Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microscopic Evaluation
2.2. Colorimetry
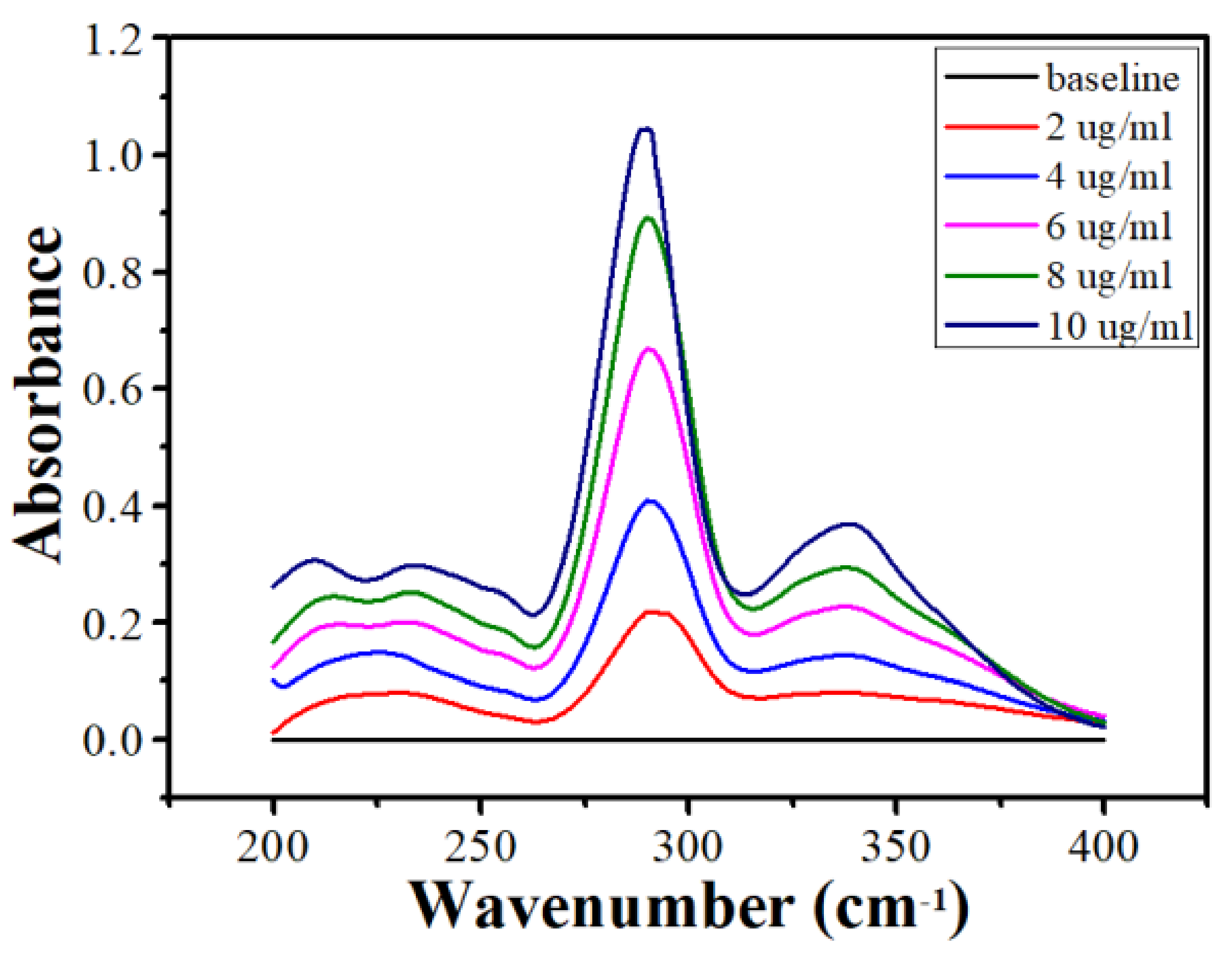
2.3. FTIR Spectroscopy
2.4. Analysis of Impedance
2.5. Stress Relaxation Studies
2.6. Drug Release Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Formulations
4.2.1. Preparation of the Oleogel
4.2.2. Preparation of the GG Hydrogels
4.2.3. Preparation of the Bigels
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as drug delivery systems: From their components to their applications. Drug Discov. Today 2021, 27, 1008–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, X.; Chen, J.; Pan, Y.; Yang, M.; Lu, L.; Yang, J.; Suo, Z.; Lu, T. Hydrogel–mesh composite for wound closure. Proc. Natl. Acad. Sci. USA 2021, 118, e2103457118. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Samui, T.; Goldenisky, D.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The development and characterization of novel in-situ bigel formulation. Food Hydrocoll. 2021, 113, 106416. [Google Scholar] [CrossRef]
- Soni, K.; Gour, V.; Agrawal, P.; Haider, T.; Kanwar, I.L.; Bakshi, A.; Soni, V. Carbopol-olive oil-based bigel drug delivery system of doxycycline hyclate for the treatment of acne. Drug Dev. Ind. Pharm. 2021, 47, 954–962. [Google Scholar] [CrossRef]
- Zhuang, X.; Clark, S.; Acevedo, N. Bigels—Oleocolloid matrices—As probiotic protective systems in yogurt. J. Food Sci. 2021, 86, 4892–4900. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf. B Biointerfaces 2014, 123, 582–592. [Google Scholar] [CrossRef]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.; Pal, K. Stearate organogel–gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Kodela, S.P.; Pandey, P.M.; Nayak, S.K.; Uvanesh, K.; Anis, A.; Pal, K. Novel agar–stearyl alcohol oleogel-based bigels as structured delivery vehicles. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 669–678. [Google Scholar] [CrossRef]
