In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
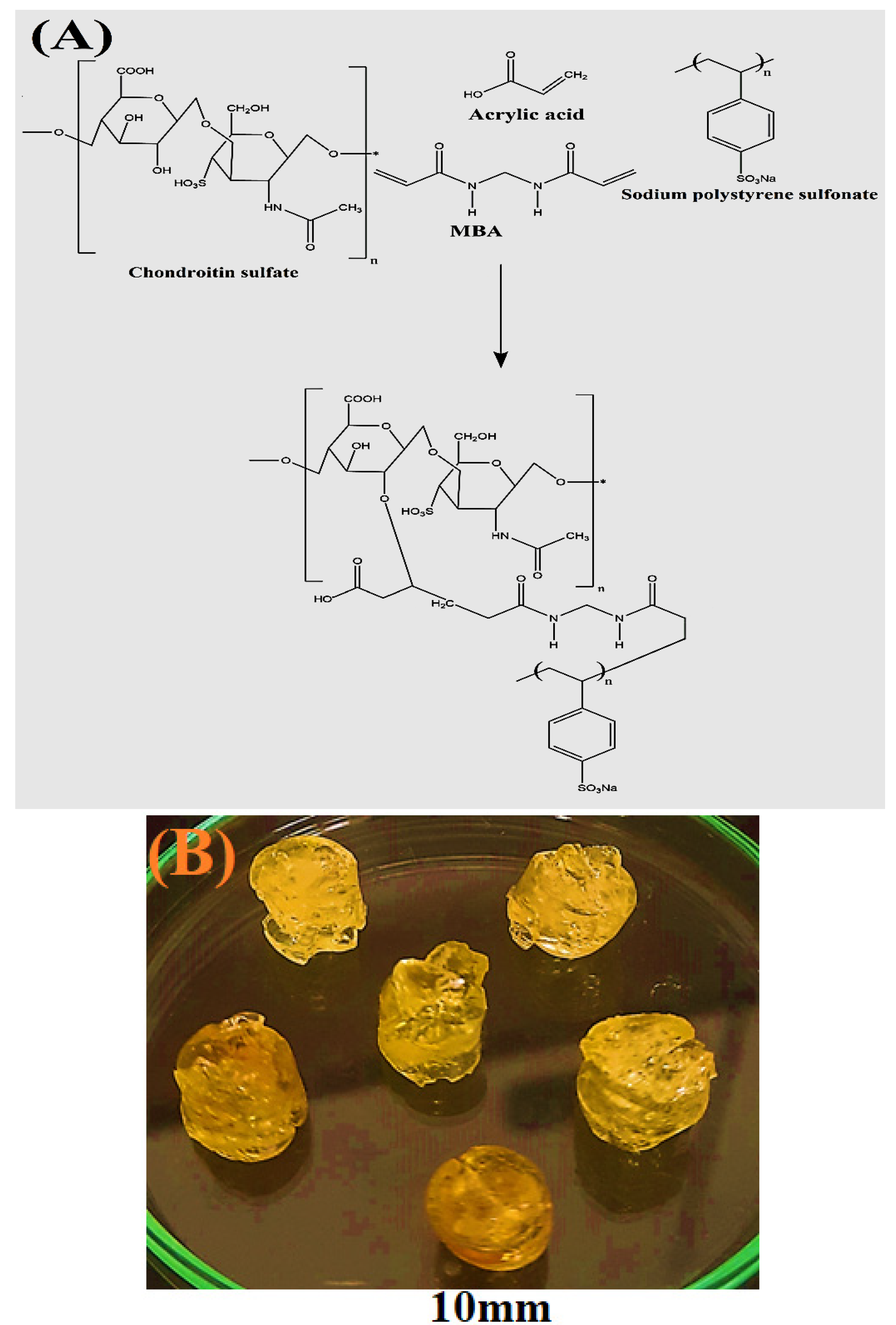
2.1. Fabrication of Polymeric Hydrogels
2.2. Sol-Gel Analysis
2.3. Porosity Study
2.4. Polymer Volume Fraction
2.5. Dynamic Swelling/Drug Loading and Drug Release Studies
2.6. Kinetic Modeling
2.7. FTIR Analysis
2.8. SEM
2.9. TGA
2.10. DSC Analysis
2.11. PXRD Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Polymeric Hydrogels
4.3. Sol-Gel Analysis
4.4. Porosity Study
4.5. Dynamic Swelling
4.6. Polymer Volume Fraction
4.7. Drug Loading
4.8. Dissolution Study
4.9. Kinetic modeling
4.10. FTIR Analysis
4.11. SEM
4.12. Thermal Properties
4.13. PXRD Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, A.; Mequanint, K. Hydrogel Biomaterials; IntechOpen: London, UK, 2011. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elvira, C.; Mano, J.; San Roman, J.; Reis, R. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials 2002, 23, 1955–1966. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Stashak, T.S.; Farstvedt, E.; Othic, A. Update on wound dressings: Indications and best use. Clin. Tech. Equine Pract. 2004, 3, 148–163. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Synthetic hydrogels for biomedical applications. ACS Publ. 1976, 1–36. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliver. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliver. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.; Nakar, D.; Sintov, A. Chondroitin sulfate: A potential biodegradable carrier for colon-specific drug delivery. Int. J. Pharm. 1992, 84, 141–150. [Google Scholar] [CrossRef]
- Morris, J.D.; Smith, K.M. Chondroitin sulfate in osteoarthritis therapy. Orthopedics 2009, 32, 268–272. [Google Scholar]
- Richy, F.; Bruyère, O.; Ethgen, O.; Rabenda, V.; Bouvenot, G.; Audran, M.; Herrero Beaumont Cuenca, G.; Moore, A.; Eliakim, R.; Van De Putte, L. Time-dependent risk of gastrointestinal complications induced by NSAIDS use: A consensus statement using meta-analytic approach. Osteoporos. Int. 2003, 14, 9. [Google Scholar]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Garg, S.; Vermani, K.; Garg, A.; Anderson, R.A.; Rencher, W.B.; Zaneveld, L.J. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm. Res. 2005, 22, 584–595. [Google Scholar] [CrossRef]
- Suhail, M.; Fang, C.-W.; Minhas, M.U.; Wu, P.-C. Preparation, Characterization, Swelling Potential and In-Vitro Evaluation of Sodium Poly (Styrene Sulfonate)-Based Hydrogels for Controlled Delivery of Ketorolac Tromethamine. Pharmaceuticals 2021, 14, 350. [Google Scholar] [CrossRef]
