3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds
Abstract
:1. Introduction
2. Results
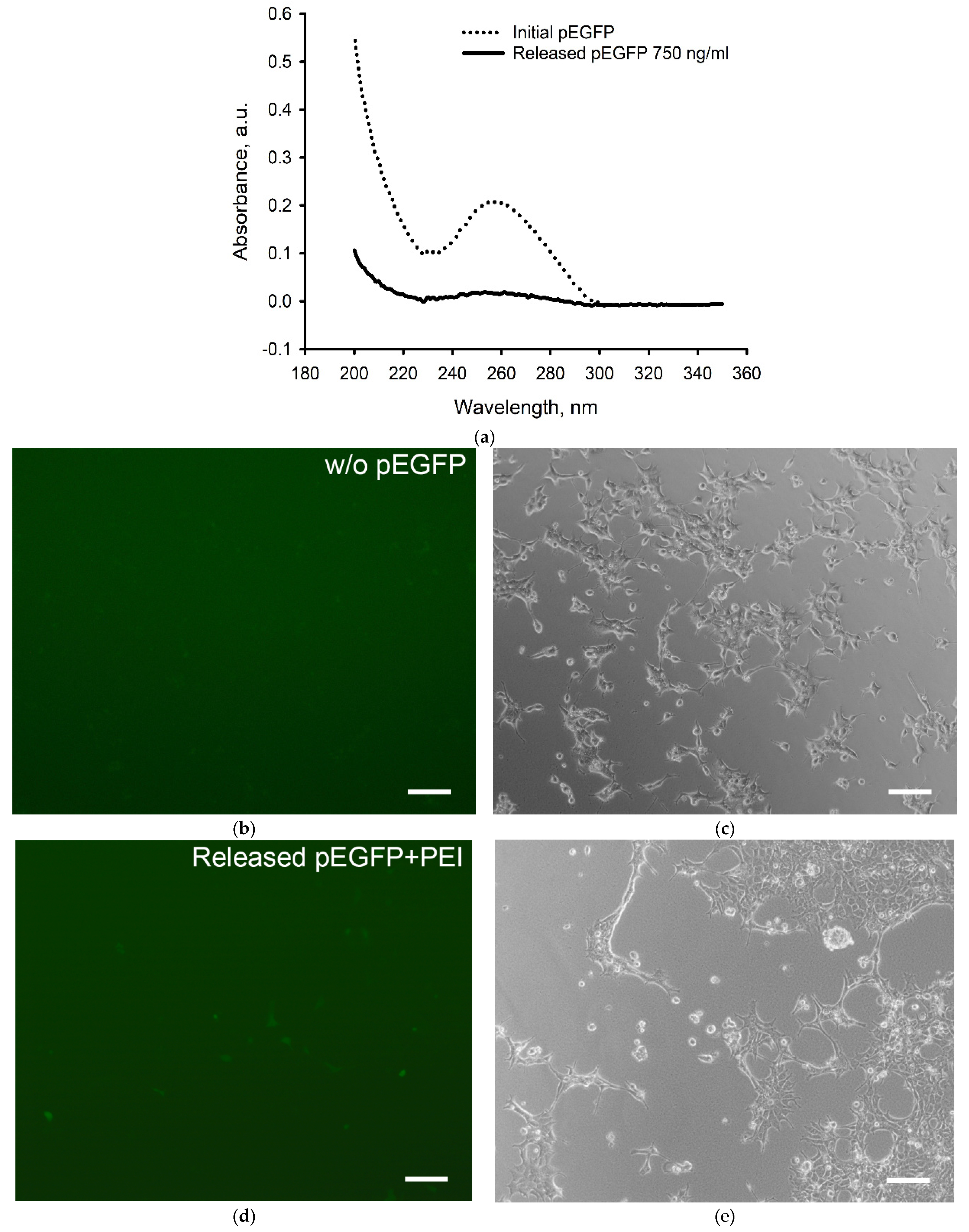