- Satapathy, S.; Singh, V.K.; Sagiri, S.S.; Agarwal, T.; Banerjee, I.; Bhattacharya, M.K.; Kumar, N.; Pal, K. Development and characterization of gelatin-based hydrogels, emulsion hydrogels, and bigels: A comparative study. J. Appl. Polym. Sci. 2015, 132, 41502. [Google Scholar] [CrossRef]
- Hasda, A.M.; Vuppaladadium, S.S.R.; Qureshi, D.; Prasad, G.; Mohanty, B.; Banerjee, I.; Shaikh, H.; Anis, A.; Sarkar, P.; Pal, K. Graphene oxide reinforced nanocomposite oleogels improves corneal permeation of drugs. J. Drug Deliv. Sci. Technol. 2020, 60, 102024. [Google Scholar] [CrossRef]
- Dhal, S.; Pal, K.; Banerjee, I.; Giri, S. Upconversion nanoparticle incorporated oleogel as probable skin tissue imaging agent. Chem. Eng. J. 2020, 379, 122272. [Google Scholar] [CrossRef]
- Lee, S. Utilization of foam structured hydroxypropyl methylcellulose for oleogels and their application as a solid fat replacer in muffins. Food Hydrocoll. 2018, 77, 796–802. [Google Scholar]
- Chen, C.; Zhang, C.; Zhang, Q.; Ju, X.; Wang, Z.; He, R. Study of monoglycerides enriched with unsaturated fatty acids at sn-2 position as oleogelators for oleogel preparation. Food Chem. 2021, 354, 129534. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of Biodegradable Hydrophilic and Hydrophobic Emulsifiers on the Oleogels Containing Sunflower Wax and Sunflower Oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef]
- Qureshi, D.; Nadikoppula, A.; Mohanty, B.; Anis, A.; Cerqueira, M.; Varshney, M.; Pal, K. Effect of carboxylated carbon nanotubes on physicochemical and drug release properties of oleogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125695. [Google Scholar] [CrossRef]