- Ali, L.; Ahmad, M.; Aamir, M.N.; Minhas, M.U.; Shah, H.H.; Shah, M.A. Cross-linked pH-sensitive pectin and acrylic acid based hydrogels for controlled delivery of metformin. Pak. J. Pharm. Sci. 2020, 33, 1483–1491. [Google Scholar]
- Sweetman, S.C. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Jiang, B.; Hu, L.; Gao, C.; Shen, J. Ibuprofen-loaded nanoparticles prepared by a co-precipitation method and their release properties. Int. J. Pharm. 2005, 304, 220–230. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Y.; Jiang, H.; Yasuda, H.; Ichimaru, A.; Yamamoto, K.; Lecomte, P.; Jérôme, R. Preparation, characterization and in vitro release properties of ibuprofen-loaded microspheres based on polylactide, poly (϶-caprolactone) and their copolymers. J. Microencapsul. 2005, 22, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rajkumar, S.; Ruckmani, K. Formulation and evaluation of ibuprofen loaded nanoparticles for improved anti-inflammatory activity. ACTA Pharm. Sci. 2003, 45, 125–130. [Google Scholar]
- Potthast, H.; Dressman, J.; Junginger, H.; Midha, K.; Oeser, H.; Shah, V.; Vogelpoel, H.; Barends, D. Biowaiver monographs for immediate release solid oral dosage forms: Ibuprofen. J. Pharm. Sci. 2005, 94, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Moore, N. Ibuprofen: A journey from prescription to over-the-counter use. J. R. Soc. Med. 2007, 100, 2–6. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. Int. J. Pharm. 2012, 436, 602–610. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Al-Lohedan, H.A.; Chaudhary, A.A.; Al-Farraj, E.; Alhokbany, N.; Issa, Z.; Alhousine, S.; Ahamad, T. Delivery of ibuprofen by natural macroporous sporopollenin exine capsules extracted from Phoenix dactylifera L. Eur. J. Pharm. Sci. 2016, 88, 158–165. [Google Scholar] [CrossRef]
- Borovac, T.; Pelage, J.; Kasselouri, A.; Prognon, P.; Guiffant, G.; Laurent, A. Release of ibuprofen from beads for embolization: In vitro and in vivo studies. J. Control. Release 2006, 115, 266–274. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently functionalized carbon nano-onions integrated gelatin methacryloyl nanocomposite hydrogel containing γ-cyclodextrin as drug carrier for high-performance pH-triggered drug release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Abbadessa, A.; Blokzijl, M.; Mouser, V.; Marica, P.; Malda, J.; Hennink, W.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; James, N.R.; Kumar, P.R.A.; Raj, D.K. Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde-gelatin nanogels. Mat. Sci. Eng. C-Mater. 2016, 68, 251–257. [Google Scholar]
