Comprehensive Review of Polymer and Polymer Gel Treatments for Natural Gas-Related Conformance Control
Abstract
:1. Introduction
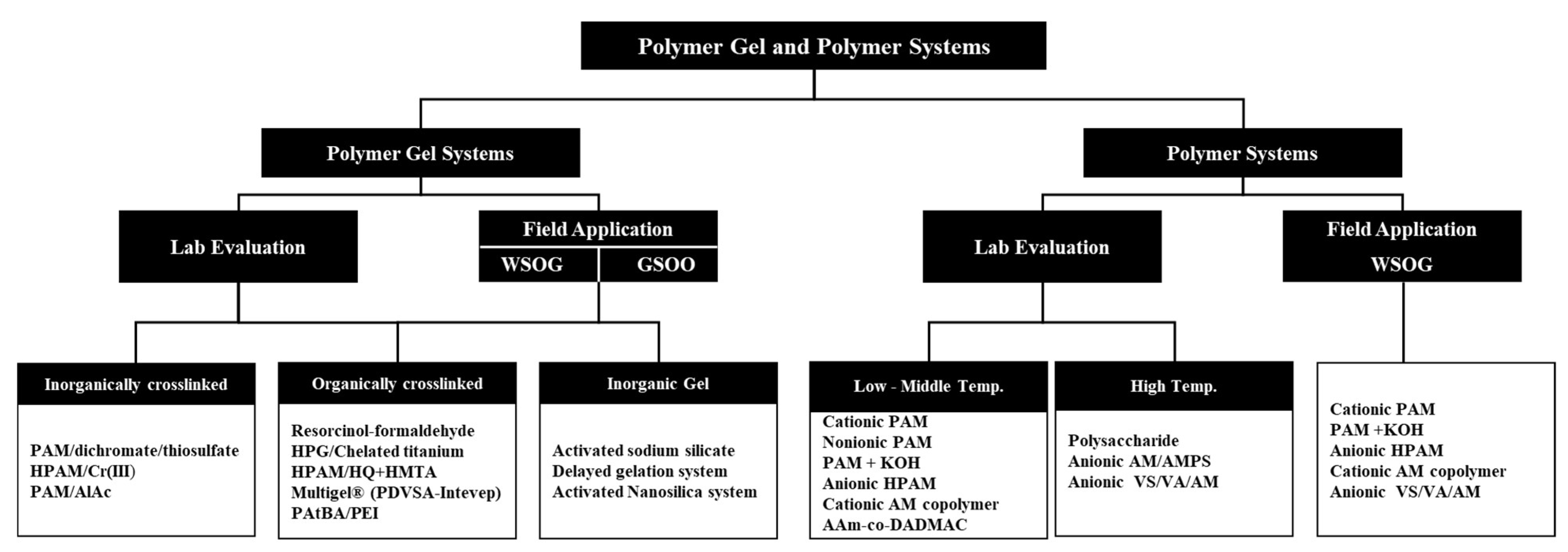
2. Polymer Gel Systems
2.1. Inorganically Crosslinked Polymer Gels
2.1.1. Laboratory Evaluation
2.1.2. Field Applications
HPAM/Cr(III)—Water Shutoff Treatments in Gas Production Wells (WSOGs)
HPAM/Cr(III)—Gas Shutoff Treatments in Oil Production Wells (GSOOs)
2.2. Organically Crosslinked Polymer Gels
2.2.1. Lab Evaluation
2.2.2. Field Applications
PAtBA/PEI—Water Shutoff Treatments in Gas Production Wells
PAtBA/PEI—Gas Shutoff Treatments in Oil Production Wells
Other Organically Crosslinked Polymer Gels
2.3. Inorganic Gel Systems
2.3.1. Lab Evaluation
2.3.2. Field Applications
3. Polymer Systems
3.1. Polymer Systems for Low- to Medium-Temperature Reservoirs—Lab Evaluation
3.1.1. Nonionic and Anionic Polymer Systems
3.1.2. Cationic Polymer Systems
3.2. Polymer Systems for High-Temperature Reservoirs—Lab Evaluation
3.3. Polymer System Applications for Water Shutoff Treatments in Gas Production Wells
4. Current Challenges and Recommendations for Future Work
4.1. Limitations of the Existing Systems
4.2. Limitations and Recommendation of Evaluation Methodologies
4.3. Recommended Future Research Directions
- Development of novel polymers and polymer gels that can be used in harsh conditions, including high temperature, high salinity, CO2 and H2S environments, large fractures, or fracture-like features such as wormholes.
- Selective shutoff treatments: Current treatments mainly consider the role of polymers and polymer gels on gas, oil, and water permeabilities modification; however, the selective penetration of a polymer or a gelant system during its injection is vital to prevent damaging the unswept zones and consequently improve the treatment efficiency.
- Systematic investigations on the feasibility of applying gels in natural gas-related conformance problems. Gels have been widely investigated and applied to control water production in oil reservoirs. However, there is a limited number of published studies regarding both water control from natural gas reservoirs and natural gas production control from oil reservoirs. A series of studies should be conducted, including the effect of the natural gas and its impurities (CO2 and H2S) on the gelation kinetics, gel strength and thermostability of the polymer gel systems, DPR/RPM performance when applied for selective water or gas shutoff, and adsorption/chromatographic effect when used for in-depth treatment.
- Development of numerical simulation tools to optimize gel treatment design. Currently, all commercial software packages are incapable of properly describing the gel or gelant injection performance during transport through common porous media and fractures and are unable to quantify the gel plugging performance, which is affected by many parameters.
