Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation
Abstract
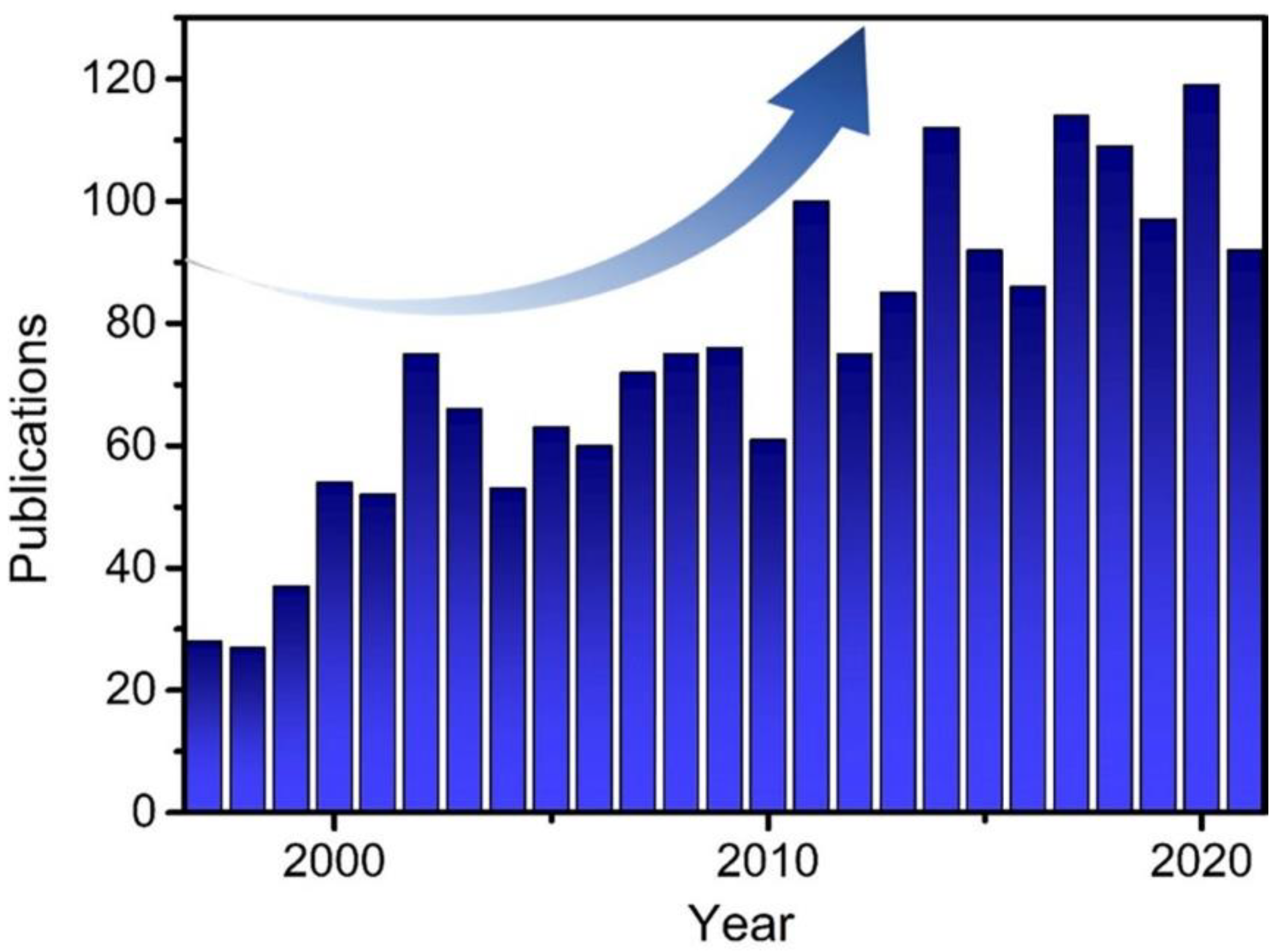
:1. Introduction
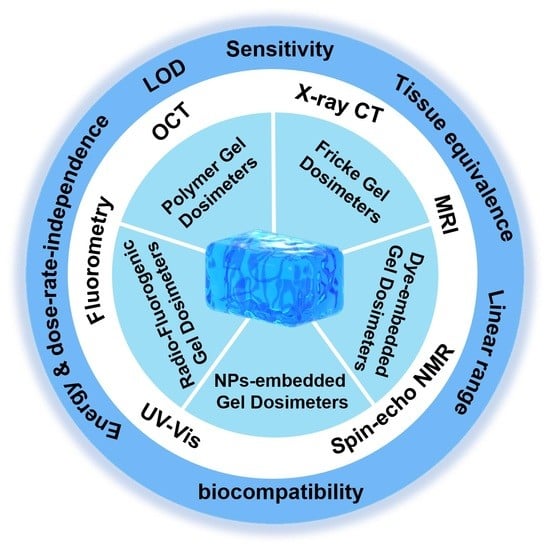
2. Gel Dosimeters
2.1. Polymer Dosimeters
2.2. Fricke Gel Dosimeters
2.3. Radio-Chromic Gel Dosimeters
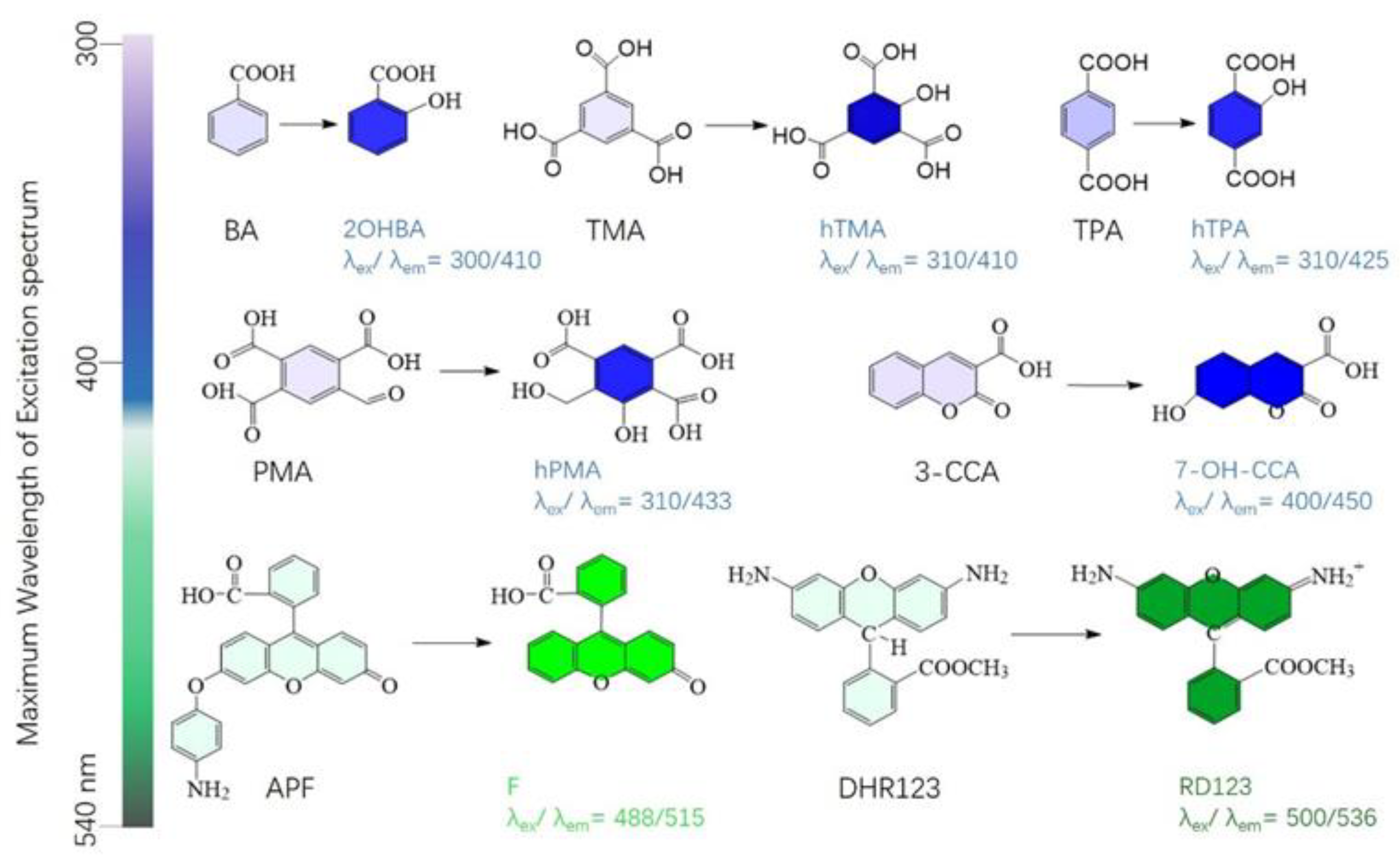
2.4. Radio-Fluorogenic Gel Dosimeters
2.5. Gel Dosimeters with Embedded NPs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dowlath, M.J.H.; Karuppannan, S.K.; Sinha, P.; Dowlath, N.S.; Arunachalam, K.D.; Ravindran, B.; Chang, S.W.; Nguyen-Tri, P.; Nguyen, D.D. Effects of radiation and role of plants in radioprotection: A critical review. Sci. Total Environ. 2021, 779, 146431. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, S.; Cheng, L.; Xie, J.; Zhai, F.; He, Y.; Wang, Y.; Chai, Z.; Wang, S. Thermoplastic membranes incorporating semiconductive metal–organic frameworks: An advance on flexible X-ray detectors. Angew. Chem. Int. Ed. 2020, 59, 11856–11860. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.; Witte, M.; Moser, T.; Lang, C.; Runz, A.; Johnen, W.; Berger, M.; Biederer, J.; Karger, C.P. 3D dosimetric validation of motion compensation concepts in radiotherapy using an anthropomorphic dynamic lung phantom. Phys. Med. Biol. 2017, 62, 573–595. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, P.; Trattner, S.; Wiegert, J.; Gerland, E.-L.; Shefer, E.; Morton, T.; Thompson, C.M.; Cheng, B.; Halliburton, S.S.; Einstein, A.J. High correlation between radiation dose estimates for 256-slice CT obtained by highly parallelized hybrid Monte Carlo computation and solid-state metal-oxide semiconductor field-effect transistor measurements in physical anthropomorphic phantoms. Med. Phys. 2019, 46, 5216–5226. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. Chapter 14—Characterisation of Radioactive Waste. In An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Ojovan, M.I., Lee, W.E., Kalmykov, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–215. [Google Scholar]
- Cho, J.D.; Son, J.; Sung, J.W.; Choi, C.H.; Kim, J.S.; Wu, H.G.; Park, J.M.; Kim, J.I. Flexible film dosimeter for in vivo dosimetry. Med. Phys. 2020, 47, 3204–3213. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, C.P.; Xin, J.; Chen, H.F.; Xing, Z.X.; Qu, W.W.; Hu, L.; Chen, X.J.; Wang, R.S. Performance of a plastic scintillation fiber dosimeter based on different photoelectric devices. Nucl. Sci. Tech. 2021, 32, 120. [Google Scholar] [CrossRef]
- Sun, L.M.; Huang, C.J.; Chen, H.Y.; Meng, F.Y.; Lu, T.H.; Tsao, M.J. Using a thermoluminescent dosimeter to evaluate the location reliability of the highest-skin dose area detected by treatment planning in radiotherapy for breast cancer. Med. Dosim. 2014, 39, 348–353. [Google Scholar] [CrossRef]
