Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy
Abstract
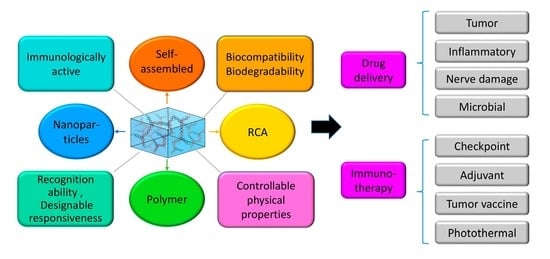
:1. Introduction
2. Classification of DNA Hydrogels
2.1. Pure DNA Hydrogel
2.1.1. Self-Assembled DNA Hydrogels
2.1.2. Rolling Circle Amplification (RCA) DNA Hydrogel
2.2. Hybrid DNA Hydrogels
2.2.1. Polymer-DNA Hybrid Hydrogels
2.2.2. Nanoparticles-DNA Hybrid Hydrogels
3. DNA Hydrogel-Based Local Drug Delivery
3.1. Local Delivery of Antitumor Drugs with DNA Hydrogels
3.2. Local Delivery for Anti-Inflammatory Drugs with DNA Hydrogels
3.3. Local Delivery of Drug against Nerve Damage with DNA Hydrogels
3.4. Local Delivery of Antimicrobial Drug with DNA Hydrogels
4. DNA Hydrogel-Based Immunotherapy
4.1. Immune Checkpoint Blocking
4.2. Immune Adjuvants Delivery
4.3. Tumor Vaccine Delivery
4.4. Photothermal Immunotherapy
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional hydrogels and their application in drug delivery, biosensors, and tissue engineering. Int. J. Polym. Sci. 2019, 2019, 3160732. [Google Scholar] [CrossRef]
- Gao, Y.F.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ formation of injectable hydrogels for chronic wound healing. J. Mat. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhu, Y.; Liu, H.; Wang, L. Tailoring DNA self-assembly to build hydrogels. Top. Curr. Chem. 2020, 378, 32. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.; Walia, S.; Mandal, B.B.; Ghoroi, C.; Bhatia, D. Functional DNA based hydrogels: Development, properties and biological applications. ACS Biomater. Sci. Eng. 2020, 6, 6021–6035. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Davies, D.R. A new two stranded helical structure: Polyadenylic acid and polyuridylic acid. J. Am. Chem. Soc. 1956, 78, 3548–3549. [Google Scholar] [CrossRef]
- Caruthers, M.H. Gene synthesis machines: DNA chemistry and its uses. Science 1985, 230, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, S.; Matsuda, T. Hydrogel formation via hybridization of oligonucleotides derivatized in water-soluble vinyl polymers. Polym. Gels Netw. 1996, 4, 111–127. [Google Scholar] [CrossRef]
- Li, C.; Faulkner-Jones, A.; Dun, A.R.; Jin, J.; Chen, P.; Xing, Y.; Yang, Z.; Li, Z.; Shu, W.; Liu, D.; et al. Rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem.-Int. Ed. 2015, 54, 3957–3961. [Google Scholar] [CrossRef]
- Stoll, H.; Steinle, H.; Stang, K.; Kunnakattu, S.; Scheideler, L.; Neumann, B.; Kurz, J.; Degenkolbe, I.; Perle, N.; Schlensak, C.; et al. Generation of large-scale DNA hydrogels with excellent blood and cell compatibility. Macromol. Biosci. 2017, 17, 1600252. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Mou, Q.; Yan, D.; Zhu, X.; Zhang, C. Floxuridine-containing nucleic acid nanogels for anticancer drug delivery. Nanoscale 2018, 10, 8367–8371. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mo, L.; Lu, C.-H.; Fu, T.; Yang, H.-H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi, M.-A.; Bauleth-Ramos, T.; Santos, H.A. DNA hydrogel assemblies: Bridging synthesis principles to biomedical applications. Adv. Therap. 2018, 1, 1800042. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Hammink, R.; Das, R.K.; Nelissen, F.H.T.; Blank, K.G.; Rowan, A.E.; Heus, H.A. DNA-responsive polyisocyanopeptide hydrogels with stress-stiffening capacity. Adv. Funct. Mater. 2016, 26, 9075–9082. [Google Scholar] [CrossRef]
- Li, C.; Chen, P.; Shao, Y.; Zhou, X.; Wu, Y.; Yang, Z.; Li, Z.; Weil, T.; Liu, D. A writable polypeptide-DNA hydrogel with rationally designed multi-modification sites. Small 2015, 11, 1138–1143. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Z.; Pan, Y.; Xie, J.; Zhou, B.; Li, Y.; Dong, Y.; Liu, D. Shear-thinning and designable responsive supramolecular DNA hydrogels based on chemically branched DNA. ACS Appl. Mater. Interfaces 2021, 13, 48414–48422. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, J.; Pei, Y.; Du, J. Aptamer functionalized DNA hydrogel for wise-stage controlled protein release. Appl. Sci. 2018, 8, 1941. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Sun, N.; Xu, L.; Chen, X.; Cheng, H.; Wang, J.; Pei, R. Dual signal amplification by an “on-command” pure DNA hydrogel encapsulating hrp for colorimetric detection of ochratoxin A. RSC Adv. 2016, 6, 114500–114504. [Google Scholar] [CrossRef]