- Dhal, S.; Qureshi, D.; Mohanty, B.; Maji, S.; Anis, A.; Kim, D.; Sarkar, P.; Pal, K. Kokum butter and rice bran oil-based oleogels as novel ocular drug delivery systems. In Advances and Challenges in Pharmaceutical Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–179. [Google Scholar]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef]
- Martinez, R.; Rosado, C.; Velasco, M.; Lannes, S.; Baby, A. Main features and applications of organogels in cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; del Pilar Medina-Herrera, N.; Castro-López, C.; Martínez-Ávila, G.C.G. Candelilla wax: Prospective suitable applications within the food field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Navarro-Guajardo, N.; García-Carrillo, E.M.; Espinoza-González, C.; Téllez-Zablah, R.; Dávila-Hernández, F.; Romero-García, J.; Ledezma-Pérez, A.; Mercado-Silva, J.A.; Torres, C.A.P.; Pariona, N. Candelilla wax as natural slow-release matrix for fertilizers encapsulated by spray chilling. J. Renew. Mater. 2018, 6, 226–236. [Google Scholar] [CrossRef]
- Redondas, C.E.; Baümler, E.R.; Carelli, A.A. Sunflower wax recovered from oil tank settlings: Revaluation of a waste product from the oilseed industry. J. Sci. Food Agric. 2020, 100, 201–211. [Google Scholar] [CrossRef]
- Temkov, M.; Mureșan, V. Tailoring the Structure of Lipids, Oleogels and Fat Replacers by Different Approaches for Solving the Trans-Fat Issue—A Review. Foods 2021, 10, 1376. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, S.; Patel, K.K. Effect of sowing dates on severity of the pathogen Myrothecium leaf spot of sesame (Sesamum indicum L.). Pharma Innov. J. 2022, 11, 504–506. [Google Scholar]