- Hill, R.; Mo, Z.; Haque, M.; Baldock, C. An evaluation of ionization chambers for the relative dosimetry of kilovoltage X-ray beams. Med. Phys. 2009, 36, 3971–3981. [Google Scholar] [CrossRef]
- Goncalves, J.A.C.; Pereira, L.N.; Potiens, M.P.A.; Vivolo, V.; Bueno, C.C. Evaluation of epitaxial silicon diodes as dosimeters in X-ray mammography. Radiat. Meas. 2014, 71, 384–388. [Google Scholar] [CrossRef]
- Marrale, M.; d’Errico, F. Hydrogels for three-dimensional ionizing-radiation dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef]
- Farhood, B.; Geraily, G.; Abtahi, S.M.M. A systematic review of clinical applications of polymer gel dosimeters in radiotherapy. Appl. Radiat. Isot. 2019, 143, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, Z.A.; Geraily, G. A review study on application of gel dosimeters in low energy radiation dosimetry. Appl. Radiat. Isot. 2022, 179, 110015. [Google Scholar] [CrossRef] [PubMed]
- Khajeali, A.; Farajollahi, A.R.; Khodadadi, R.; Kasesaz, Y.; Khalili, A. Role of gel dosimeters in boron neutron capture therapy. Appl. Radiat. Isot. 2015, 103, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ceberg, S.; Lepage, M.; Bäck, S.Å.J.; Gustafsson, H.; Ceberg, C. Modelling the dynamic dose response of an nMAG polymer gel dosimeter. Phys. Med. Biol. 2012, 57, 4845–4853. [Google Scholar] [CrossRef] [PubMed]
- Parwaie, W.; Yarahmadi, M.; Nedaie, H.A.; Zahmatkesh, M.H.; Barati, A.H.; Afkhami, M. Evaluation of MRI-based MAGIC polymer gel dosimeter in small photon fields. Int. J. Radiat. Res. 2016, 14, 59–65. [Google Scholar] [CrossRef]
- Kawamura, H.; Sakae, T.; Terunuma, T.; Ishida, M.; Shibata, Y.; Matsumura, A. Evaluation of three-dimensional polymer gel dosimetry using X-ray CT and R2 MRI. Appl. Radiat. Isot. 2013, 77, 94–102. [Google Scholar] [CrossRef]
- Campbell, W.G.; Jirasek, A.; Wells, D.M. Radiation-induced refraction artefacts in the optical CT readout of polymer gel dosimeters. Med. Phys. 2014, 41, 112102. [Google Scholar] [CrossRef]
- Mather, M.L.; Collings, A.F.; Bajenov, N.; Whittaker, A.K.; Baldock, C. Ultrasonic absorption in polymer gel dosimeters. Ultrasonics 2003, 41, 551–559. [Google Scholar] [CrossRef]
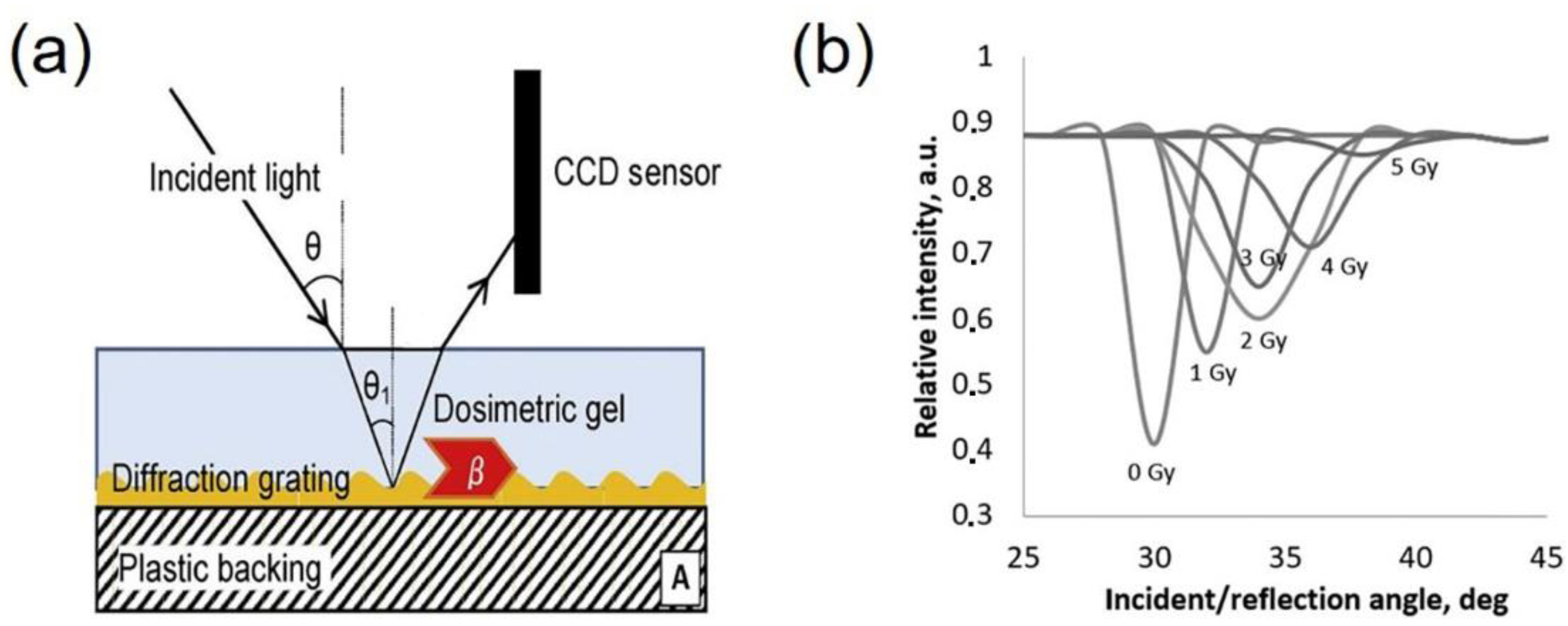