- Badshah, S.F.; Akhtar, N.; Minhas, M.U.; Khan, K.U.; Khan, S.; Abdullah, O.; Naeem, A. Porous and highly responsive cross-linked β-cyclodextrin based nanomatrices for improvement in drug dissolution and absorption. Life Sci. 2021, 267, 118931. [Google Scholar] [CrossRef] [PubMed]
- Hu, X. Synthesis and properties of silk sericin-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogel. Polym. Bull. 2011, 66, 447–462. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I.; Malik, N.S. Chondroitin sulfate-based smart hydrogels for targeted delivery of oxaliplatin in colorectal cancer: Preparation, characterization and toxicity evaluation. Polym. Bull. 2019, 77, 1–27. [Google Scholar] [CrossRef]
- Atta, A.M. Swelling behaviors of polyelectrolyte hydrogels containing sulfonate groups. Polym. Adv. Technol. 2002, 13, 567–576. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Wang, T.; Yan, X. Synthesis and investigation of a self-assembled hydrogel based on hydroxyethyl cellulose and its in vitro ibuprofen drug release characteristics. RSC Adv. 2017, 7, 9500–9511. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.K.; Mohan, Y.M.; Sreeramulu, J.; Raju, K.M. Semi-IPNs of starch and poly (acrylamide-co-sodium methacrylate): Preparation, swelling and diffusion characteristics evaluation. React. Funct. Polym. 2006, 66, 1482–1493. [Google Scholar] [CrossRef]
- Oprea, A.-M.; Ciolacu, D.; Neamtu, A.; Mungiu, O.C.; Stoica, B.; Vasile, C. Cellulose/chondroitin sulfate hydrogels: Synthesis, drug loading/release properties and biocompatibility. Cellul. Chem. Technol. 2010, 44, 369. [Google Scholar]
- Şanlı, O.; Ay, N.; Işıklan, N. Release characteristics of diclofenac sodium from poly (vinyl alcohol)/sodium alginate and poly (vinyl alcohol)-grafted-poly (acrylamide)/sodium alginate blend beads. Eur. J. Pharm. Biopharm. 2007, 65, 204–214. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals 2021, 14, 234. [Google Scholar] [CrossRef]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH-sensitive hydrogels prepared from the blends of poly (vinyl alcohol) with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- El-Hag Ali, A. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 2012, 123, 763–769. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: Effect of entrapped air. J. Pharm. Sci. 1983, 72, 1189–1191. [Google Scholar] [CrossRef]
- Crispim, E.; Piai, J.; Fajardo, A.; Ramos, E.; Nakamura, T.; Nakamura, C.; Rubira, A.; Muniz, E. Hydrogels based on chemically modified poly (vinyl alcohol) (PVA-GMA) and PVA-GMA/chondroitin sulfate: Preparation and characterization. Express Polym. Lett. 2012, 6. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar] [CrossRef]
