0.4 | 0.20 |
| 2002 | Hill et al. [50] | PVA | 20.0 | 0.4 | 0.40 |
| 2000 | Chu et al. [24] | PVA | 15.0, 20.0, 25.0 | 0.2–0.8 | 0.20–0.80 |
| 1997 | Pedersen et al. [51] | Gelatin | 4.0 | 1.5 | 1.50 |
| Agarose | 1.5–3.0 | ||||
| 1997 | Kron et al. [52] | Gelatin | 2.0–10.0 | 0.5–1.0 | 0.02–025 |
| Agarose | 1.0–1.5 | 0.25 | |||
| 1996 | Rae et al. [53] | Gelatin | 4.0 | 0.2 | 0.20 |
| 1996 | Tarte et al. [54] | Agarose | 1.0 | 0.4 | 0.20 |
| 1991 | Appleby et al. [55] | Agarose | 1.5 | 0.4 | 0.04–0.06 |
| 1987 | Appleby et al. [56] | Agarose | 1.5 | 0.2 | 0.0 |
| 1984 | Gore et al. [57] | Gelatin | 4.0 | 1.0 | 0.0 |
| SET | XO (mM) | FAS (mM) | [FAS]/[XO] Ratio |
|---|---|---|---|
| 1 | 0.200 | 0.05 | 0.25 |
| 2 | 0.200 | 0.10 | 0.50 |
| 3 | 0.200 | 0.40 | 2.00 |
| 4 | 0.200 | 0.60 | 3.00 |
| 5 | 0.200 | 1.00 | 5.00 |
| 6 | 0.200 | 5.00 | 25.00 |
| 7 | 0.020 | 0.40 | 20.00 |
| 8 | 0.040 | 0.40 | 10.00 |
| 9 | 0.080 | 0.40 | 5.00 |
| 10 | 0.133 | 0.40 | 3.00 |
| 11 | 0.166 | 0.40 | 2.40 |
| 12 | 0.200 | 0.40 | 2.00 |
| 13 | 0.240 | 0.40 | 1.67 |
| 14 | 0.400 | 0.40 | 1.00 |
| 15 | 0.800 | 0.40 | 0.50 |
| 16 | 0.133 | 0.40 | 3.00 |
| 17 | 0.166 | 0.50 | 3.00 |
| 18 | 0.200 | 0.60 | 3.00 |
| 19 | 0.166 | 0.40 | 2.40 |
| 20 | 0.166 | 0.50 | 3.00 |
| 21 | 0.166 | 0.60 | 3.60 |
| (FAS) mM | Slope (Gy−1) | R2 |
|---|---|---|
| 5.00 | 6.99 ± 0.05 | 0.9997 |
| 1.00 | 7.78 ± 0.06 | 0.9997 |
| 0.60 | 7.98 ± 0.06 | 0.9998 |
| 0.40 | 8.22 ± 0.07 | 0.9996 |
| (XO) mM | Slope (Gy−1) | Linear Dose Interval (Gy) | R2 |
|---|---|---|---|
| 0.800 | 4.97 ± 0.02 | 0–42 | 0.9999 |
| 0.400 | 6.52 ± 0.04 | 0–35 | 0.9998 |
| 0.240 | 7.88 ± 0.06 | 0–28 | 0.9997 |
| 0.200 | 8.22 ± 0.06 | 0–28 | 0.9998 |
| 0.166 | 8.37 ± 0.11 | 0–28 | 0.9991 |
| 0.133 | 8.78 ± 0.05 | 0–21 | 0.9999 |
| 0.080 | 9.07 ± 0.38 | 0–14 | 0.9966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, M.; Arosio, P.; Brambilla, E.; Gallo, S.; Lenardi, C.; Locarno, S.; Orsini, F.; Pignoli, E.; Pedicone, L.; Veronese, I. How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels 2022, 8, 204. https://doi.org/10.3390/gels8040204
Scotti M, Arosio P, Brambilla E, Gallo S, Lenardi C, Locarno S, Orsini F, Pignoli E, Pedicone L, Veronese I. How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels. 2022; 8(4):204. https://doi.org/10.3390/gels8040204
Chicago/Turabian StyleScotti, Martina, Paolo Arosio, Elisa Brambilla, Salvatore Gallo, Cristina Lenardi, Silvia Locarno, Francesco Orsini, Emanuele Pignoli, Luca Pedicone, and Ivan Veronese. 2022. "How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study" Gels 8, no. 4: 204. https://doi.org/10.3390/gels8040204
APA StyleScotti, M., Arosio, P., Brambilla, E., Gallo, S., Lenardi, C., Locarno, S., Orsini, F., Pignoli, E., Pedicone, L., & Veronese, I. (2022). How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels, 8(4), 204. https://doi.org/10.3390/gels8040204