- Adliene, D.; Jakstas, K.; Vaiciunaite, N. Application of optical methods for dose evaluation in normoxic polyacrylamide gels irradiated at two different geometries. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 741, 88–94. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Gore, J.C.; Kennan, R.P.; Schulz, R.J. NMR relaxation enhancement in gels polymerized and cross-linked by ionizing radiation: A new approach to 3D dosimetry by MRI. Magn. Reson. Imaging 1993, 11, 253–258. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic resonance imaging of radiation dose distributions using a polymer-gel dosimeter. Phys. Med. Biol. 1994, 39, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Simola, J.; Savolainen, S.; Kangasmaki, A.; Heikkinen, S.; Perkio, J.; Ramadan, U.A.; Seppala, T.; Karila, J.; Seren, T.; Kotiluoto, P.; et al. Study of the relative dose-response of BANG-3((R)) polymer gel dosimeters in epithermal neutron irradiation. Phys. Med. Biol. 2003, 48, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Adliene, D.; Urbonavicius, B.G.; Laurikaitiene, J.; Puiso, J. New application of polymer gels in medical radiation dosimetry: Plasmonic sensors. Radiat. Phys. Chem. 2020, 168, 108609. [Google Scholar] [CrossRef]
- Abtahi, S.M.M.; Anaraki, V.; Farhood, B.; Mahdavi, S.R. Assessment of photon energy and dose rate dependence of U-NIPAM polymer gel dosimeter. Radiat. Phys. Chem. 2020, 172, 108784. [Google Scholar] [CrossRef]
- Abtahi, S.M. Characteristics of a novel polymer gel dosimeter formula for MRI scanning: Dosimetry, toxicity and temporal stability of response. Phys. Med. Eur. J. Med. Phys. 2016, 32, 1156–1161. [Google Scholar] [CrossRef]
- Rajaee, A.; Wang, S.; Zhao, L.; Liu, Y. Gel dosimetry measurement of dose enhancement bismuth-based nanoparticles in radiation therapy. J. Phys. Conf. Ser. 2019, 1305, 012046. [Google Scholar] [CrossRef] [Green Version]
- Fuse, H.; Oyama, S.; Yasue, K.; Ito, S.; Sato, T.; Fujisaki, T.; Abe, S.; Oyama, K.; Suzuki, A.; Yoshizaw, T.; et al. Design and characteristics of an agar additive polymer gel dosimeter. Appl. Radiat. Isot. 2019, 151, 62–66. [Google Scholar] [CrossRef]
- Mustaqim, A.S.; Yahaya, N.Z.; Razak, N.N.A.; Zin, H. The dose enhancement of MAGAT gel dosimeter doped with zinc oxide at 6 MV photon beam. Radiat. Phys. Chem. 2020, 172, 108739. [Google Scholar] [CrossRef]
- Hsieh, B.T.; Chiang, C.T.; Hung, P.H.; Kao, C.H.; Liang, J.A. Preliminary investigation of a new type of propylene based gel dosimeter (DEMBIG). J. Radioanal. Nucl. Chem. 2011, 288, 799–803. [Google Scholar] [CrossRef]
- Chiang, C.-T.; Chang, Y.-J.; Huang, S.-K.; Jang, C.-J.; Hsieh, B.-T. Optimal composition of a new polymer gel dosimeter-DEMBIG. J. Radioanal. Nucl. Chem. 2011, 290, 59–65. [Google Scholar] [CrossRef]