- De, R.; Lee, H.; Das, B. Exploring the interactions in binary mixtures of polyelectrolytes: Influence of mixture composition, concentration, and temperature on counterion condensation. J. Mol. Liq. 2018, 251, 94–99. [Google Scholar] [CrossRef]
- Moharram, M.; Khafagi, M. Application of FTIR spectroscopy for structural characterization of ternary poly (acrylic acid)–metal–poly (vinyl pyrrolidone) complexes. J. Appl. Polym. Sci. 2007, 105, 1888–1893. [Google Scholar] [CrossRef]
- Omwoyo, W.N.; Moloto, M.J. Encapsulation of ibuprofen into solid lipid nanoparticles for controlled and sustained release using emulsification solvent evaporation technique. J. Nanomed Nanotechnol. 2019, 10, 40. [Google Scholar]
- Wang, L.-F.; Shen, S.-S.; Lu, S.-C. Synthesis and characterization of chondroitin sulfate–methacrylate hydrogels. Carbohydr. Polym. 2003, 52, 389–396. [Google Scholar] [CrossRef]
- Liu, H.; Gong, B.; Zhou, Y.; Sun, Z.; Wang, X.; Zhao, S. Preparation of high-capacity magnetic polystyrene sulfonate sodium material based on SI-ATRP method and its adsorption property research for sulfonamide antibiotics. BMC Chem. 2020, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Kim, S.; Noh, I. Synthesis of in situ chondroitin sulfate hydrogel through phosphine-mediated Michael type addition reaction. Macromol. Res. 2012, 20, 968–976. [Google Scholar] [CrossRef]
- Amrutkar, J.R.; Gattani, S.G. Chitosan–chondroitin sulfate based matrix tablets for colon specific delivery of indomethacin. AAPS Pharmscitech 2009, 10, 670–677. [Google Scholar] [CrossRef]
- Varghese, J.M.; Ismail, Y.A.; Lee, C.K.; Shin, K.M.; Shin, M.K.; Kim, S.I.; So, I.; Kim, S.J. Thermoresponsive hydrogels based on poly (N-isopropylacrylamide)/chondroitin sulfate. Sens. Actuators B Chem. 2008, 135, 336–341. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I. Oxaliplatin-loaded crosslinked polymeric network of chondroitin sulfate-co-poly (methacrylic acid) for colorectal cancer: Its toxicological evaluation. J. Appl. Polym. Sci. 2017, 134, 45312. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Usman Minhas, M.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly (methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Chaopanich, P.; Siriphannon, P. Facile refluxing synthesis of hydroxyapatite nanoparticles. Aust. J. Chem. 2015, 68, 1293–1298. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, C.-P.; Lee, Y.-D. Synthesis and characterizations of amphiphilic poly (l-lactide)-grafted chondroitin sulfate copolymer and its application as drug carrier. Biomol. Eng. 2007, 24, 131–139. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef]