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| WSOG | water shutoff treatment in natural gas production wells |
| GSOO | gas shutoff treatment in oil production wells |
| BHT | bottomhole temperature (°C) |
| BHP | bottomhole pressure (psi) |
| TDS | total dissolved solids (ppm) |
| CT | coiled tubing |
| Frrw | water residual resistance factor |
| Frrg | gas residual resistance factor |
| Fr | resistance factor |
| ICGP | inside casing gravel pack |
| PCon | polymer concentration (ppm) |
| Kf | fracture permeability (md) |
| Mscf/d | thousand standard cubic feet per day |
| RT | room temperature (°C) |
| BWPD | barrel of water per day |
| WGR | water–gas ratio (bbl/Mscf) |
| GOC | gas–oil contact |
| Qg | gas production rate |
| Qw | water production rate |
References
- Bai, B.; Sun, X. Development of swelling-rate controllable particle gels to control the conformance of CO2 flooding. In Proceedings of the SPE Symposium on Improved Oil Recovery, Virtual, 31 August–4 September 2020. [Google Scholar] [CrossRef]
- Seright, R.; Brattekas, B. Water shutoff and conformance improvement: An introduction. Pet. Sci. 2021, 18, 450–478. [Google Scholar] [CrossRef]
- Seright, R.S.; Lane, R.H.; Sydansk, R.D. A Strategy for Attacking Excess Water Production. SPE Prod. Facil. 2003, 18, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Nigmatullin, T.E.; Nikulin, V.Y.; Shaymardanov, A.R.; Mukminov, R.R.; Ivanov, A.Y.; Akhmadullin, M.E.; Soltanov, D.K. Water-and-gas shutoff technologies in horizontal wells on North Komsomolskoe field: Screening and successful trial. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 12–15 October 2021. [Google Scholar] [CrossRef]
- Hidayat, R.; Kurniawati, P.S.; Kurniawan, A.T.; Setyaji, I.; Pancawisna, G.P.; Marindha, R.; Umar, K.; Dahnil, G.T.; Rahman, R.; Jamal, M.N.; et al. Mechanical Isolation of a Challenging Reservoir: First Installation of High Expansion Straddle Packer in Indonesia. In Proceedings of the Offshore Technology Conference Asia, Virtual and Kuala Lumpur, Malaysia, 22–25 March 2022. [Google Scholar] [CrossRef]
- Mishra, A.; Abbas, S.; Braden, J.; Hazen, M.; Li, G.; Peirce, J.; Smith, D.D.; Lantz, M. Comprehensive Review of Fracture Control for Conformance Improvement in the Kuparuk River Unit—Alaska. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Casero, A.; Gomaa, A.M. Several Decades of Fluid Diversion Evolution, Is There a Good Solution? In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 15–18 November 2021. [Google Scholar] [CrossRef]
- Kabir, A. Chemical Water & Gas Shutoff Technology—An Overview. In Proceedings of the SPE Asia Pacific Improved Oil Recovery Conference, APIORC, Kuala Lumpur, Malaysia, 8–9 October 2001. [Google Scholar] [CrossRef]
- Sinha, G.; Sinha, R. Water and Gas Control Jobs in Indian Offshore Field (Bombay High)—Trends and Case Studies. In Proceedings of the SPE Middle East Oil Show, Manama, Bahrain, 17–20 March 2001. [Google Scholar] [CrossRef]
- Syahruddin, A.; Riyanto, L. Tackling Gas Field Decline with Efficient Chemical Water Shut-off: Successful Application on Peciko Field (East Kalimantan, Indonesia). In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 3–5 December 2008. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer systems for water shutoff and profile modification: A review over the last decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Di Lullo, G.; Rae, P.; Curtis, J. New Insights into Water Control—A Review of the State of the Art–Part II. Control. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium and International Horizontal Well Technology Conference, Calgary, AB, Canada, 4–7 November 2008. [Google Scholar] [CrossRef]
- Tessarolli, F.G.C.; Gomes, A.S.; Mansur, C.R. Hydrogels applied for conformance-improvement treatment of oil reservoirs. In Hydrogels; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Amir, Z.; Said, I.M.; Jan, B.M. In situ organically cross-linked polymer gel for high-temperature reservoir conformance control: A review. Polym. Adv. Technol. 2018, 30, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oilfield. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef]
- Pusch, G.; Kohler, N.; Kretzschmar, H.J. Practical Experience with Water Control in Gas Wells by Polymer Treatments. In Proceedings of the IOR 1995—8th European Symposium on Improved Oil Recovery, Vienna, Austria, 15–17 May 1995. [Google Scholar] [CrossRef] [Green Version]
- Al-Shajalee, F.; Saeedi, A.; Wood, C.D. A New Dimensionless Approach to Assess Relative Permeability Modifiers. Energy Fuels 2019, 33, 3448–3455. [Google Scholar] [CrossRef]
- Chan, K.; Bond, A.; Keese, R.; Lai, Q. Diagnostic Plots Evaluate Gas Shut-Off Gel Treatments at Prudhoe Bay, Alaska. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 6–9 October 1996; pp. 421–428. [Google Scholar] [CrossRef]
- Wawro, K.; Wassmuth, F.; Smith, J. Reducing Water Production in a Naturally Fractured Gas Well Using Sequential Gel/Gas Slug Injection. In Proceedings of the Society of Petroleum Engineers–SPE/CERI Gas Technology Symposium, Calgary, AB, Canada, 3–5 April 2000. [Google Scholar] [CrossRef]