- Shih, T.Y.; Shih, C.T.; Chang, Y.J.; Yu, C.Y.; Hsieh, B.T.; Chang, S.J.; Liang, J.A.; Wu, J. Evaluating the characteristics of a novel DEMBIG gel dosimeter using computed tomography. IEEE Trans. Nucl. Sci. 2013, 60, 716–721. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Al-Ajaleen, M.S.; Abuzayed, M.H.; Aldweri, F.M.; Eyadeh, M.M. High dose sensitivity of N-(isobutoxymethyl)acrylamide polymer gel dosimeters with improved monomer solubility using acetone co-solvent. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 442, 67–72. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Smadi, S.A.; Rabaeh, K.A.; Oglat, A.A.; Diamond, K.R. Effect of lithium chloride inorganic salt on the performance of N-(Hydroxymethyl)acrylamide polymer-gel dosimeter in radiation therapy. J. Radioanal. Nucl. Chem. 2021, 330, 1255–1261. [Google Scholar] [CrossRef]
- Maraghechi, B.; Gach, H.M.; Setianegara, J.; Yang, D.; Li, H.H. Dose uncertainty and resolution of polymer gel dosimetry using an MRI guided radiation therapy system’s onboard 0.35 T scanner. Phys. Med. 2020, 73, 8–12. [Google Scholar] [CrossRef]
- Mariotti, V.; Gayol, A.; Pianoschi, T.; Mattea, F.; Vedelago, J.; Pérez, P.; Valente, M.; Alva-Sánchez, M. Radiotherapy dosimetry parameters intercomparison among eight gel dosimeters by Monte Carlo simulation. Radiat. Phys. Chem. 2022, 190, 109782. [Google Scholar] [CrossRef]
- Kudrevicius, L.; Jaselske, E.; Adliene, D.; Rudzianskas, V.; Radziunas, A.; Tamasauskas, A. Application of 3D Gel Dosimetry as a Quality Assurance Tool in Functional Leksell Gamma Knife Radiosurgery. Gels 2022, 8, 69. [Google Scholar] [CrossRef]
- Elter, A.; Dorsch, S.; Marot, M.; Gillmann, C.; Johnen, W.; Runz, A.; Spindeldreier, C.K.; Klüter, S.; Karger, C.P.; Mann, P. RSC: Gel dosimetry as a tool for clinical implementation of image-guided radiotherapy. J. Phys. Conf. Ser. 2022, 2167, 012020. [Google Scholar] [CrossRef]
- Jordan, K.; De Deene, Y. Making and assessing 3D dosimeters. J. Phys. Conf. Ser. 2019, 1305, 012037. [Google Scholar] [CrossRef]
- Deene, Y.D.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A basic study of some normoxic polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 3441–3463. [Google Scholar] [CrossRef]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys. Med. Biol. 2001, 46, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak, M.; Maras, P.; Kozicki, M. Characterization of a new N-vinylpyrrolidone-containing polymer gel dosimeter with Pluronic F-127 gel matrix. Radiat. Phys. Chem. 2020, 177, 109125. [Google Scholar] [CrossRef]