- Feng, W.; Qin, C.; Chu, Y.; Berton, M.; Lee, J.B.; Zgair, A.; Bettonte, S.; Stocks, M.J.; Constantinescu, C.S.; Barrett, D.A.; et al. Natural sesame oil is superior to pre-digested lipid formulations and purified triglycerides in promoting the intestinal lymphatic transport and systemic bioavailability of cannabidiol. Eur. J. Pharm. Biopharm. 2021, 162, 43–49. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Moon, K.-D. Non-destructive discrimination of sesame oils via hyperspectral image analysis. J. Food Compos. Anal. 2020, 90, 103505. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2019, 112, 722–735. [Google Scholar] [CrossRef]
- Abdel-raouf, M.E.-S.; Sayed, A.; Mostafa, M. Application of Guar Gum and Its Derivatives in Agriculture. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–17. [Google Scholar]
- Palumbo, G.; Berent, K.; Proniewicz, E.; Banaś, J. Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials 2019, 12, 2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.; Xu, X.; Babiker, D.M.; Xu, J.; Yang, S. Complex membrane of cellulose and chitin nanocrystals with cationic guar gum for oil/water separation. J. Appl. Polym. Sci. 2019, 136, 47947. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and Characterization of Alpha and Nanocrystalline Cellulose from Date Palm (Phoenix dactylifera L.) Trunk Mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Maharana, V.; Gaur, D.; Nayak, S.K.; Singh, V.K.; Chakraborty, S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing the inner phase of the filled hydrogels with CNTs alters drug release properties and human keratinocyte morphology: A study on the gelatin- tamarind gum filled hydrogels. J. Mech. Behav. Biomed. Mater. 2017, 75, 538–548. [Google Scholar] [CrossRef]
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of bigels based on stearic acid-rice bran oil oleogels and tamarind gum hydrogels for controlled delivery applications. J. Surfactants Deterg. 2018, 21, 17–29. [Google Scholar] [CrossRef]