- Zia, M.A.; Sohail, M.; Minhas, M.U.; Sarfraz, R.M.; Khan, S.; de Matas, M.; Hussain, Z.; Abbasi, M.; Shah, S.A.; Kousar, M. HEMA based pH-sensitive semi IPN microgels for oral delivery; a rationale approach for ketoprofen. Drug Dev. Ind. Pharm. 2020, 46, 272–282. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Azam, F.; Qureshi, J. Thiolation of arabinoxylan and its application in the fabrication of pH-sensitive thiolated arabinoxylan grafted acrylic acid copolymer. Drug Dev. Ind. Pharm. 2019, 45, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Hussain, A.; Khalid, S.; Qadir, M.; Massud, A.; Ali, M.; Khan, I.; Saleem, M.; Iqbal, M.; Asghar, S.; Gul, H. Water uptake and drug release behaviour of methyl methacrylate-co-itaconic acid [P (MMA/IA)] hydrogels cross-linked with methylene bis-acrylamide. J. Drug Deliv. Sci. Technol. 2011, 21, 249. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Sohail, M.; Ahmad, M.; Minhas, M.U.; Ali, L.; Khalid, I.; Rashid, H. Controlled delivery of valsartan by cross-linked polymeric matrices: Synthesis, in vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 110–119. [Google Scholar] [CrossRef]
- Sarfraz, R.; Khan, H.; Mahmood, A.; Ahmad, M.; Maheen, S.; Sher, M. Formulation and evaluation of mouth disintegrating tablets of atenolol and atorvastatin. Indian J. Pharm. Sci. 2015, 77, 83. [Google Scholar] [CrossRef] [Green Version]
- Ullah, K.; Sohail, M.; Buabeid, M.A.; Murtaza, G.; Ullah, A.; Rashid, H.; Khan, M.A.; Khan, S.A. Pectin-based (LA-co-MAA) semi-IPNS as a potential biomaterial for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 569, 118557. [Google Scholar] [CrossRef]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, Optimization and in-vitro characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Mannan, A.; Rashid, H.; Shah, A.; Murtaza, G.; Khan, S.A. Facile synthesis of chitosan based-(AMPS-co-AA) semi-IPNs as a potential drug carrier: Enzymatic degradation, cytotoxicity, and preliminary safety evaluation. Curr. Drug Deliv. 2019, 16, 242–253. [Google Scholar] [CrossRef]








| Formulation Code | Dynamic Swelling up to 72 h | Polymer Volume Fraction | ||||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.6 | pH 7.4 | pH 1.2 | pH 4.6 | pH 7.4 | |
| N-1 | 3.58 ± 0.20 | 5.42 ± 0.18 | 10.78 ± 0.26 | 0.279 | 0.184 | 0.092 |
| N-2 | 3.72 ± 0.18 | 6.20 ± 0.22 | 11.80 ± 0.19 | 0.268 | 0.161 | 0.084 |
| N-3 | 3.80 ± 0.23 | 6.60 ± 0.20 | 12.30 ± 0.17 | 0.263 | 0.151 | 0.081 |
| N-4 | 3.58 ± 0.20 | 5.42 ± 0.18 | 10.78 ± 0.26 | 0.279 | 0.184 | 0.092 |
| N-5 | 3.76 ± 0.16 | 6.33 ± 0.23 | 11.94 ± 0.14 | 0.265 | 0.157 | 0.083 |
| N-6 | 3.84 ± 0.22 | 6.72 ± 0.21 | 12.45 ± 0.22 | 0.260 | 0.148 | 0.080 |