2.1. Transfection Efficiency of pEGFP with TF and PEI
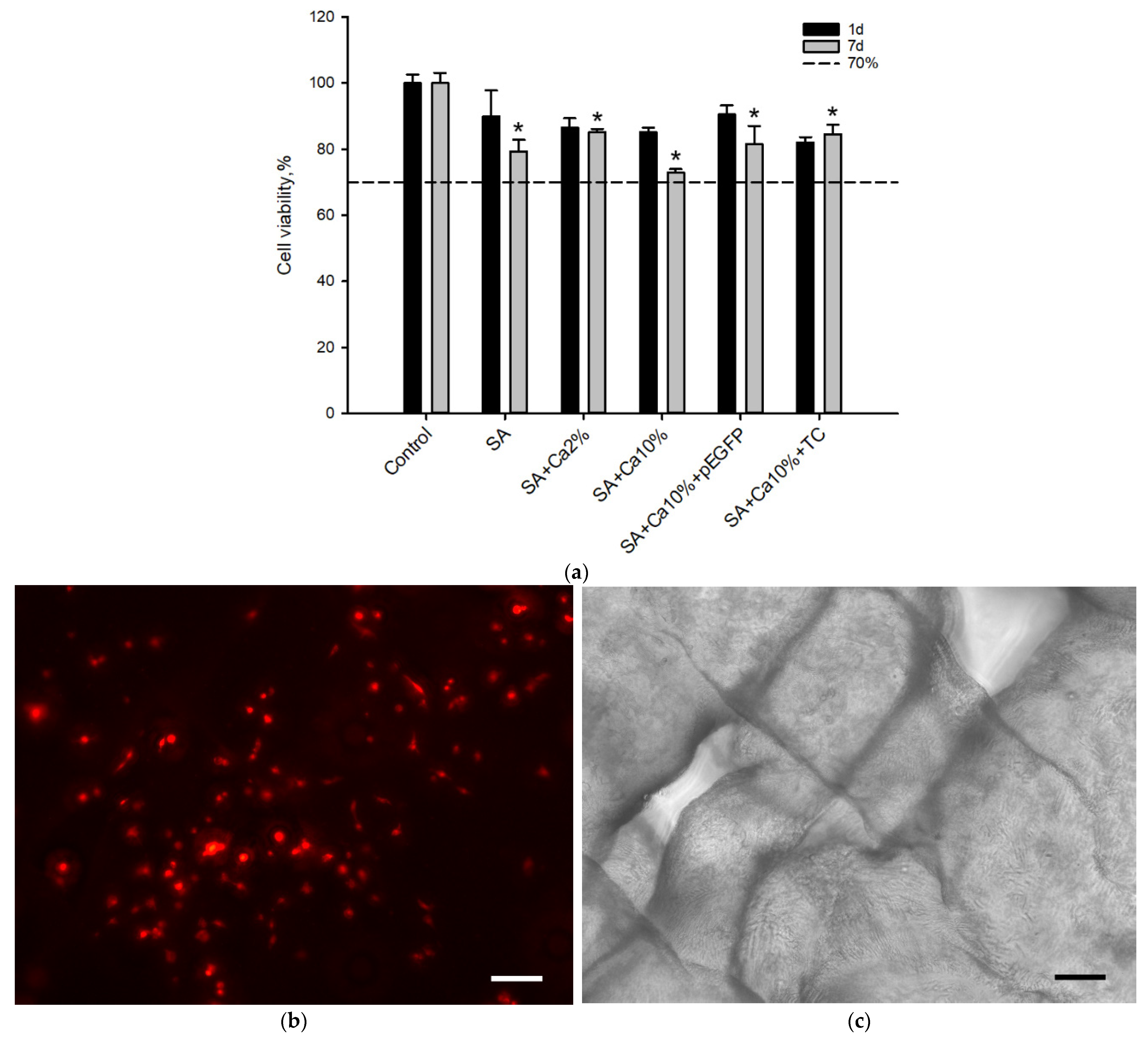
2.2. In Vitro Biocompatibility Assessment