- Roozshenas, A.A.; Hematpur, H.; Abdollahi, R.; Esfandyari, H. Water Production Problem in Gas Reservoirs: Concepts, Challenges, and Practical Solutions. Math. Probl. Eng. 2021, 2021, 9075560. [Google Scholar] [CrossRef]
- Amirov, A.; Hadiaman, F.; Parra, D.E.; Zeynalov, J.; Kok, A. Successful gas shut off operation using a coiled tubing telemetry System: A case history. In Proceedings of the SPE/ICoTA Well Intervention Conference and Exhibition, Virtual, 22–25 March 2021. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Sydansk, R. A New Conformance-Improvement-Treatment Chromium(III) Gel Technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 16–21 April 1988. [Google Scholar] [CrossRef]
- Sydansk, R.D. A Newly Developed Chromium(lll) Gel Technology. SPE Reserv. Eng. 1990, 5, 346–352. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Smith, T.B. Field Testing of a New Conformance-Improvement-Treatment Chromium(III) Gel Technology. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 16–21 April 1988; pp. 700–707. [Google Scholar] [CrossRef]
- Stavland, A.; Jonsbraten, H.C. New Insight into Aluminium Citrate/Polyacrylamide Gels for Fluid Control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar] [CrossRef]
- Moffitt, P.; Moradi-Araghi, A.; Ahmed, I.; Janway, V.; Young, G. Development and Field Testing of a New Low Toxicity Polymer Crosslinking System. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 27–29 March 1996. [Google Scholar] [CrossRef]
- Hardy, M.; Botermans, W.; Hamouda, A.A.; Valdal, J.; Warren, J. The First Carbonate Field Application of a New Organically Crosslinked Water Shutoff Polymer System. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1999. [Google Scholar] [CrossRef]
- Ghriga, M.A.; Grassl, B.; Gareche, M.; Khodja, M.; Lebouachera, S.E.I.; Andreu, N.; Drouiche, N. Review of recent advances in polyethylenimine crosslinked polymer gels used for conformance control applications. Polym. Bull. 2019, 76, 6001–6029. [Google Scholar] [CrossRef]
- Dalrymple, E.D.; Everett, D.M.; Eoff, L.S. Global field results of a polymeric gel system in conformance applications. In Proceedings of the SPE Russian Oil and Gas Technical Conference and Exhibition, Moscow, Russia, 3–6 October 2006. [Google Scholar] [CrossRef]
- Vasquez, J.E.; Eoff, L.S. Laboratory development and successful field application of a conformance polymer system for low-, medium-, and high-temperature applications. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Lima, Peru, 1–3 December 2010. [Google Scholar] [CrossRef]
- Dovan, H.; Hutchins, R.; Sandiford, B. Delaying Gelation of Aqueous Polymers at Elevated Temperatures Using Novel Organic Crosslinkers. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997. [Google Scholar] [CrossRef]
- Perdomo, L.; Rodriguez, H.A.; Llamedo, M.A.; Oliveros, L.; Gonzalez, E.R.; Molina, O.; Giovingo, C. Successful experiences for water and gas shutoff treatments in North Monagas, Venezuela. In Proceedings of the Latin American & Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 15–18 April 2007. [Google Scholar] [CrossRef]
- Dovan, H.; Hutchins, R. New Polymer Technology for Water Control in Gas Wells. SPE Prod. Facil. 1994, 9, 280–286. [Google Scholar] [CrossRef]
- Seright, R.S. Reduction of Gas and Water Permeabilities Using Gels. SPE Prod. Facil. 1995, 10, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Kantzas, A.; Marentette, D.; Allsopp, K. Utilization of Polymer Gels, Polymer Enhanced Foams, And Foamed Gels For Improving Reservoir Conformance. J. Can. Pet. Technol. 1999, 38, 1–8. [Google Scholar] [CrossRef]
- Burrafato, G.; Pitoni, E.; Vietina, G.; Mauri, L.; Chiappa, L. Rigless WSO treatments in gas fields. Bullheading gels and polymers in shaly sands: Italian case histories. In Proceedings of the SPE—European Formation Damage Conference, Proceedings, EFDC, The Hague, The Netherlands, 31 May–1 June 1999. [Google Scholar] [CrossRef]
- Wassmuth, F.; Green, K.; Hodgins, L. Water shutoff in gas wells: Proper gel placement is the key to success. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004. [Google Scholar] [CrossRef]
- Al-Shajalee, F.; Arif, M.; Machale, J.; Verrall, M.; Almobarak, M.; Iglauer, S.; Wood, C. A Multiscale Investigation of Cross-Linked Polymer Gel Injection in Sandstone Gas Reservoirs: Implications for Water Shutoff Treatment. Energy Fuels 2020, 34, 14046–14057. [Google Scholar] [CrossRef]
- Shamlooh, M.; Hussein, I.A.; Nasser, M.S.; Magzoub, M.; Salehi, S. Development of pH-Controlled Aluminum-Based Polymeric Gel for Conformance Control in Sour Gas Reservoirs. ACS Omega 2020, 5, 24504–24512. [Google Scholar] [CrossRef]
- Brady, J.W.; Shattuck, D.P.; Parker, M.H.; Sramek, C.S.; Jones, A.M.; Gould, J.; Jayakumar, S. Video production logging and polymer gel yields successful water isolation in the Fayetteville shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar] [CrossRef]
- Sanders, G.; Chambers, M.; Lane, R. Successful Gas Shutoff with Polymer Gel Using Temperature Modeling and Selective Placement in the Prudhoe Bay Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994. [Google Scholar] [CrossRef]