- Xiang, B.; He, K.; Zhu, R.; Liu, Z.; Zeng, S.; Huang, Y.; Nie, Z.; Yao, S. Self-assembled DNA hydrogel based on enzymatically polymerized DNA for protein encapsulation and enzyme/dnazyme hybrid cascade reaction. ACS Appl. Mater. Interfaces 2016, 8, 22801–22807. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Ma, X.; Zhou, B.; Faulkner-Jones, A.; Shu, W.; Liu, D. Constructing tissuelike complex structures using cell-laden DNA hydrogel bricks. ACS Appl. Mater. Interfaces 2017, 9, 12311–12315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Ohtsuki, S.; Araie, Y.; Umeki, Y.; Endo, M.; Emura, T.; Hidaka, K.; Sugiyama, H.; Takahashi, Y.; Takakura, Y.; et al. Self-assembling DNA hydrogel-based delivery of immunoinhibitory nucleic acids to immune cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, W.; Shen, X.; Wang, S.; Li, Q.; An, F.; Wu, S. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of ochratoxin a. J. Agric. Food. Chem. 2020, 68, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tian, R.; Liu, G.; Wen, Y.; Bian, X.; Luan, D.; Wang, H.; Lai, K.; Yan, J. Fishing unfunctionalized sers tags with DNA hydrogel network generated by ligation-rolling circle amplification for simple and ultrasensitive detection of kanamycin. Biosens. Bioelectron. 2022, 207, 114187. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, S.; Huang, T.; Xiao, F.; Wu, Z.; Yu, R. Construction and research of multiple stimuli-responsive 2D photonic crystal DNA hydrogel sensing platform with double-network structure and signal self-expression. Anal. Chem. 2022, 94, 5530–5537. [Google Scholar] [CrossRef]
- Nomura, D.; Saito, M.; Takahashi, Y.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Development of orally-deliverable DNA hydrogel by microemulsification and chitosan coating. Int. J. Pharm. 2018, 547, 556–562. [Google Scholar] [CrossRef]
- Lian, S.W.M.; Guo, S.; Ren, K.; Xu, Y.; Ho, J.S.; Chen, C.-H. Heterogeneous multi-compartmental DNA hydrogel particles prepared via microfluidic assembly for lymphocyte-inspired precision medicine. Nanoscale 2021, 13, 20531–20540. [Google Scholar] [CrossRef]
- Lyu, D.; Chen, S.; Guo, W. Liposome crosslinked polyacrylamide/DNA hydrogel: A smart controlled-release system for small molecular payloads. Small 2018, 14, 1704039. [Google Scholar] [CrossRef]
- Zhou, L.; Pi, W.; Hao, M.; Li, Y.; An, H.; Li, Q.; Zhang, P.; Wen, Y. An injectable and biodegradable nano-photothermal DNA hydrogel enhances penetration and efficacy of tumor therapy. Biomater. Sci. 2021, 9, 4904–4921. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Kumari, R.; Sinha, K.K.; Singh, M.K.; Das, P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon 2017, 114, 169–176. [Google Scholar] [CrossRef]
- Bagley, R.H.T.; Jones, S.T. Deoxyribonucleic acid polymer nanoparticle hydrogels. Chem. Commun. 2021, 57, 12111–12114. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.O.; Kim, S.; Park, N. Light-responsible DNA hydrogel-gold nanoparticle assembly for synergistic cancer therapy. J. Mat. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, G.; Grainger-Boultby, C.; Francini, N.; Magnusson, J.P.; Saeed, A.O.; Fernandez-Trillo, F.; Spain, S.G.; Alexander, C. Programmable polymer-DNA hydrogels with dual input and multiscale responses. Biomater. Sci. 2014, 2, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, M.; Mizuno, Y.; Mohri, K.; Matsuoka, N.; Rattanakiat, S.; Takahashi, Y.; Funabashi, H.; Luo, D.; Takakura, Y. Biodegradable CpG DNA hydrogels for sustained delivery of doxorubicin and immunostimulatory signals in tumor-bearing mice. Biomaterials 2011, 32, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Yang, Z.; Wang, Y.; Zhang, G.; Shao, Y.; Jia, H.; Cao, T.; Wang, R.; Liu, D. Remote controlling DNA hydrogel by magnetic field. ACS Appl. Mater. Interfaces 2017, 9, 1995–2000. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA hydrogel-based gene editing and drug delivery systems. Adv. Drug Deliv. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Yata, T.; Takahashi, Y.; Tan, M.; Hidaka, K.; Sugiyama, H.; Endo, M.; Takakura, Y.; Nishikawa, M. Efficient amplification of self-gelling polypod-like structured DNA by rolling circle amplification and enzymatic digestion. Sci. Rep. 2015, 5, 14979. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, R.; Tang, J.; Yang, D. Rolling circle amplification (rca)-based DNA hydrogel. Nat. Protoc. 2021, 16, 5460–5483. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019, 98, 101163. [Google Scholar] [CrossRef]