| N-7 | 3.45 ± 0.25 | 5.13 ± 0.28 | 08.94 ± 0.27 | 0.289 | 0.194 | 0.111 |
| N-8 | 3.58 ± 0.20 | 5.42 ± 0.18 | 10.78 ± 0.26 | 0.279 | 0.184 | 0.092 |
| N-9 | 3.88 ± 0.19 | 6.78 ± 0.25 | 12.90 ± 0.24 | 0.257 | 0.147 | 0.077 |
| Formulation Code | Drug Loaded (mg)/500 mg of Dry Gels | |
|---|---|---|
| Weight Method | Extraction Method | |
| N-1 | 130.8 ± 0.9 | 128.9 ± 1.1 |
| N-2 | 171.6 ± 0.8 | 170.1 ± 0.9 |
| N-3 | 198.1 ± 0.9 | 196.8 ± 1 |
| N-4 | 130.8 ± 0.9 | 128.9 ± 1.1 |
| N-5 | 180.2 ± 1 | 179.1 ± 0.9 |
| N-6 | 205.2 ± 1 | 203.2 ± 0.9 |
| N-7 | 92.7 ± 1 | 91.5 ± 1 |
| N-8 | 130.8 ± 0.9 | 128.9 ± 1.1 |
| N-9 | 212.4 ± 0.9 | 210.9 ± 0.9 |
| F. Code | Zero Order r2 | First Order r2 | Higuchi r2 | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| r2 | n | ||||
| N-1 | 0.9477 | 0.9864 | 0.8563 | 0.9740 | 0.7738 |
| N-2 | 0.9554 | 0.9930 | 0.9910 | 0.9846 | 0.7093 |
| N-3 | 0.9710 | 0.9980 | 0.9947 | 0.9918 | 0.5937 |
| N-4 | 0.9477 | 0.9864 | 0.8563 | 0.9740 | 0.7738 |
| N-5 | 0.8829 | 0.9953 | 0.9729 | 0.9687 | 0.5687 |
| N-6 | 0.8946 | 0.9827 | 0.9795 | 0.9817 | 0.5250 |
| N-7 | 0.9528 | 0.9903 | 0.9840 | 0.9677 | 0.5558 |
| N-8 | 0.9477 | 0.9864 | 0.8563 | 0.9740 | 0.7738 |
| N-9 | 0.9566 | 0.9948 | 0.9915 | 0.9877 | 0.7498 |
| F. Code | Polymer Cs g/100 g | Polymer Sps g/100 g | Monomer Aca g/100 g | Initiator Aps g/100 g | Crosslinker Mba g/100 g |
|---|---|---|---|---|---|
| N-1 | 0.24 | 0.24 | 20 | 0.4 | 0.4 |
| N-2 | 0.48 | 0.24 | 20 | 0.4 | 0.4 |
| N-3 | 0.72 | 0.24 | 20 | 0.4 | 0.4 |
| N-4 | 0.24 | 0.24 | 20 | 0.4 | 0.4 |
| N-5 | 0.24 | 0.52 | 20 | 0.4 | 0.4 |
| N-6 | 0.24 | 0.80 | 20 | 0.4 | 0.4 |
| N-7 | 0.24 | 0.24 | 16 | 0.4 | 0.4 |
| N-8 | 0.24 | 0.24 | 20 | 0.4 | 0.4 |
| N-9 | 0.24 | 0.24 | 24 | 0.4 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhail, M.; Chiu, I.-H.; Hung, M.-C.; Vu, Q.L.; Lin, I.-L.; Wu, P.-C. In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery. Gels 2022, 8, 406. https://doi.org/10.3390/gels8070406
Suhail M, Chiu I-H, Hung M-C, Vu QL, Lin I-L, Wu P-C. In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery. Gels. 2022; 8(7):406. https://doi.org/10.3390/gels8070406
Chicago/Turabian StyleSuhail, Muhammad, I-Hui Chiu, Ming-Chia Hung, Quoc Lam Vu, I-Ling Lin, and Pao-Chu Wu. 2022. "In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery" Gels 8, no. 7: 406. https://doi.org/10.3390/gels8070406
APA StyleSuhail, M., Chiu, I.-H., Hung, M.-C., Vu, Q. L., Lin, I.-L., & Wu, P.-C. (2022). In Vitro Evaluation of Smart and pH-Sensitive Chondroitin Sulfate/Sodium Polystyrene Sulfonate Hydrogels for Controlled Drug Delivery. Gels, 8(7), 406. https://doi.org/10.3390/gels8070406