- Lai, Q.; Bond, A.; Cahalane, T.; Carpenter, R.; Newhouse, D.; Singh, T.; Styler, J. Gel-cement combination squeezes for gas shutoff. In Proceedings of the SPE Western Regional Meeting, Anchorage, Alaska, 26–27 May 1999. [Google Scholar] [CrossRef]
- Okasha, T.; Nasr-El-Din, H.; Al-Khudair, W. Abatement of Water Production from Upper Permian Gas Wells in Saudi Arabia Using a New Polymer Treatment. In Proceedings of the SPE Middle East Oil Show, Manama, Bahrain, 17–20 March 2001. [Google Scholar] [CrossRef]
- Eoff, L.; Dalrymple, D.; Everett, D.; Vasquez, J. Worldwide Field Applications of a Polymeric Gel System for Conformance Applications. SPE Prod. Oper. 2007, 22, 231–235. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Sierra, L.; Garzon, F.O.; Lynn, J.D.; Izquierdo, G.A. Water Shut-off with Polymer Gels in A High Temperature Horizontal Gas Well: A Success Story. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar] [CrossRef]
- Wattanasuwankorn, R.; Jiemsawat, N.; Dunlop, T.; Kanchiak, S. First Achievement Using Water Shutoff Polymer in Monobore Well Completion, Gulf of Thailand. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Bangkok, Thailand, 25–27 August 2014. [Google Scholar] [CrossRef]
- Bach, T.; Wennberg, K.E.; Mebratu, A.; Hendriks, W.P.; Warren, J.M.; Rolfsvaag, T. Polymer Sealant for Unwanted Gas in Openhole Gravel-Pack Completion. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar] [CrossRef]
- Mebratu, A.; Nerland, B.; Kleppan, T. Annular Barrier Re-Establishment Using a Long-Life, High-Strength Polymer Gel System. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2004. [Google Scholar] [CrossRef]
- Diaz, I.; Nava, T.; Deolarte, C.; Castillo, O.; Vasquez, J.; Cancino, V.; Caballero, C. Successfully Controlling Unwanted Gas Production in a Highly Naturally Fractured Carbonate Reservoir. In Proceedings of the SPE Western Venezuela Section South American Oil and Gas Congress, Maracaibo, Venezuela, 18–21 October 2011. [Google Scholar] [CrossRef]
- Hutchins, R.; Dovan, H.; Sandiford, B. Field Applications of High Temperature Organic Gels for Water Control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996; pp. 419–426. [Google Scholar] [CrossRef]
- Llamedo, M.A.; Mejias, F.; Gonzalez, E.R.; Espinoza, J.; Valero, E.M. Successful Gas Shutoff with Gel, Evaluation and Implementation, North East, Venezuela. In Proceedings of the SPE Offshore Europe Oil and Gas Exhibition and Conference, Aberdeen, UK, 6–9 September 2005. [Google Scholar] [CrossRef]
- Avery, M.; Wells, T.; Chang, P.; Millican, J. Field Evaluation of a New Gelant for Water Control in Production Wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar] [CrossRef]
- Karadkar, P.; Almohsin, A.; Bataweel, M.; Huang, J. In-situ pore plugging using nanosilica based fluid system for gas shutoff. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019. [Google Scholar] [CrossRef]
- Al-Dhafeeri, A.M.; Nasr-El-Din, H.A.; Al-Harith, A.M. Evaluation of rigless water shutoff treatments to be used in Arab-C carbonate reservoir in Saudi Arabia. In Proceedings of the Society of Petroleum Engineers—CIPC/SPE Gas Technology Symposium, Calgary, AB, Canada, 16–19 June 2008. [Google Scholar] [CrossRef]
- Herring, G.D.; Milloway, J.T.; Wilson, W.N. Selective Gas Shut-Off Using Sodium Silicate in the Prudhoe Bay Field, AK. In Proceedings of the SPE Formation Damage Control Symposium, Bakersfield, CA, USA, 13–14 February 1984. [Google Scholar] [CrossRef]
- Chenevière, P.; Falxa, P.; Alfenore, J.; Poirault, D.; Enkababian, P.G.; Chan, K.S. Chemical water shut off interventions in the tunu gas field: Optimisation of the treatment fluids, well interventions and operational challenges. In Proceedings of the SPE—European Formation Damage Conference, EFDC, Sheveningen, The Netherlands, 25–27 May 2005. [Google Scholar] [CrossRef]
- Zaitoun, A.; Kohler, N. Two-Phase Flow Through Porous Media: Effect of an Adsorbed Polymer Layer. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar] [CrossRef]
- Zaitoun, A.; Kohler, N.; Guerrinl, Y. Improved Polyacrylamide Treatments for Water Control in Producing Wells. J. Pet. Technol. 1991, 43, 862–867. [Google Scholar] [CrossRef]
- Qin, L.; Arjomand, E.; Myers, M.B.; Otto, C.; Pejcic, B.; Heath, C.; Saeedi, A.; Wood, C. Mechanistic Aspects of Polymeric Relative Permeability Modifier Adsorption onto Carbonate Rocks. Energy Fuels 2020, 34, 12065–12077. [Google Scholar] [CrossRef]
- Mennella, A.; Chiappa, L.; Bryant, S.L.; Burrafato, G. Pore-scale Mechanism for Selective Permeability Reduction by Polymer Injection. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar] [CrossRef]
- Stavland, A.; Nilsson, S. Segregated Flow is the Governing Mechanism of Disproportionate Permeability Reduction in Water and Gas Shutoff. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001. [Google Scholar] [CrossRef]
- Zaitoun, A.; Bertin, H.; Lasseux, D. Two-Phase Flow Property Modifications by Polymer Adsorption. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar] [CrossRef]
- Chatterji, J.; Borchardt, J. Applications of Water-Soluble Polymers in the Oil Field. J. Pet. Technol. 1981, 33, 2042–2056. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Cleveland, D.; Westerman, I. Development and Evaluation of EOR Polymers Suitable for Hostile Environments: II-Copolymers of Acrylamide and Sodium AMPS. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 4–6 February 1987. [Google Scholar] [CrossRef]