- Da Pieve, S.; Calligaris, S.; Co, E.; Nicoli, M.C.; Marangoni, A.G. Shear Nanostructuring of Monoglyceride Organogels. Food Biophys. 2010, 5, 211–217. [Google Scholar] [CrossRef]
- Masaoka, K.; Jiang, F.; Fairchild, M.D.; Heckaman, R.L. Analysis of color volume of multi-chromatic displays using gamut rings. J. Soc. Inf. Disp. 2020, 28, 273–286. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar]
- Anwarul, S. An Efficient Minimum Spanning Tree-Based Color Image Segmentation Approach. In International Advanced Computing Conference; Springer: Cham, Switzerland, 2021; pp. 588–598. [Google Scholar]
- Palugan, L.; Spoldi, M.; Rizzuto, F.; Guerra, N.; Uboldi, M.; Cerea, M.; Moutaharrik, S.; Melocchi, A.; Gazzaniga, A.; Zema, L. What’s next in the use of opacifiers for cosmetic coatings of solid dosage forms? Insights on current titanium dioxide alternatives. Int. J. Pharm. 2022, 616, 121550. [Google Scholar] [CrossRef]
- Ware, C.; Turton, T.L.; Bujack, R.; Samsel, F.; Shrivastava, P.; Rogers, D.H. Measuring and modeling the feature detection threshold functions of colormaps. IEEE Trans. Vis. Comput. Graph. 2018, 25, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastav, P.P.; Pravitha, M.; Hasan, M.; Mangaraj, S.; Prithviraj, V.; Verma, D.K. Comparative study on the optimization and characterization of soybean aqueous extract based composite film using response surface methodology (RSM) and artificial neural network (ANN). Food Packag. Shelf Life 2022, 31, 100778. [Google Scholar]
- Tenyang, N.; Tiencheu, B.; Tonfack Djikeng, F.; Morfor, A.T.; Womeni, H.M. Alteration of the lipid of red carp (Cyprinus carpio) during frozen storage. Food Sci. Nutr. 2019, 7, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S.; Mukherjee, N.; Ahmed, S.F. Optical properties of diamond like carbon nanocomposite thin films. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 090018. [Google Scholar]