- Wu, J.; Liyarita, B.R.; Zhu, H.; Liu, M.; Hu, X.; Shao, F. Self-assembly of dendritic DNA into a hydrogel: Application in three-dimensional cell culture. ACS Appl. Mater. Interfaces 2021, 13, 49705–49712. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Z.; Liu, D. DNA nanotechnology based on i-motif structures. Acc. Chem. Res. 2014, 47, 1853–1860. [Google Scholar] [CrossRef]
- Helwa, Y.; Dave, N.; Froidevaux, R.; Samadi, A.; Liu, J. Aptamer-functionalized hydrogel microparticles for fast visual detection of mercury(ii) and adenosine. ACS Appl. Mater. Interfaces 2012, 4, 2228–2233. [Google Scholar] [CrossRef] [Green Version]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, L.; Fan, Q.; Liu, D. A ph-triggered, fast-responding DNA hydrogel. Angew. Chem.-Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, S.; Lu, H.; Ying, J.Y. Acid-resistant and physiological ph-responsive DNA hydrogel composed of a-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.-K.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Y.; Cheng, P.; Chen, X.; Luo, Y.; Xu, W. A rapidly self-assembling soft-brush DNA hydrogel based on rca products. Chem. Commun. 2019, 55, 5375–5378. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Zeng, Q.; Qian, H.; Zhang, D.; Wei, Y.; Wang, Y.; Chai, C.; Cheng, W.; Ding, S.; Chen, T. Dual-targeted self-assembled DNA hydrogels decorated with multivalent aptamers loaded with dox for anticancer therapy. Front. Pharmacol. 2022, 13, 807498. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Li, Y.; Jiang, C.; Chen, D.; Wen, Y.; Li, Z. Capillarity self-driven DNA hydrogel sensor for visual quantification of microrna. Sens. Actuator B-Chem. 2020, 313, 128036. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.-H.; Qi, X.-J.; Orbach, R.; Fadeev, M.; Yang, H.-H.; Willner, I. Switchable bifunctional stimuli-triggered poly-n-isopropylacrylamide/DNA hydrogels. Angew. Chem.-Int. Ed. 2014, 53, 10134–10138. [Google Scholar] [CrossRef]
- Lu, C.-H.; Guo, W.; Hu, Y.; Qi, X.-J.; Willner, I. Multitriggered shape-memory acrylamide-DNA hydrogels. J. Am. Chem. Soc. 2015, 137, 15723–15731. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Yan, H.; Liu, Y. Soft robotics programmed with double crosslinking DNA hydrogels. Adv. Funct. Mater. 2019, 29, 1905911. [Google Scholar] [CrossRef]
- Chen, F.; He, Y.; Li, Z.; Xu, B.; Ye, Q.; Li, X.; Ma, Z.; Song, W.; Zhang, Y. A novel tunable, highly biocompatible and injectable DNA-chitosan hybrid hydrogel fabricated by electrostatic interaction between chitosan and DNA backbone. Int. J. Pharm. 2021, 606, 120938. [Google Scholar] [CrossRef]
- Ishii-Mizuno, Y.; Umeki, Y.; Onuki, Y.; Watanabe, H.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Improved sustained release of antigen from immunostimulatory DNA hydrogel by electrostatic interaction with chitosan. Int. J. Pharm. 2017, 516, 392–400. [Google Scholar] [CrossRef]
- Ren, N.; Sun, R.; Xia, K.; Zhang, Q.; Li, W.; Wang, F.; Zhang, X.; Ge, Z.; Wang, L.; Fan, C.; et al. DNA-based hybrid hydrogels sustain water-insoluble ophthalmic therapeutic delivery against allergic conjunctivitis. ACS Appl. Mater. Interfaces 2019, 11, 26704–26710. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Pan, G.; Wang, P.; Li, Y.; Zhu, X.; Zhang, C. Injectable drug-conjugated DNA hydrogel for local chemotherapy to prevent tumor recurrence. ACS Appl. Mater. Interfaces 2020, 12, 21441–21449. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Zhou, L.; Li, C.; Zhang, M.; Zhang, F.; Ding, Z.; Wen, Y.; Zhang, P. Xt-type DNA hydrogels loaded with vegf and ngf promote peripheral nerve regeneration via a biphasic release profile. Biomater. Sci. 2021, 9, 8221–8234. [Google Scholar] [CrossRef] [PubMed]