- Scott, A.J.; Romero-Zerón, L.; Penlidis, A. Evaluation of polymeric materials for chemical enhanced oil recovery. Processes 2020, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Al-Taq, A.; Alrustum, A.; Alfakher, B.; Al-Ibrahim, H. Relative Permeability Modifiers as a Chemical Means to Control Water Production in Oil and Gas Reservoirs. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Event Canceled, 28 November–1 December 2021. [Google Scholar] [CrossRef]
- Mishra, S.; Bera, A.; Mandal, A. Effect of Polymer Adsorption on Permeability Reduction in Enhanced Oil Recovery. J. Pet. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zaitoun, A.; Pichery, T. A Successful Polymer Treatment for Water Coning Abatement in Gas Storage Reservoir. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001. [Google Scholar] [CrossRef]
- Tielong, C.; Yong, Z.; Kezong, P.; Wanfeng, P. A Relative Permeability Modifier for Water Control of Gas Wells in a Low-Permeability Reservoir. SPE Reserv. Eng. 1996, 11, 168–173. [Google Scholar] [CrossRef]
- Chiappa, L.; Mennalla, A.; Ortolani, M. Role of Polymer Adsorption and of Petrophysical Properties in Water-Cut Control Treatments by Polymer Injection in Gas Wells. In Proceedings of the IOR 1997—9th European Symposium on Improved Oil Recovery, The Hague, The Netherlands, 20–22 October 1997. [Google Scholar] [CrossRef]
- Ranjbar, M.; Schaffie, M. Improved treatment of acrylamide co- and terpolymers for water control in gas-producing and storage wells. J. Pet. Sci. Eng. 2000, 26, 133–141. [Google Scholar] [CrossRef]
- Al-Shajalee, F.; Wood, C.; Xie, Q.; Saeedi, A. Effective Mechanisms to Relate Initial Rock Permeability to Outcome of Relative Permeability Modification. Energies 2019, 12, 4688. [Google Scholar] [CrossRef]
- Zaitoun, A.; Templier, A.; Hernando, L. Gas production enhancement by polymer treatment. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2020. [Google Scholar] [CrossRef]
- Ranjbar, M.; Clausthal, T.; Czolbe, P.; Kohler, N. Comparative Laboratory Selection and Field Testing of Polymers for Selective Control of Water Production in Gas Wells. Oil Gas Eur. Mag. 1995, 22, 38–41. [Google Scholar] [CrossRef]
- Zaitoun, A.; Kohler, N.; Bossie-Codreanu, D.; Denys, K. Water shutoff by relative permeability modifiers: Lessons from several field applications. In Proceeding of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999. [Google Scholar] [CrossRef]
- Elmkies, P.; Lasseux, D.; Bertin, H.; Pichery, T.; Zaitoun, A. Polymer Effect on Gas/Water Flow in Porous Media. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar] [CrossRef]
- Fernandez, I. Evaluation of Cationic Water-Soluble Polymers with Improved Thermal Stability. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar] [CrossRef]
- Denys, K.; Fichen, C.; Zaitoun, A. Bridging adsorption of cationic polyacrylamides in porous media. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar] [CrossRef]
- Kalfayan, L.J.; Dawson, J.C. Successful implementation of resurgent Relative Permeability Modifier (RPM) technology in well treatments requires realistic expectations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar] [CrossRef]
- Dupuis, G.; Bouillot, J.; Zaitoun, A.; Caremi, G.; Burrafato, G. Combined water/sand control polymer treatments in offshore gas wells. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, OGWA, Muscat, Oman, 21–23 March 2016. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Seright, R.S. When and where relative permeability modification water-shutoff treatments can be successfully applied. SPE Prod. Oper. 2007, 22, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Botermans, C.W.; Van Batenburg, D.W.; Bruining, J. Relative Permeability Modifiers: Myth or Reality? In Proceedings of the SPE—European Formation Damage Control Conference, the SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar] [CrossRef]
- Ligthelm, D. Water Shut Off in Gas Wells: Is there Scope for a Chemical Treatment. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar] [CrossRef]
- Zaitoun, A.; Pichery, T.R. New polymer technology for sand control treatments of gas storage wells. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009. [Google Scholar] [CrossRef]
- Vargas-Vasquez, S.M.; Romero-Zerón, L.B. A Review of the Partly Hydrolyzed Polyacrylamide Cr(III) Acetate Polymer Gels. Pet. Sci. Technol. 2008, 26, 481–498. [Google Scholar] [CrossRef]
- Albonico, P.; Lockhart, T.P. Stabilization of Polymer Gels Against Divalent Ion-Induced Syneresis. J. Pet. Sci. Engineering. 1997, 18, 61–71. [Google Scholar] [CrossRef]
- Jin, H.; McCool, C.; Willhite, G.; Green, D.; Michnick, M. Propagation of Chromium (III) Acetate Solutions Through Dolomite Rock. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.; Al Brahim, A.; Bai, B. Ultra-high temperature resistant preformed particle gels for enhanced oil recovery. Chem. Eng. J. 2021, 426, 130712. [Google Scholar] [CrossRef]
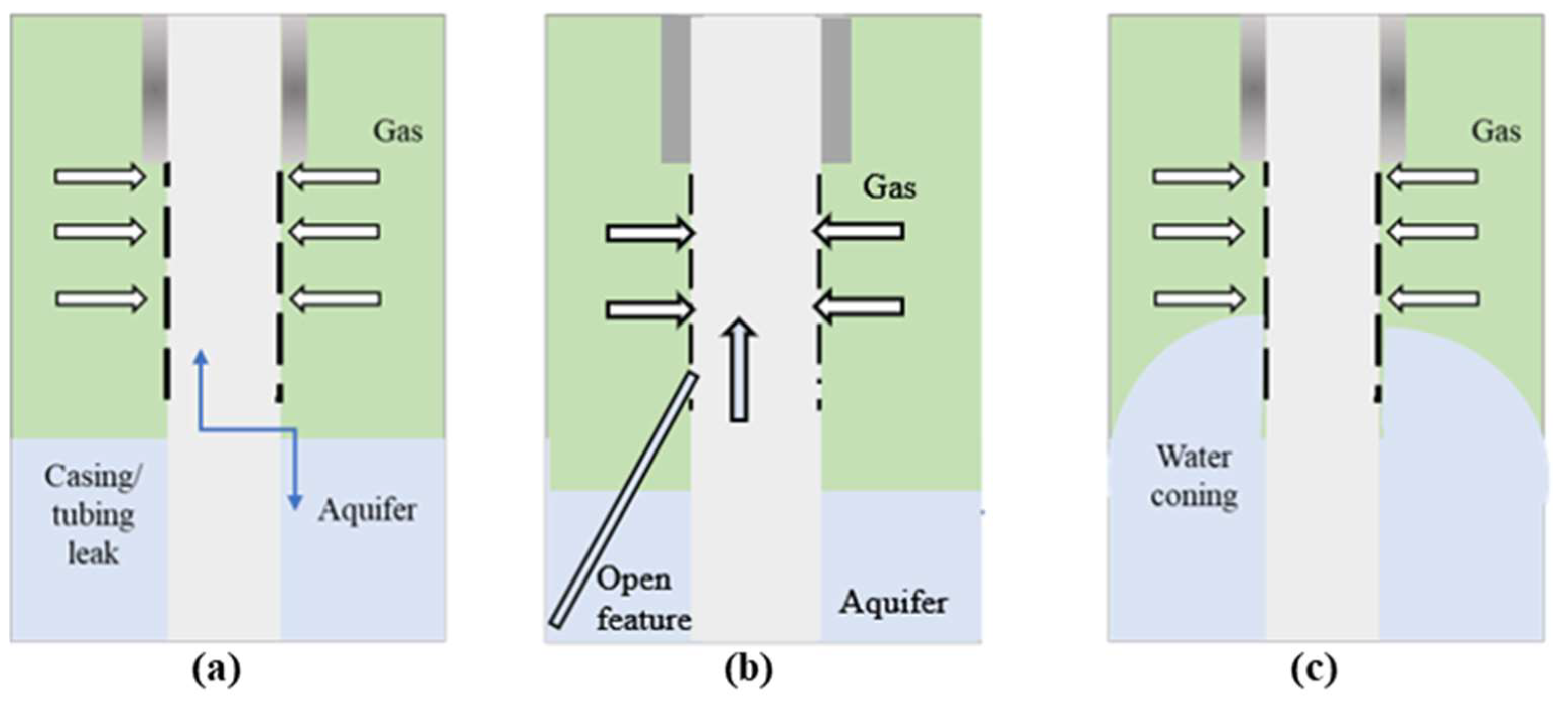