- Deyhimihaghighi, N.; Noor, N.M.; Soltani, N.; Jorfi, R.; Haghir, M.E.; Adenan, M.Z.; Saion, E.; Khandaker, M.U. Contrast enhancement of magnetic resonance imaging (MRI) of polymer gel dosimeter by adding Platinum nano- particles. J. Phys. Conf. Ser. 2014, 546, 012013. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Akasaka, H.; Geso, M.; Morita, K.; Yada, R.; Uehara, K.; Sasaki, R. Utilisation of the chemiluminescence method to measure the radiation dose enhancement caused by gold nanoparticles: A phantom-based study. Radiat. Meas. 2020, 134, 106317. [Google Scholar] [CrossRef]
- Sabbaghizadeh, R.; Shamsudin, R.; Deyhimihaghighi, N.; Sedghi, A. Enhancement of dose response and nuclear magnetic resonance image of PAGAT polymer gel dosimeter by adding silver nanoparticles. PLoS ONE 2017, 12, e0168737. [Google Scholar] [CrossRef] [PubMed]
- Rabaeh, K.A.; Hammoudeh, I.M.E.; Eyadeh, M.M.; Aldweri, F.M.; Awad, S.I.; Oglat, A.A.; Shatnawi, M.T.M. Improved performance of N-(Hydroxymethyl)acrylamide gel dosimeter using potassium chloride for radiotherapy. Radiat. Meas. 2021, 142, 106542. [Google Scholar] [CrossRef]
- Chacón, D.; Strumia, M.; Valente, M.; Mattea, F. Effect of inorganic salts and matrix crosslinking on the dose response of polymer gel dosimeters based on acrylamide. Radiat. Meas. 2018, 117, 7–18. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S.; Schulz, R.J. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef]
- Araujo, B.C.R.; Ferreira, B.D.L.; Virtuoso, L.S.; Meira-Belo, L.C.; Fonseca, T.C.F.; Santos, Â.M.M.; Lula, I.; Sebastião, R.C.O. A new formulation for polymer fricke dosimeter and an innovative application of neural network to study dose profile from spin-echo NMR data. Radiat. Phys. Chem. 2021, 184, 109444. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Preparation of W1/O/W2 emulsion to limit the diffusion of Fe3+ in the Fricke gel 3D dosimeter. Polym. Adv. Technol. 2020, 31, 2127–2135. [Google Scholar]
- Gallo, S.; Bettega, D.; Gambarini, G.; Lenardi, C.; Veronese, I. Studies of Fricke-PVA-GTA xylenol orange hydrogels for 3D measurements in radiotherapy dosimetry. AIP Conf. Proc. 2019, 2160, 050007. [Google Scholar]
- Chu, K.C.; Jordan, K.J.; Battista, J.J.; Van Dyk, J.; Rutt, B.K. Polyvinyl alcohol-Fricke hydrogel and cryogel: Two new gel dosimetry systems with low Fe3+ diffusion. Phys. Med. Biol. 2000, 45, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Gambarini, G.; Veronese, I.; Argentiere, S.; Gargano, M.; Ianni, L.; Lenardi, C.; Ludwig, N.; Pignoli, E.; d’Errico, F. Does the gelation temperature or the sulfuric acid concentration influence the dosimetric properties of radiochromic PVA-GTA Xylenol Orange Fricke gels? Radiat. Phys. Chem. 2019, 160, 35–40. [Google Scholar] [CrossRef]