- Obuobi, S.; Tay, H.K.-L.; Nhan Dai Thien, T.; Selvarajan, V.; Khara, J.S.; Wang, Y.; Ee, P.L.R. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control. Release 2019, 313, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.M.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann.Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Aebi, S.; Gelber, S.; Anderson, S.J.; Lang, I.; Robidoux, A.; Martin, M.; Nortier, J.W.R.; Paterson, A.H.G.; Rimawi, M.F.; Canada, J.M.B.; et al. Chemotherapy for isolated locoregional recurrence of breast cancer (calor): A randomised trial. Lancet Oncol. 2014, 15, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, Y.; Kim, J.; Jeong, J.; Han, S.; Lee, J.S.; Lee, J.B. Nucleic acid engineering: Rna following the trail of DNA. ACS Comb. Sci. 2016, 18, 87–99. [Google Scholar] [CrossRef]
- Han, S.; Park, Y.; Kim, H.; Nam, H.; Ko, O.; Lee, J.B. Double controlled release of therapeutic rna modules through injectable DNA-rna hybrid hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 55554–55563. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mat. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Dupuis, P.; Prokopich, C.L.; Hynes, A.; Kim, H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020, 16, 5. [Google Scholar] [CrossRef]
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.A.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D. Spinal-cord injury: Spurring regrowth. Nature 2017, 552, S49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Shao, Y.; Zhou, X.; Liu, Q.; Zhu, Z.; Zhou, B.; Dong, Y.; Stephanopoulos, N.; Gui, S.; Yan, H.; et al. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv. Mater. 2021, 33, 2102428. [Google Scholar] [CrossRef] [PubMed]
- Perpoint, T.; Lina, G.; Poyart, C.; de Barbeyrac, B.; Traineau, R.; Jeanne, M.; Vandenesch, F.O.; Etienne, J. Two cases of fatal shock after transfusion of platelets contaminated by Staphylococcus aureus: Role of superantigenic toxins. Clin. Infect. Dis. 2004, 39, E106–E109. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Chamoto, K.; Tsuji, T.; Suzuki, Y.; Wakita, D.; Takeshima, T.; Nishimura, T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004, 95, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Musetti, S.N.; Huang, L. Nanomaterials for cancer immunotherapy. Biomaterials 2017, 148, 16–30. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the pd-l1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Lee, J.; Le, Q.-V.; Yang, G.; Oh, Y.-K. Cas9-edited immune checkpoint blockade pd-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials 2019, 218, 119359. [Google Scholar] [CrossRef]
- Yao, C.; Zhu, C.; Tang, J.; Ou, J.; Zhang, R.; Yang, D. T lymphocyte-captured DNA network for localized immunotherapy. J. Am. Chem. Soc. 2021, 143, 19330–19340. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ogawa, K.; Umeki, Y.; Mohri, K.; Kawasaki, Y.; Watanabe, H.; Takahashi, N.; Kusuki, E.; Takahashi, R.; Takahashi, Y.; et al. Injectable, self-gelling, biodegradable, and immunomodulatory DNA hydrogel for antigen delivery. J. Control. Release 2014, 180, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, H. Interactions between bacterial CpG-DNA and tlr9 bridge innate and adaptive immunity. Curr. Opin. Microbiol. 2002, 5, 62–69. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takeuchi, O.; Akira, S. Tlr9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008, 60, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded rna via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [Green Version]
- Komura, F.; Okuzumi, K.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Development of rna/DNA hydrogel targeting toll-like receptor 7/8 for sustained rna release and potent immune activation. Molecules 2020, 25, 728. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, B.L.; Day, S.; Malins, L.R.; Apostolopoulos, V.; Payne, R.J. Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated muc1 glycopeptides and the toll-like receptor 2 agonist pam(3)cysser. Angew. Chem.-Int. Ed. 2011, 50, 1635–1639. [Google Scholar] [CrossRef]