- Makarem, M.; Lee, C.M.; Sawada, D.; O’Neill, H.M.; Kim, S.H. Distinguishing surface versus bulk hydroxyl groups of cellulose nanocrystals using vibrational sum frequency generation spectroscopy. J. Phys. Chem. Lett. 2018, 9, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Arstad, B.; Muggerud, A.M.F.; Erbe, A. Structural evolution of water and hydroxyl groups during thermal, mechanical and chemical treatment of high purity natural quartz. RSC Adv. 2020, 10, 29018–29030. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, E.; Magazù, S. Methyl and methylene vibrations response in amino acids of typical proteins in water solution under high-frequency electromagnetic field. Electromagn. Biol. Med. 2019, 38, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Mech-Dorosz, A.; Khan, M.S.; Mateiu, R.V.; Hélix-Nielsen, C.; Emnéus, J.; Heiskanen, A. Impedance characterization of biocompatible hydrogel suitable for biomimetic lipid membrane applications. Electrochim. Acta 2021, 373, 137917. [Google Scholar] [CrossRef]
- Wei, C.; Wu, M. An Eulerian nonlinear elastic model for compressible and fluidic tissue with radially symmetric growth. arXiv 2021, arXiv:2103.09427. [Google Scholar]
- Joshi, Y.M. Thixotropy, nonmonotonic stress relaxation, and the second law of thermodynamics. J. Rheol. 2022, 66, 111–123. [Google Scholar] [CrossRef]
- Balfour, J.A.B.; Wiseman, L.R. Moxifloxacin. Drugs 1999, 57, 363–373. [Google Scholar] [CrossRef]