- Smith, S.T.; Boase, N.R.B.; Masters, K.S.; Hosokawa, K.; Asena, A.; Crowe, S.B.; Kairn, T.; Trapp, J.V. A very low diffusion Fricke gel dosimeter with functionalised xylenol orange-PVA (XOPVA). Phys. Med. Biol. 2019, 64, 205017. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Liosi, G.M.; Mariani, M.; Dondi, D. An innovative Fe3+ selective ligand for Fricke-gel dosimeter. Radiat. Phys. Chem. 2020, 171, 108733. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.L.; Fukasaku, K.; Fukuda, S. Radiological properties of nanocomposite Fricke gel dosimeters for heavy ion beams. J. Radiat. Res. 2016, 57, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Soliman, Y.S.; El Gohary, M.I.; Abdel Gawad, M.H.; Amin, E.A.; Desouky, O.S. Fricke gel dosimeter as a tool in quality assurance of the radiotherapy treatment plans. Appl. Radiat. Isot. 2017, 120, 126–132. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Hailat, T.F.; Aldweri, F.M. Evaluation of ferrous Methylthymol blue gelatin gel dosimeters using nuclear magnetic resonance and optical techniques. Radiat. Meas. 2018, 108, 26–33. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Hailat, T.F.; Al-Shorman, M.Y.; Aldweri, F.M.; Kanan, H.M.; Awad, S.I. Investigation of a novel chemically cross-linked fricke-Methylthymol blue-synthetic polymer gel dosimeter with glutaraldehyde cross-linker. Radiat. Meas. 2018, 118, 77–85. [Google Scholar] [CrossRef]
- Parwaie, W.; Geraily, G.; Shirazi, A.; Mehri-Kakavand, G.; Farzin, M. Evaluation of ferrous benzoic methylthymol-blue gel as a dosimeter via magnetic resonance imaging. Phys. Med. 2020, 80, 47–56. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Aldweri, F.M.; Al-Shorman, M.Y.; Alheet, S.M.; Awad, S.I.; Hailat, T.F. Nuclear magnetic resonance analysis of a chemically cross-linked ferrous-methylthymol blue-polyvinyl alcohol radiochromic gel dosimeter. Appl. Radiat. Isot. 2019, 153, 108812. [Google Scholar] [CrossRef] [PubMed]
- Colnot, J.; Huet, C.; Gschwind, R.; Clairand, I. Characterisation of two new radiochromic gel dosimeters TruView™ and ClearView™ in combination with the vista™ optical CT scanner: A feasibility study. Phys. Med. 2018, 52, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, A.A.; Beshir, W.B.; Hassan, H.M.; Soliman, Y.S. Radiation-induced coloration of nitro blue tetrazolium gel dosimeter for low dose applications. Radiat. Meas. 2017, 100, 18–26. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Aljammal, S.A.; Eyadeh, M.M.; Abumurad, K.M. Methyl thymol blue solution and film dosimeter for high dose measurements. Results Phys. 2021, 23, 103980. [Google Scholar] [CrossRef]
- Pilařová, K.; Kozubíková, P.; Šolc, J.; Spěváček, V. Characteristics of polyacrylamide gel with THPC and Turnbull Blue gel dosimeters evaluated using optical tomography. Radiat. Phys. Chem. 2014, 104, 283–286. [Google Scholar] [CrossRef]