- Galonic, D.P.; Gin, D.Y. Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature 2007, 446, 1000–1007. [Google Scholar] [CrossRef]
- Danishefsky, S.J.; Shue, Y.-K.; Chang, M.N.; Wong, C.-H. Development of globo-h cancer vaccine. Acc. Chem. Res. 2015, 48, 643–652. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Z.-Y.; Wang, Y.; Zhang, B.-D.; Liu, D.; Li, Y.-M. Designable immune therapeutical vaccine system based on DNA supramolecular hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 9310–9314. [Google Scholar] [CrossRef]
- Mohri, K.; Nishikawa, M.; Takahashi, N.; Shiomi, T.; Matsuoka, N.; Ogawa, K.; Endo, M.; Hidaka, K.; Sugiyama, H.; Takahashi, Y.; et al. Design and development of nanosized DNA assemblies in polypod-like structures as efficient vehicles for immunostimulatory CpG motifs to immune cells. ACS Nano 2012, 6, 5931–5940. [Google Scholar] [CrossRef]
- Uno, S.; Nishikawa, M.; Mohri, K.; Umeki, Y.; Matsuzaki, N.; Takahashi, Y.; Fujita, H.; Kadowaki, N.; Takakura, Y. Efficient delivery of immunostimulatory DNA to mouse and human immune cells through the construction of polypod-like structured DNA. Nanomed.-Nanotechnol. Biol. Med. 2014, 10, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeki, Y.; Mohri, K.; Kawasaki, Y.; Watanabe, H.; Takahashi, R.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Induction of potent antitumor immunity by sustained release of cationic antigen from a DNA-based hydrogel with adjuvant activity. Adv. Funct. Mater. 2015, 25, 5758–5767. [Google Scholar] [CrossRef] [Green Version]
- Umeki, Y.; Saito, M.; Kusamori, K.; Tsujimura, M.; Nishimura, M.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Combined encapsulation of a tumor antigen and immune cells using a self-assembling immunostimulatory DNA hydrogel to enhance antigen-specific tumor immunity. J. Control. Release 2018, 288, 189–198. [Google Scholar] [CrossRef]
- Umeki, Y.; Saito, M.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Retardation of antigen release from DNA hydrogel using cholesterol-modified DNA for increased antigen-specific immune response. Adv. Healthc Mater. 2017, 6, 1700355. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Adams, R.L.; Carubelli, R.; Nordquist, R.E. Laser-photosensitizer assisted immunotherapy: A novel modality for cancer treatment. Cancer Lett. 1997, 115, 25–30. [Google Scholar] [CrossRef]
- Li, X.; Min, M.; Gu, Y.; Du, N.; Hode, T.; Nordquist, R.E.; Wolf, R.F.; Howard, E.; Lunn, J.A.; Adalsteinsson, O.; et al. Laser immunotherapy: Concept, possible mechanism, clinical applications, and recent experimental results. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1434–1438. [Google Scholar] [CrossRef]
- Yata, T.; Takahashi, Y.; Tan, M.; Nakatsuji, H.; Ohtsuki, S.; Murakami, T.; Imahori, H.; Umeki, Y.; Shiomi, T.; Takakura, Y.; et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials 2017, 146, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Shim, G.; Oh, Y.-K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Control. Release 2021, 330, 540–553. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Qu, Y.; Zhang, Z.; Huang, H.; Xu, Y.; Shen, F.; Wang, L.; Sun, L. Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels 2022, 8, 400. https://doi.org/10.3390/gels8070400
Wang Q, Qu Y, Zhang Z, Huang H, Xu Y, Shen F, Wang L, Sun L. Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels. 2022; 8(7):400. https://doi.org/10.3390/gels8070400
Chicago/Turabian StyleWang, Qi, Yanfei Qu, Ziyi Zhang, Hao Huang, Yufei Xu, Fengyun Shen, Lihua Wang, and Lele Sun. 2022. "Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy" Gels 8, no. 7: 400. https://doi.org/10.3390/gels8070400
APA StyleWang, Q., Qu, Y., Zhang, Z., Huang, H., Xu, Y., Shen, F., Wang, L., & Sun, L. (2022). Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels, 8(7), 400. https://doi.org/10.3390/gels8070400