- Muijsers, R.B.R.; Jarvis, B. Moxifloxacin in uncomplicated skin and skin structure infections. Drugs 2002, 62, 967–973; discussion 974–965. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.; Jaafari, D.S.; Al-Sou’od, K.A.; Badwan, A.A. Moxifloxacin hydrochloride. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 299–431. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H.; Abd El-Alim, S.H.; Awad, G.E.A.; Kassem, A.A. Chitosan/β-glycerophosphate in situ forming thermo-sensitive hydrogel for improved ocular delivery of moxifloxacin hydrochloride. Eur. J. Pharm. Sci. 2021, 167, 106041. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Li, Y.; Zhu, C.; Liang, G.; SM, J.A.; Hu, G.; Gui, Y. Preparation of organic-modified magadiite–magnetic nanocomposite particles as an effective nanohybrid drug carrier material for cancer treatment and its properties of sustained release mechanism by Korsmeyer–Peppas kinetic model. J. Mater. Sci. 2021, 56, 14270–14286. [Google Scholar] [CrossRef]

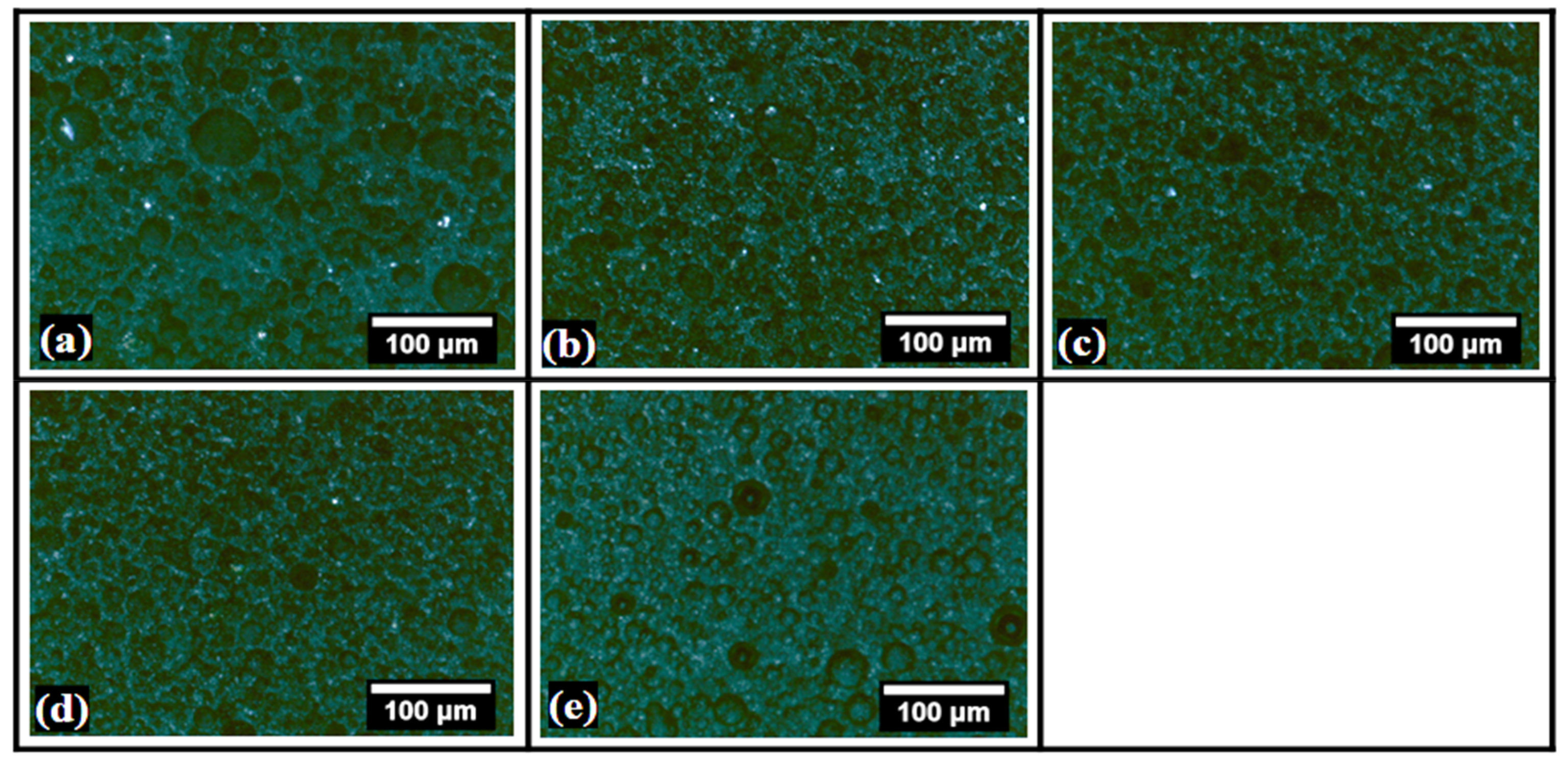
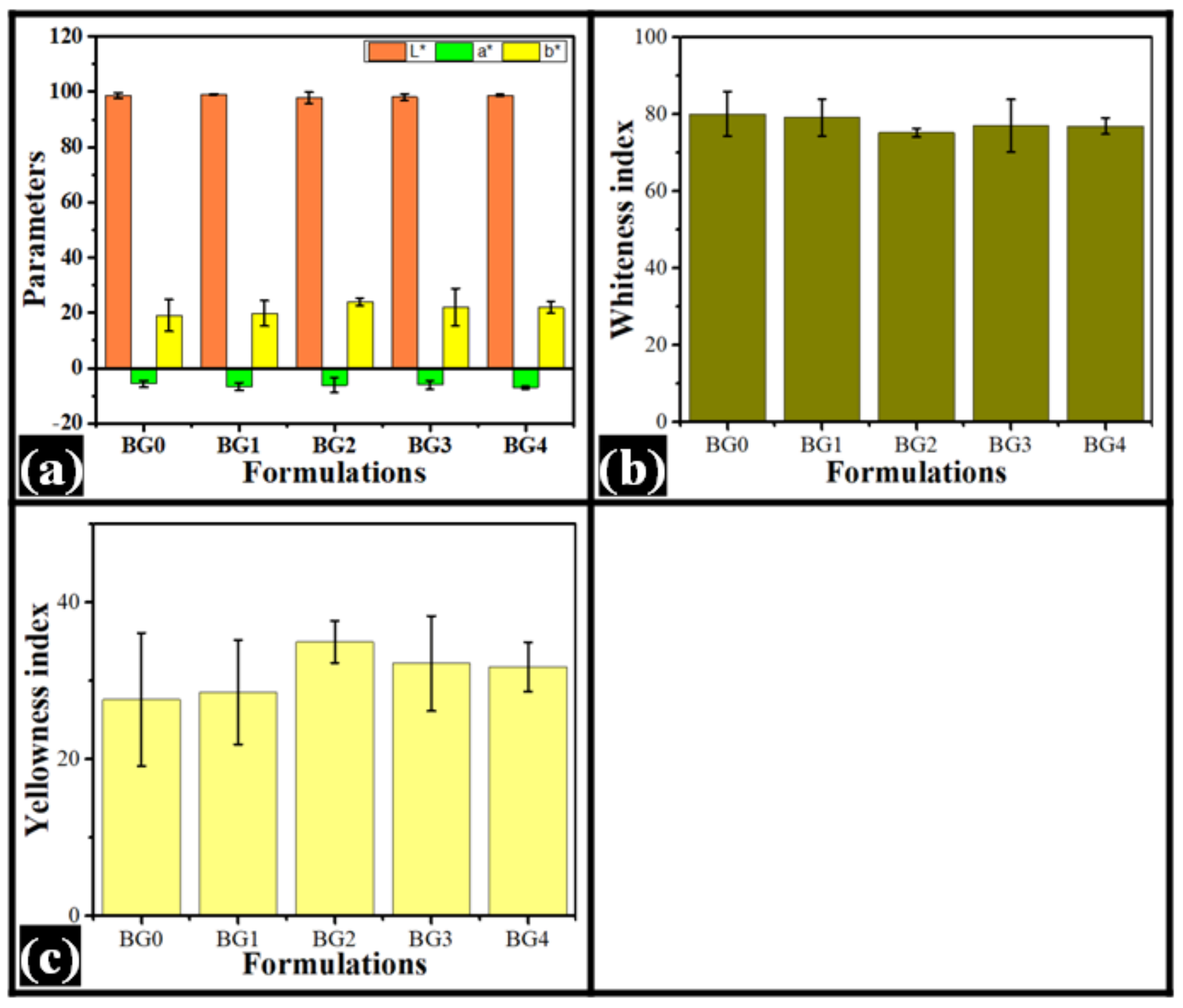
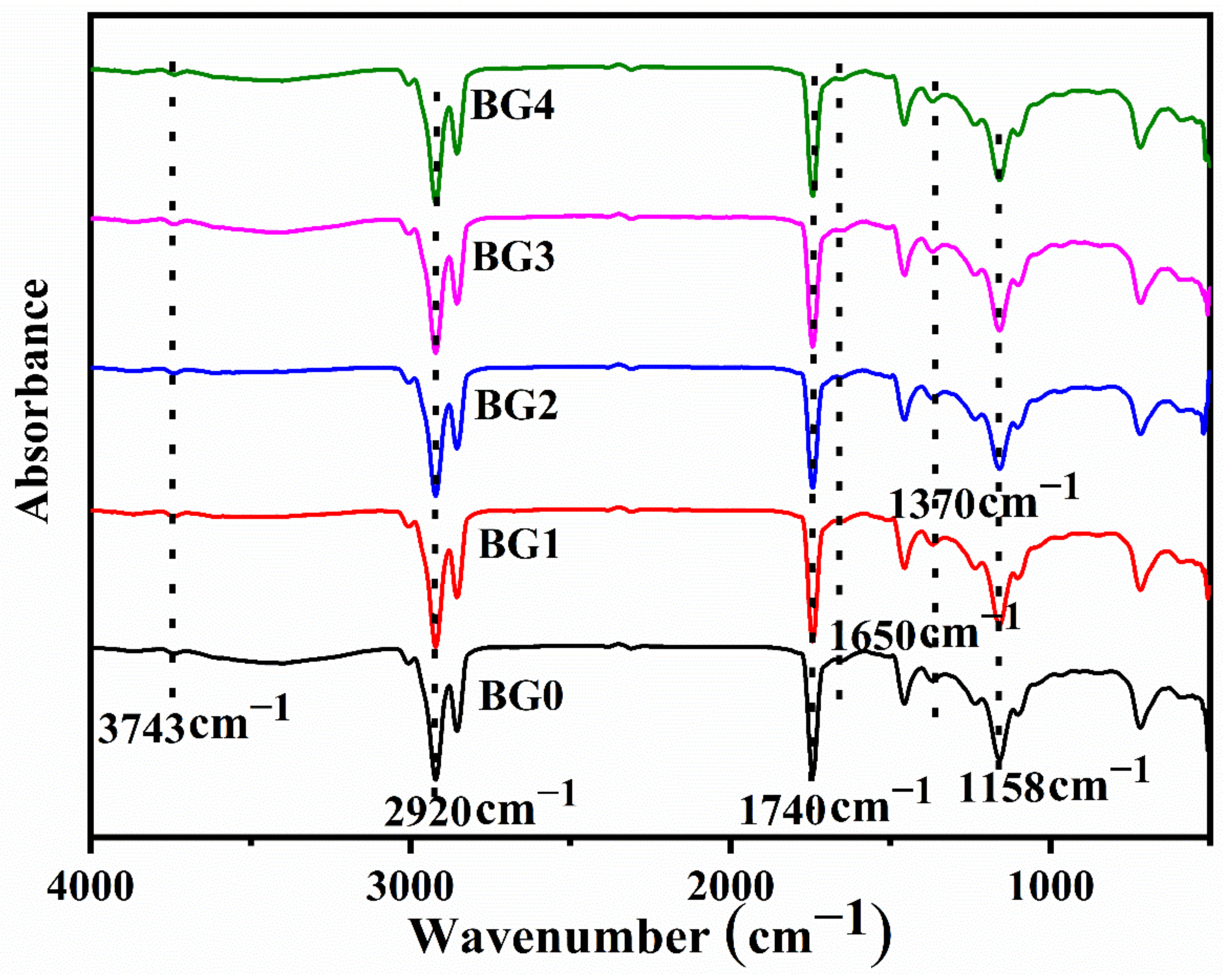
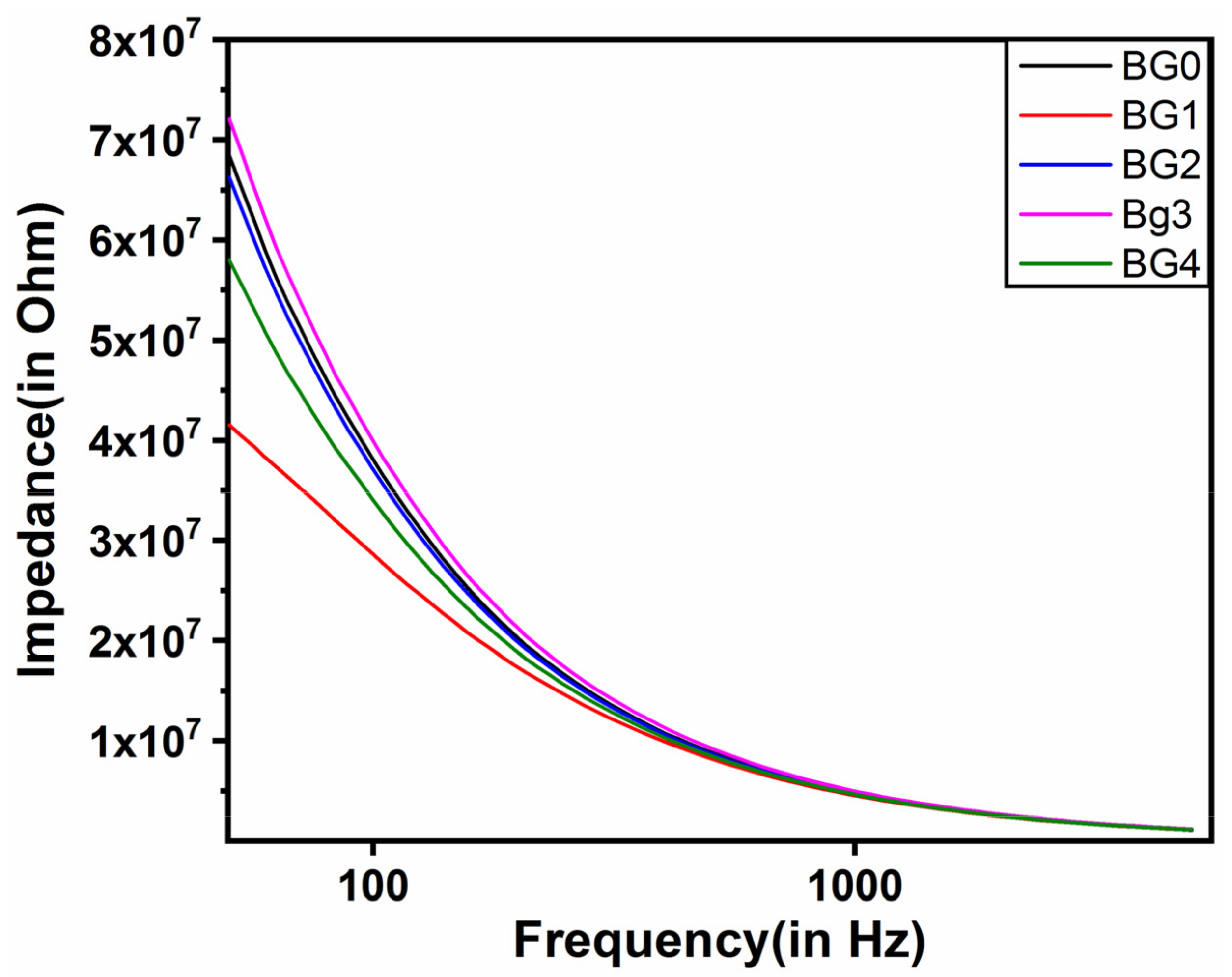


| Sample | L * | a * | b * | WI | YI |
|---|---|---|---|---|---|
| BG0 | 98.67 ± 0.96 | −5.74 ± 1.16 | 19.06 ± 5.70 | 80.02 ± 5.77 | 27.60 ± 8.49 |
| BG1 | 99.07 ± 0.19 | −6.64 ± 1.40 | 19.80 ± 4.57 | 79.10 ± 4.78 | 28.55 ± 6.64 |
| BG2 | 97.76 ± 2.03 | −6.16 ± 2.67 | 23.92 ± 1.38 | 75.20 ± 1.09 | 34.95 ± 2.68 |
| BG3 | 98.19 ± 1.17 | −6.12 ± 1.45 | 22.13 ± 6.74 | 76.97 ± 6.82 | 32.20 ± 6.06 |
| BG4 | 98.81 ± 0.37 | −7.05 ± 0.44 | 21.97 ± 2.11 | 76.90 ± 2.12 | 31.77 ± 3.15 |
| Formulations | F0 | F60 | %SR |
|---|---|---|---|
| BG0 | 205.37 ± 26.95 | 78.85 ± 8.09 | 61.60 ± 2.43 |
| BG1 | 1349.64 ± 133.67 | 739.42 ± 105.43 | 45.21 ± 3.00 |
| BG2 | 1569.40 ± 219.49 | 877.00 ± 77.08 | 44.18 ± 2.94 |
| BG3 | 2548.71 ± 106.68 | 1123.40 ± 25.01 | 55.92 ± 2.60 |
| BG4 | 2186.90 ± 328.30 | 971.89 ± 79.98 | 55.55 ± 3.45 |
| Formulations | Model Parameters | ||
|---|---|---|---|
| K | n | R2 | |