| Ref. | Polymer Gel | Model Parameters | Operating Conditions | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Lithology | D in. | L in. | Gas | p psi | T °C | |||
| [34] | 4000 ppm Anionic PAM (dichromate/thiosulfate) Cationic PAM (chromium or aldehydes) Lignosulfonates/chromium Silicates/acidic salts TDS: seawater | Sandstone K (383–541 md) Φ (23%) | 1.0 | 5.0 | N2 | 290 | 59 | Anionic PAM gel Frrw (17.4): Frrg (2.6) Frrw/Frrg (6.7) Cationic PAM gel Frrw (13.4): Frrg (2.0) Frrw/Frrg (6.7) |
| [35] | 13,900 ppm HPAM/212 ppm Cr(llI) (66/1) TDS: 10,000 ppm | Sandstone K (650 md) Φ (21%) | 1.4 | 5.5 | N2 | 1500 | 41 | Frrw (170,000): Frrg (284) Frrw/Frrg (599) ↑WAGcycles ↓Frrw/Frrg (22) |
| [36] | 5000 ppm HPAM/800 ppm Cr(llI) (7/1) | Artificially fractured carbonate K (54.3–156 md) | 1.5 | - | CH4 | - | - | Frrw/Frrg (8.75) ↑WAGcycles ↓Frrw/Frrg (7.75) |
| [37] | 35,000 ppm HPAM/900 ppm Cr(llI) (40/1) | Sandstone K (170 md) | - | - | - | - | 37 | ↓ Kw (70%) ↑ Kg |
| [19] | 3000–6000 ppm HPAM/Cr(III) (40/1) | Crushed carbonate core K (10.000–20,000 md) | - | - | - | - | - | Frrw/Frrg (1.5 to 2) |
| [38] | HPAM/Cr(III) (40/1) | Berea cores K (400–600 md) Fractured cores Kf (6000 md) Φ (24%) | 1.5 | 4 | N2 | 507 | RT | Frr f(remaining gelant in the core and flooding history) |
| Sandpack Crushed limestone K (24 d) Φ (36%) | 1.5 | 11.4 | N2 | 507 | ||||
| [39] | 20,000 ppm P(AAM-co-AA)Na/200–600 ppm Cr(llI) (100/1) TDS: 20,000 ppm | Sandstone K (140–170 md) Φ (18.7–20%) | 1.5 | 3 | N2 | 500–1500 | 60 | ↑ Cr(llI)con ↑ Frr ↑ Qw ↓ Frrw (shear thinning) ↑ Qg ↑Frrg (shear thickening) ↓ Frrw/Frrg ↑ Cr(llI)con ↓ Q |
| 20,000 ppm P(AAM-co-AA)Na/300 ppm Cr(llI) (66/1) TDS: 20,000 ppm | Micromodel K (2500 D) Φ (48%) | 0.4 | 0.8 | N2 | 500 | 24 | ||
| 20,000 ppm P(AAM-co-AA)Na/600 ppm Cr(llI) (33/1) TDS: 20,000 ppm | Capillary tube | 0.02 | 6 | N2 | - | - | ||
| [40] | 90,000 ppm PAM with aluminum (acetate, amino-acetate, nitrate, and lactate) | Plugging efficiency using API PPT with fracture disc of 1 mm width | - | - | - | - | - | PAM/aluminum acetate was selected Gelation time (50 min) at 75 °C It crosslinked with PAM at a wide range of pH (3.5 to 8.5) Sealed the fracture under 700 and 2000 psi |
| Ref. | Field/ Wellbore | Mechanism of Excess Water | Reservoir Parameters | Treatment Design | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Salinity ppm | Type | PCon ppm | TDS ppm | Vol bbl | ||||
| [34] | North Mexico /vertical | Water influx (fracture-related) | Sandstone | 1500 | 59 | - | Seawater | Anionic PAM (dichromate/ thiosulfate) | 4000 | - | 634 | Qw↓(93.8%) Qg ↓(84%) 3 years |
| [19] | Canada/ deviated openhole | Water influx (fracture-related) | - | 2031 | 77 | - | - | HPAM/ Cr(III) (40/1) | 3000 to 8000 | - | 802 | WGR↓(58%) Qg ↑(71%) 6 months |
| [37] | Italy/vertical with ICGP | Water table close to perforations | Shaly sands 100 md | 1465 | 37 | 3727 | - | HPAM/ Cr(III) (40/1) | 35,000 | - | 314 | Successful for two weeks only |
| [41] | Northern Arkansas/horizontal casedhole | Layer communication (fracture-related) | Shale | 3000 | 79 | 7000 | - | HPAM/ Cr(III) | 5500 to 50,000 | - | 440 | Qw ↓ (97%) Qg ↑ (17%) 2 months |
| Ref. | Field | Mechanism of Excess Gas | Reservoir Parameters | Treatment Design | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Salinity ppm | Type | PCon ppm | TDS ppm | Vol bbl | ||||
| [42] | Prudhoe Bay, AK | Gas coning + channeling (matrix-related) | Sandstone | 3500 | 104 | 8800 | - | HPAM/ Cr(III) (60/1) | 40,000 50,000 | - | 93–120 | GOR ↓ 6 months then increased to pretreatment level |
| [43] | Prudhoe Bay, AK | Leaking cement-squeezed perforations | Sandstone K (150–300 md) | 3400 | 85–99 | 8800 | - | HPAM/ Cr(III) | 50,000 70,000 | - | - | 85% success rate based on covering the treatment cost |
| Ref. | Polymer Gel | Model Parameters | Operating Conditions | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Lithology | D in. | L in. | Gas | p psi | T °C | |||
| [35] | 30,000 ppm resorcinol/30,000 ppm formaldehyde TDS: 5000 ppm KCl, 4200 ppm NaHCO3 | Sandstone K (650 md) Φ (21%) | 1.4 | 5.5 | N2 | 900 | 41 | Frrw (10,400): Frrg (126) Frrw/Frrg (83) ↑WAGcycles ↓ Frrw/Frrg (7.9) |
| [44] | 1000–2000 ppm PAtBA/PEI TDS: 218,000 ppm | Carbonate K (10–3000 md) Φ (20%) | 1.5 | 2.0 | N2 | 1500 | 90 | Frrw (2.75): Frrg (1.25) Frrw/Frrg (2.2) |
| [45] | PAtBA/PEI | Sandpack (100 mesh, 7.8 md) | - | 20 | N2 | 865 | 132 | PStable (425 psi): Frrg (6555) |
| Carbonate fractured core (fracture width = 0.002 in.) | 0.9 | 3.3 | - | - | 132 | PBreakthrough (196 psi) | ||
| [46] | PAtBA/PEI 150 gpt polymer, 10 gpt crosslinker, 814 gpt of field mixing water, 686 Ib/1000 gal of NaCl (retarder) compared with a new retarder | Carbonate K (2.7 md) Φ (18.7%) | - | - | N2 | 900 | 116 | The new retarder Gelation time (90 min) at 150 °C PInjection (32 psi) |
| Ref. | Field/ Wellbore | Mechanism of Excess Water | Reservoir Parameters | Treatment Design | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Salinity ppm | Type | PCon ppm | TDS g/l | Vol bbl | ||||
| [10] | Indonesia /deviated vertical monobore | Water source located at the top of perforation in a well that is still producing | Sandstone K (500 md) | 2200 | 150 | 11,830 | - | PAtBA/ PEI | - | - | - | Qw↓(97.5%) Qg ↑(83.3%) 2 months |