- Maeyama, T.; Hase, S. Nanoclay gel-based radio-fluorogenic gel dosimeters using various fluorescence probes. Radiat. Phys. Chem. 2018, 151, 42–46. [Google Scholar] [CrossRef]
- Maeyama, T.; Kato, A.; Mochizuki, A.; Sato, N.; Watanabe, Y.; Mizukami, S. Dose-rate-independent and diffusion-free nanoclay-based radio-fluorogenic gel dosimeter. Sens. Actuators A Phys. 2019, 298, 111435. [Google Scholar] [CrossRef]
- Mochizuki, A.; Maeyama, T.; Watanabe, Y.; Mizukami, S. Sensitivity enhancement of DHR123 radio-fluorogenic nanoclay gel dosimeter by incorporating surfactants and halogenides. RSC Adv. 2020, 10, 28798–28806. [Google Scholar] [CrossRef]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-Fluorogenic Gel Dosimetry with Coumarin. Gels 2018, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Nie, J.; Hu, R.; Zhao, R.; Zhu, W.; Chen, X.; Li, D.; Wang, L.; Hu, L. A nanogel sensor for colorimetric fluorescence measurement of ionizing radiation doses. Chem. Commun. 2019, 55, 9614–9617. [Google Scholar] [CrossRef]
- Jiang, L.; Li, W.X.; Nie, J.; Wang, R.S.; Chen, X.J.; Fan, W.H.; Hu, L. Fluorescent Nanogel Sensors for X-ray Dosimetry. ACS Sens. 2021, 6, 1643–1648. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, X.; Yang, N.; Huang, Z.; Huang, L.; Fang, Z.; Zhang, C.; Li, L.; Yu, C. Surface engineering strategies of gold nanomaterials and their applications in biomedicine and detection. J. Mater. Chem. B 2021, 9, 5583–5598. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.R.; Pushpavanam, K.; Nair, D.G.; Potta, T.; Sutiyoso, C.; Kodibagkar, V.D.; Sapareto, S.; Chang, J.; Rege, K. Generation of polypeptide-templated gold nanoparticles using ionizing radiation. Langmuir 2013, 29, 10166–10173. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Pushpavanam, K.; Lentz, J.M.; Bues, M.; Anand, A.; Rege, K. Hydrogel nanosensors for colorimetric detection and dosimetry in proton beam radiotherapy. ACS Appl. Mater. Interfaces 2018, 10, 3274–3281. [Google Scholar] [CrossRef] [PubMed]
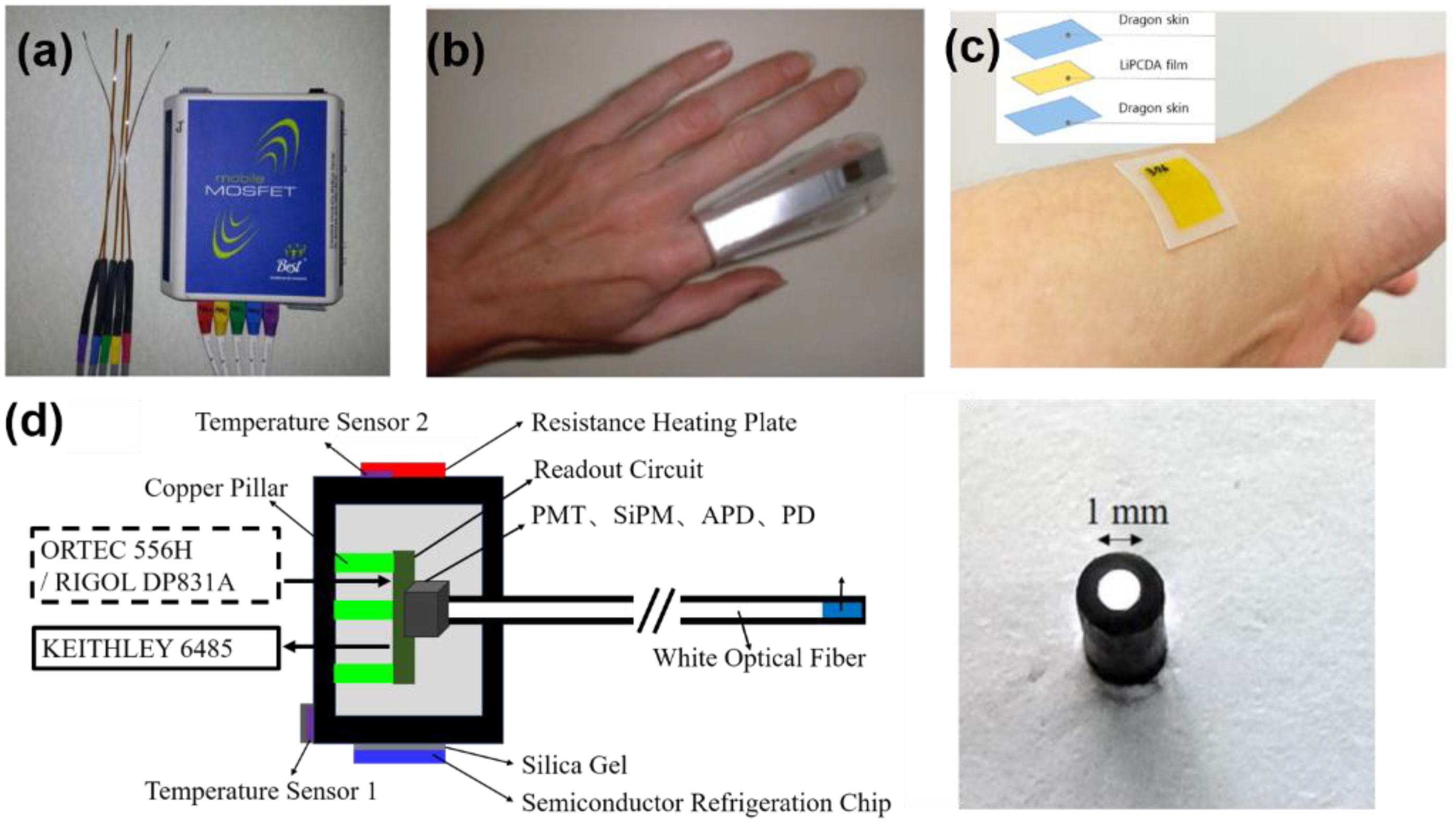
- Kohno, R.; Hotta, K.; Matsubara, K.; Nishioka, S.; Matsuura, T.; Kawashima, M. In vivo proton dosimetry using a MOSFET detector in an anthropomorphic phantom with tissue inhomogeneity. J. Appl. Clin. Med. Phys. 2012, 13, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pushpavanam, K.; Inamdar, S.; Dutta, S.; Bista, T.; Sokolowski, T.; Boshoven, E.; Sapareto, S.; Rege, K. Determination of topographical radiation dose profiles using gel nanosensors. Sci. Adv. 2019, 5, eaaw8704. [Google Scholar] [CrossRef] [Green Version]