| BG0D | 2.294 ± 0.037 | 0.267 ± 0.016 | 0.995 |
| BG1D | 2.186 ± 0.188 | 0.308 ± 0.017 | 0.993 |
| BG2D | 3.045 ± 0.227 | 0.256 ± 0.007 | 0.988 |
| BG3D | 6.023 ± 1.726 | 0.193 ± 0.050 | 0.987 |
| BG4D | 4.362 ± 0.394 | 0.232 ± 0.020 | 0.992 |
| Formulations | Mean Droplet Size | %SR | Diffusion Factor (K) |
|---|---|---|---|
| B0 | 22.42 ± 0.42 | 61.60 ± 2.43 | 2.294 ± 0.037 |
| B1 | 20.53 ± 0.16 | 45.21 ± 3.00 | 2.186 ± 0.188 |
| B2 | 20.45 ± 0.53 | 44.18 ± 2.94 | 3.045 ± 0.227 |
| B3 | 20.30 ± 0.14 | 55.92 ± 2.60 | 6.023 ± 1.726 |
| B4 | 20.45 ± 0.41 | 55.55 ± 3.45 | 4.362 ± 0.394 |
| Code | Hydrogel (g) | dp-CNC (mg) | Oleogel (g) | Moxifloxacin HCl (% w/w) |
|---|---|---|---|---|
| BG0 | 25 | - | 75 | - |
| BG1 | 25 | 5 | 75 | - |
| BG2 | 25 | 10 | 75 | - |
| BG3 | 25 | 15 | 75 | - |
| BG4 | 25 | 20 | 75 | - |
| BG0D | 25 | - | 75 | 0.25 |
| BG1D | 25 | 5 | 75 | 0.25 |
| BG2D | 25 | 10 | 75 | 0.25 |
| BG3D | 25 | 15 | 75 | 0.25 |
| BG4D | 25 | 20 | 75 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, H.M.; Anis, A.; Poulose, A.M.; Madhar, N.A.; Al-Zahrani, S.M. Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin. Gels 2022, 8, 330. https://doi.org/10.3390/gels8060330
Shaikh HM, Anis A, Poulose AM, Madhar NA, Al-Zahrani SM. Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin. Gels. 2022; 8(6):330. https://doi.org/10.3390/gels8060330
Chicago/Turabian StyleShaikh, Hamid M., Arfat Anis, Anesh Manjaly Poulose, Niyaz Ahamad Madhar, and Saeed M. Al-Zahrani. 2022. "Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin" Gels 8, no. 6: 330. https://doi.org/10.3390/gels8060330
APA StyleShaikh, H. M., Anis, A., Poulose, A. M., Madhar, N. A., & Al-Zahrani, S. M. (2022). Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin. Gels, 8(6), 330. https://doi.org/10.3390/gels8060330