| [46] | Middle East/openhole horizontal | Water was entering the openhole at the toe | Carbonate K (2–3 md) | 7000 | 149 | 13,611 | - | PAtBA/ PEI | 250 gal/1000 gal | 20 | 155 | Qw↓(58%) Qg ↑(672%) 8 months |
| [47] | Gulf of Thailand/vertical monobore | Water production from top perf zones (matrix-related) | - | - | - | - | - | PAtBA/ PEI | - | - | 10 | Qw↓(49%) |
| Ref. | Field/ Wellbore | Mechanism of Excess Gas | Reservoir Parameters | Treatment Design | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Salinity ppm | Type | PCon ppm | TDS g/l | Vol bbl | ||||
| [48] | North Sea/vertical openhole gravel pack | High K channel between casing shoe bottom and top of gravel pack | Sandstone K (17–340 md) | 3539 | 88 | - | - | PAtBA/ PEI | - | - | 638 | GOR ↓ (70%) Qo ↓ 12 months Payout (less than a month) |
| [49] | North Sea/vertical casedhole | Communication between the tubing and casing | Chalk K (1–340 md) | 1900 | 93 | 5980 | - | PAtBA/ PEI | - | - | 20 | The annulus pressure ↓ from 1305 to 350 psi with minimal leak |
| [50] | Southern Mexico/vertical casedhole | Perforated interval close to GOC/high K streak (fracture-related) | Carbonate K (1–10) md | 1400 | 93 | 8645 | - | PAtBA/ PEI | - | - | 660 | GOR ↓ (79%) Qo = 3900 BOPD 6 months |
| Ref. | Field/Wellbore | Mechanism of Excess Water/Gas | Reservoir Parameters | Treatment Design | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Type | PCon ppm | Vol bbl | ||||
| [34] | Northern California/vertical | - | - | - | - | - | Hydroxypropyl guar (HPG) crosslinked with chelated titanium | - | - | No improvement in gas and water rates |
| [51] | New Mexico | Water influx through crack/fracture in cement | Sandstone | - | 121 | 17,000 | HPAM/HQ+HMTA | - | - | Qw ↓ 60%) Qg ↔ 8 months |
| Canada | Water influx through fracture | Carbonate K (200 md) | - | 113 | - | HPAM/HQ+HMTA | - | 620 | 3 wells Qw ↓ (65%) Qg ↑ (315%) | |
| [52] | Venezuela/vertical | Gas channeling and coning | K (54–180 md) | - | 148 | 14,080 | Multigel (PDVSA-Intevep) | - | - | GOR ↓ (70%) Qo ↑ by (22%) |
| Ref. | Polymer Gel | Model Parameters | Operating Conditions | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Lithology | D in. | L in. | Gas | p psi | T °C | |||
| [54] | Activated nanosilica system 78.5 (wt.%) nanosilica, 0.2 (vol%) surfactant, 0.2 (vol%) clay control | Sandstone K (370 md) Φ (24%) | - | - | N2 | 500 | 93 | Complete pore plugging PBrine = 1500 psi PN2 = 600 psi |
| Ref. | Field/Wellbore | Mechanism of Excess Water/Gas | Reservoir Parameters | Treatment Design | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Type | PCon ppm | Vol bbl | ||||
| [56] | Prudhoe Bay, AK/ vertical | Gas coning/tonguing through high K sands | Sandstone K (100–4000) md | - | 93 | 15,000 | Activated sodium silicate | - | 192 | Incomplete shutoff |
| [57] | Indonesia/vertical monobore | Crossflow from watered-out to gas-producing interval | Shaly sands K (1–100) md | 2815 | 118 | 10,658 | Inorganic gel—delayed gelation system | - | 139 | Qw ↓ (65%) Qg ↓ (33%) 3 months |
| Ref. | Polymer Gel | Model Parameters | Operating Conditions | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Lithology | D In. | L In. | Gas | p psi | T °C | |||
| [34] | Cationic PAM | Sandstone K (383–54 md) Φ (23%) | 1.0 | 5.0 | N2 | 290 | 59 | Frrw (2.1): Frrg (1) Frrw/Frrg (2.1) After 20 PV Frrw (1.5): Frrg (1.4) Frrw/Frrg (1.05) |
| [59] | 2500 ppm HPAM 8 × 106 MW TDS: 972 → 8243 ppm | Sandstone K (280 md) Φ (24%) | 1.6 | 3.1 | N2 | - | 35 | Adsorption (192.4) µg/g Before swelling Frrw (1.8): Frrg (0.5) Frrw/Frrg (3.6) After swelling Frrw (28.6): Frrg (0.5) Frrw/Frrg (57.2) Swi ↑ |
| [70] | 2500 ppm PAM + 500 ppm Activator TDS: 14,000 ppm | Limestone K (108 md) 23% | 1.6 | 2.3 | N2 | 435 | 36 | Adsorption (150)µg/g Before swelling Frrw (13.8): Frrg (1.5) Frrw/Frrg (9.2) After swelling Frrw (52.8): Frrg (1.3) Frrw/Frrg (40.6) Swi ↑ |
| [71] | 1200 ppm Anionic HPAM-2 17 × 106 MW TDS:2362.5 ppm | Sandstone Φ (14.5–16.7%) K (0.0123–0.0870 md) | 1.0 | 2–3 | N2 | - | 72 | Adsorption (174.5–120.4) µg/g Kab ↓: µg/g ↑ Frrw ↑ Frrg ↓ Frrw/Frrg (5.2–5.0) Swi ↑ |
| 800–1200 ppm Anionic HPAM-1 14 × 106 MW TDS:2362.5 ppm | K (0.0212—0.0224 md) | 1.0 | 2–3 | N2 | - | 72 | Adsorption (132.6–138.9) µg/g PCON: µg/g ↑ Frrw ↑ Frrg ↑ Frrw/Frrg (2.7–1.7) Swi ↑ | |
| [37] | 2000 ppm Cationic PAM CAT1: 4 × 106 MW TDS: 20,000 ppm | Reservoir sands K (15–474 md) Φ (16%) | 1.0 | 2.6 | N2 | - | 48 | CAT1 Kab ↑ Frrw ↑ Frrg ↓ Sheared CAT1 Kabs < 300 md: Frrw/Frrg (5.4) Kabs > 300 md Frrw/Frrg (1.4) |
| [72] | 1000–2000 ppm TDS: 20,000 ppm Cationic PAM CAT1: 4 × 106 MW | Sandpack Reservoir sands Φ (16%) | 1.0 | 2.6 | N2 | - | 48 | Adsorption (2691–3694) µg/g Frrw/Frrg (2.9–3.4) Swi ↑ |
| Cationic PAM CAT2: 0.8 × 106 MW | (2152–3187) µg/g Frrw/Frrg (2.6) Swi ↑ | |||||||
| Nonionic PAM PAM: 5 × 106 MW | (1634–2477) µg/g Frrw/Frrg (1.8) | |||||||
| [73] | 1000–2000 ppm Cationic acrylamide co-polymer 6 × 106 MW TDS: 216,000–53,000 ppm | Reservoir cores K (938–1473 md) Φ (21–26%) | - | - | N2 | - | 36 23 | Adsorption (1637–941) µg/g Fr (25.07–15.04) Frrw (11.34–6.92) Frrg (1.14–1.07) |