- Tadros, S.M.; Soliman, Y.S.; Beshir, W.B.; Saad, G.R.; Ali, L. Dosimetric investigations on radiation-induced Ag nanoparticles in a gel dosimeter. J. Radioanal. Nucl. Chem. 2021, 329, 463–473. [Google Scholar] [CrossRef]
- Naghavi, K.; Saion, E.; Rezaee, K.; Yunus, W.M.M. Influence of dose on particle size of colloidal silver nanoparticles synthesized by gamma radiation. Radiat. Phys. Chem. 2010, 79, 1203–1208. [Google Scholar] [CrossRef]
- Eisa, W.H.; Abdel-Moneam, Y.K.; Shaaban, Y.; Abdel-Fattah, A.A.; Abou Zeid, A.M. Gamma-irradiation assisted seeded growth of Ag nanoparticles within PVA matrix. Mater. Chem. Phys. 2011, 128, 109–113. [Google Scholar] [CrossRef]
- Soliman, Y.S. Gamma-radiation induced synthesis of silver nanoparticles in gelatin and its application for radiotherapy dose measurements. Radiat. Phys. Chem. 2014, 102, 60–67. [Google Scholar] [CrossRef]
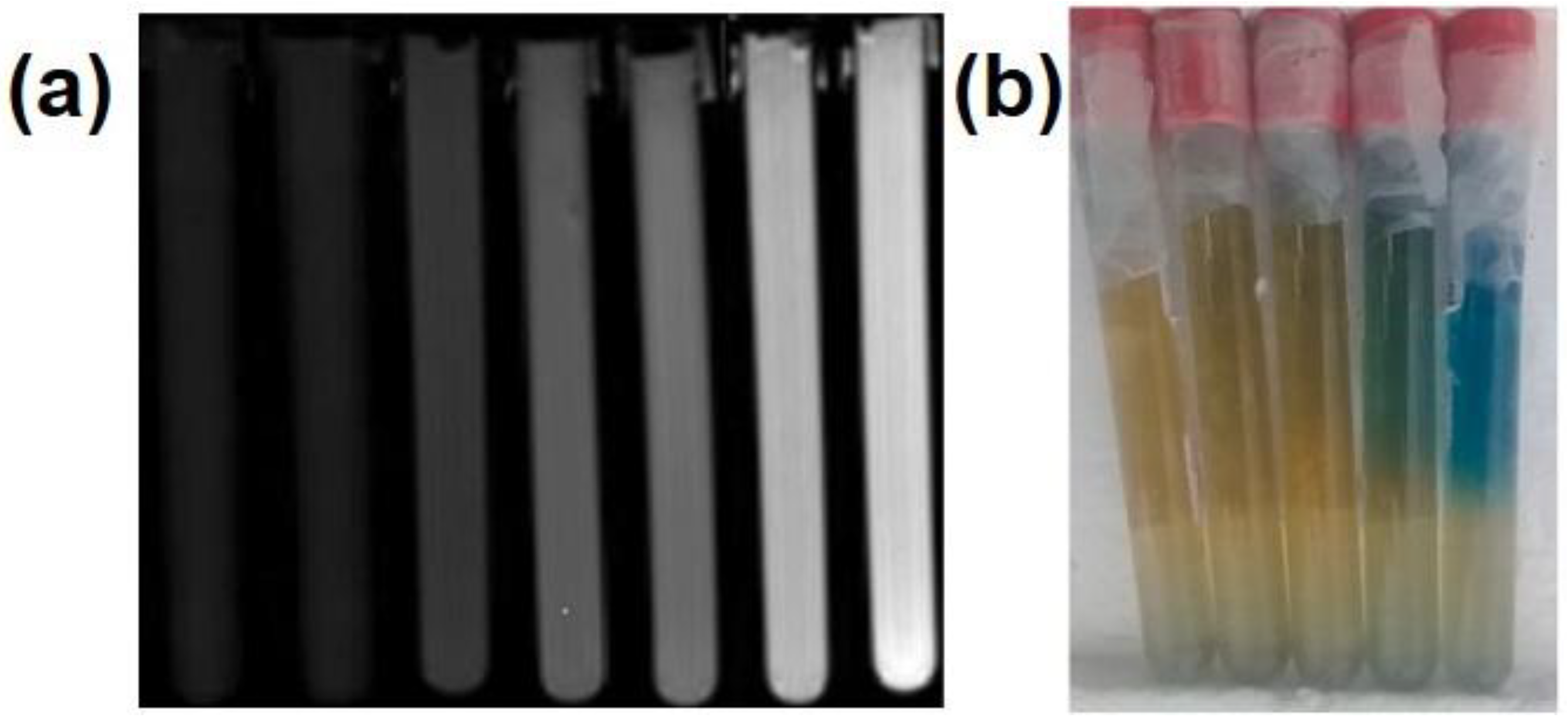



| Dosimeter | Main Composition | Beam | Linear Range (Gy) | LOD (Gy) | Measurement |
|---|---|---|---|---|---|
| BANANA | AAm, BIS, agarose [22] | X-ray | 0–20 | ~4 | MRI |
| BANG | AAm, BIS, gelatin [23] | X-ray | 0–8 | 1 | MRI |
| BANG-3 | AAc, BIS, gelatin [24] | γ-ray | 0.5–3.5 | 0.5 | MRI |
| neutron | 0–2.5 | <0.5 | |||
| nPAG | AAm, BIS, gelatin [25] | X-ray | 0–5 | 0.2 | SPR |
| U-NIPAM | NIPAm, BIS, agarose [26] | X-ray | 0–7 | 1 | MRI |
| PAMPSGAT | AMPS, BIS, gelatin [27] | γ-ray | 10–40 | 10 | MRI |
| MAGIC | MAA, AA, gelatin [28] | X-ray | 0–16 | 4 | MRI |
| MAGT-A | MAA, gelatin, agar [29] | X-ray | 0–10 | 2 | MRI |
| MAGAT | MAA, gelatin [30] | X-ray | 1–10 | 1 | UV-Vis |
| DEMBIG | DEMA, BIS, gelatin | X-ray | 0–20 | 5 | MRI [31] |
| DEMBIG | DEMA, BIS, gelatin | X-ray | 0–30 | 1 | Laser optic tomography [32] |
| DEMBIG | DEMA, BIS, gelatin | X-ray | 1–25 | 1 | CT [33] |
| NIBMAGAT | NIBMA, BIS, gelatin [34] | X-ray | 0–10 | 2 | MRI |
| NHMA | NHMA, BIS, gelatin [35] | X-ray | 0–6 | 1 | MRI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Jiang, L.; Chen, H.; Hu, L. Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels 2022, 8, 238. https://doi.org/10.3390/gels8040238
Zhang P, Jiang L, Chen H, Hu L. Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels. 2022; 8(4):238. https://doi.org/10.3390/gels8040238
Chicago/Turabian StyleZhang, Ping, Li Jiang, Hong Chen, and Liang Hu. 2022. "Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation" Gels 8, no. 4: 238. https://doi.org/10.3390/gels8040238
APA StyleZhang, P., Jiang, L., Chen, H., & Hu, L. (2022). Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels, 8(4), 238. https://doi.org/10.3390/gels8040238