| [74] | 1000 ppm Cationic poly(acrylamide-co-diallyldimethylammonium chloride) 25,000 MW TDS: 20,000 | Sandstone K (2.7–66.4 md) Φ (17.6–19.5%) | 1.5 | 3.0 | N2 | - | - | Frrw (1.44–2.35) Frrg (4.60–7.60) Frrw/Frrg (0.19–0.43) |
| K (350–385 md) Φ (21%) | Frrw (2.3–2.86) Frrg (0.90–0.928) Frrw/Frrg (2.5–3.08) | |||||||
| K (3001–5053 md) Φ (23–29%) | Frrw (1.21–1.75) Frrg (1.0–1.32) Frrw/Frrg (1.03–1.75) | |||||||
| [17] | 1000–8000 ppm Cationic poly(acrylamide-co-diallyldimethylammonium chloride) 25,000 MW TDS: 20,000 | Sandstone K (350–426 md) Φ (21%) | 1.5 | 2 | N2 | 1000 | RT | Frrw (1.15–2.75) Frrg (0.6–2) Frrw/Frrg (1.38–3.96) Frrw/Frrg ↑Pcon ↓ Kabs ↓ |
| [75] | 500–5000 ppm Copolymer (POWELGEL P321) TDS: 33,000 ppm | Sandstone K (360 md) md Φ (29%) | 1.5 | 2.3 | N2 | - | 40 | Adsorption (600) µg/g Fr (2.4–76.6) Frrw (1.5–6.5) @ 5000 ppm Frrw/Frrg (6.5/1.1) 5.90 Swi ↑ |
| [76] | 1000 ppm Polysaccharide 14 × 106 MW TDS: 60,000 ppm | Vosges sandstone K (40–60 md) Obernkirchr sandstone K (4–9 md) Reservoir core K (10–20 md) | - | - | N2 | - | 90 130 | Vosges: 90 °C Adsorption (50) µg/g Fr (35.8) |
| 1000 ppm Vinyl sulfonate/acrylamide copolymer (AM/AMPS) HMW TDS: 20,000 ppm | Vosges: 90 °C Adsorption (10) µg/g Fr (25.7) | |||||||
| 1000 ppm Vinyl sulfonate/vinyl amide/acrylamide Terpolymers (VS/VA/AM) Hostadrill: 0.5 × 106 MW Hostamer: 1.0 × 106 MW TDS: 300,000 ppm | Vosges reservoir core: 130 °C Adsorption (135–185) µg/g Fr (7–9.5): (5.6–7.6) Frrw/Frrg (3.8–5.8): (2.3–5.3) Adsorption (148–203) µg/g Fr (11–16): (8.8–12.8) Frrw/Frrg (4.6–6.8): (3.0–6.1) | |||||||
| [44] | 1000 to 2000 ppm Biopolymer(Polymer B) HMW TDS: 217,000 ppm | Carbonate K (12–138.7 md) Φ (20)% | 1.5 | 2.0 | N2 | 1500 | 90 | Adsorption (21–94) µg/g ↑ PCON↑ Fr (25–44) Frrw (18.5–20.3) Frrg (1.6–2) Frrw/Frrg (11.6–10.15) |
| 1000 to 2000 ppm Vinyl amide/vinyl sulfonate Terpolymer (Polymer A) TDS: 217,000 ppm | Adsorption (20.3–75.4) µg/g Fr (2.4–1.9) Frrw (1.6–1.9) Frrg (1.4–1.4) Frrw/Frrg (1.14–10.1) | |||||||
| Ref. | Location | Mechanism of Excess Water | Reservoir Parameters | Treatment Design | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formation | BHP Psi | BHT °C | Depth ft | Salinity ppm | Type | PCon ppm | TDS ppm | Vol bbl | ||||
| [59] | France/gas storage | Water encroachment through active water aquifer to high-permeability streak | Sandstone K (100–5000 md) | - | 30 | 1640 | 972 | HPAM | 3000 | 8209 | 4400 | WGR ↓ and WProd ↓ GProd and GInjt unchanged |
| [77] | France/gas storage | Water coning to multiple layers with good vertical communication | Limestone K (10–700 md) | 1450 | 36 | 2300 | 14,015 | Nonionic PAM + KOH | 2000 | River water | 1600 | No improvement |
| [71] | China | Water influx through high-K streak | Sandstone K (7–124 md) Φ (11.2%) | - | 75 | 7546 | - | Anionic HPAM-2 | 1000 | 2363 | 4088 | WGR ↓ (68%) GWR ↑ (230%) 1.5 years |
| [37] | Italy/ vertical | - | Shaly sands K (44 md) | 985 | 48 | 3170 | - | Cationic polymer CAT1 | 1500 | 30,000 | 345 | GWR ↑ (4.1–11.2) Mscf/bbl WGR ↓ (0.24–0.09) bbl/Mscf 8 months |
| [73] | Germany/gas storage | - | Sandstone (50–2000 md) Φ (17.4–23.2%) | 1015 | 36 | x | 216,000 | Cationic acrylamide copolymer | 1000 | - | 1415 | GWR ↑ (269.5–898) Mscf/bbl 5 years |
| Germany/gas storage | - | Sandstone K (40–3600 md) Φ (24–27)% | 551 | 23 | x | 53,000 | Cationic acrylamide copolymer | 750–2000 | - | 1258 | GWR ↑ (330–1061) Mscf/bbl 6 years | |
| Germany/gas storage | - | Sandstone K (50–90) md Φ (14.6–21.3%) | 3988 | 90 | 7195 | 270,000 | Anionic VS/VA/AM terpolymer (Hostadrill) | 500–2000 | - | 917 | GWR ↑ (390–1605) Mscf/bbl 6 years | |
| Germany | Abandoned gas well loaded up with water | Sandstone K (10–15 md) Φ (11.5–14.7%) | 2030 | 130 | 11,286 | 300,000 | Anionic VS/VA/AM terpolymer (Hostamer) | 1000 | 180,000 | 1258 | GWR ↑ (0–1) Mscf/bbl 4.5 years | |
| [81] | Canada | - | Sandstone K (25 md) | - | - | - | - | Terpolymer | 30,000 | - | - | WGR ↓ (0.06–0) bbl/Mscf 5 months |
| [82] | Adriatic Sea | Water encroachment | Sandstone K (10–50 md) | - | 40–55 | 5584–10,653 | 30,000–40,000 | Copolymer | 750 to 1000 | 30,000 | 251–403 | 9 wells were treated |
| [75] | Adriatic Sea | Water encroachment through high-K streak | Sandstone K (660 md) Φ (36%) | 2343 | 40 | 1924 | 33,000 | Copolymer | 1500 to 3000 | 30,000 | 283 | Improved the gas production decline rate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Brahim, A.; Bai, B.; Schuman, T. Comprehensive Review of Polymer and Polymer Gel Treatments for Natural Gas-Related Conformance Control. Gels 2022, 8, 353. https://doi.org/10.3390/gels8060353
Al Brahim A, Bai B, Schuman T. Comprehensive Review of Polymer and Polymer Gel Treatments for Natural Gas-Related Conformance Control. Gels. 2022; 8(6):353. https://doi.org/10.3390/gels8060353
Chicago/Turabian StyleAl Brahim, Ali, Baojun Bai, and Thomas Schuman. 2022. "Comprehensive Review of Polymer and Polymer Gel Treatments for Natural Gas-Related Conformance Control" Gels 8, no. 6: 353. https://doi.org/10.3390/gels8060353
APA StyleAl Brahim, A., Bai, B., & Schuman, T. (2022). Comprehensive Review of Polymer and Polymer Gel Treatments for Natural Gas-Related Conformance Control. Gels, 8(6), 353. https://doi.org/10.3390/gels8060353