2.3. Bioresorption of Gene-Activated Structures
2.4. The Genetic Constructs Stability in GAS
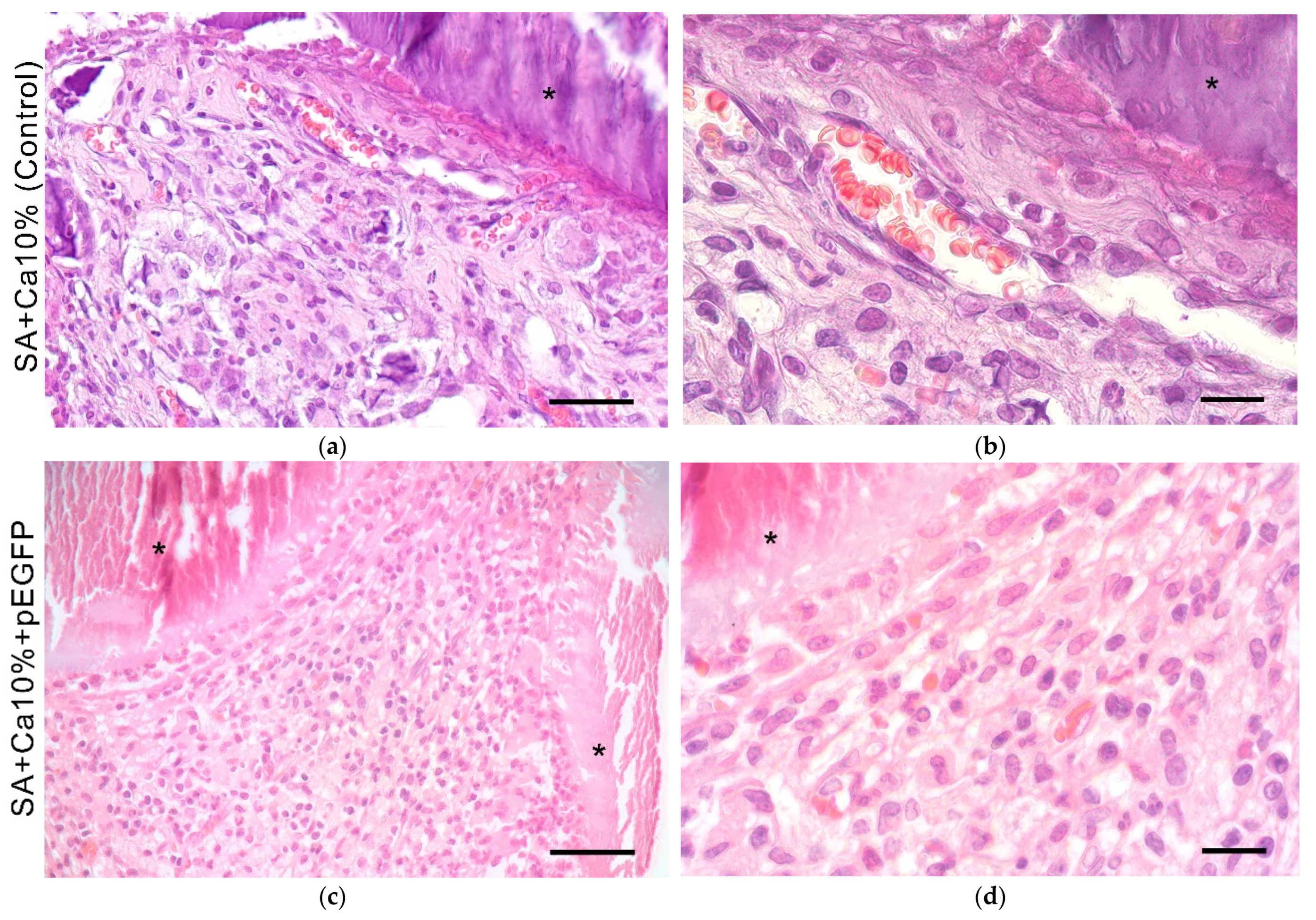
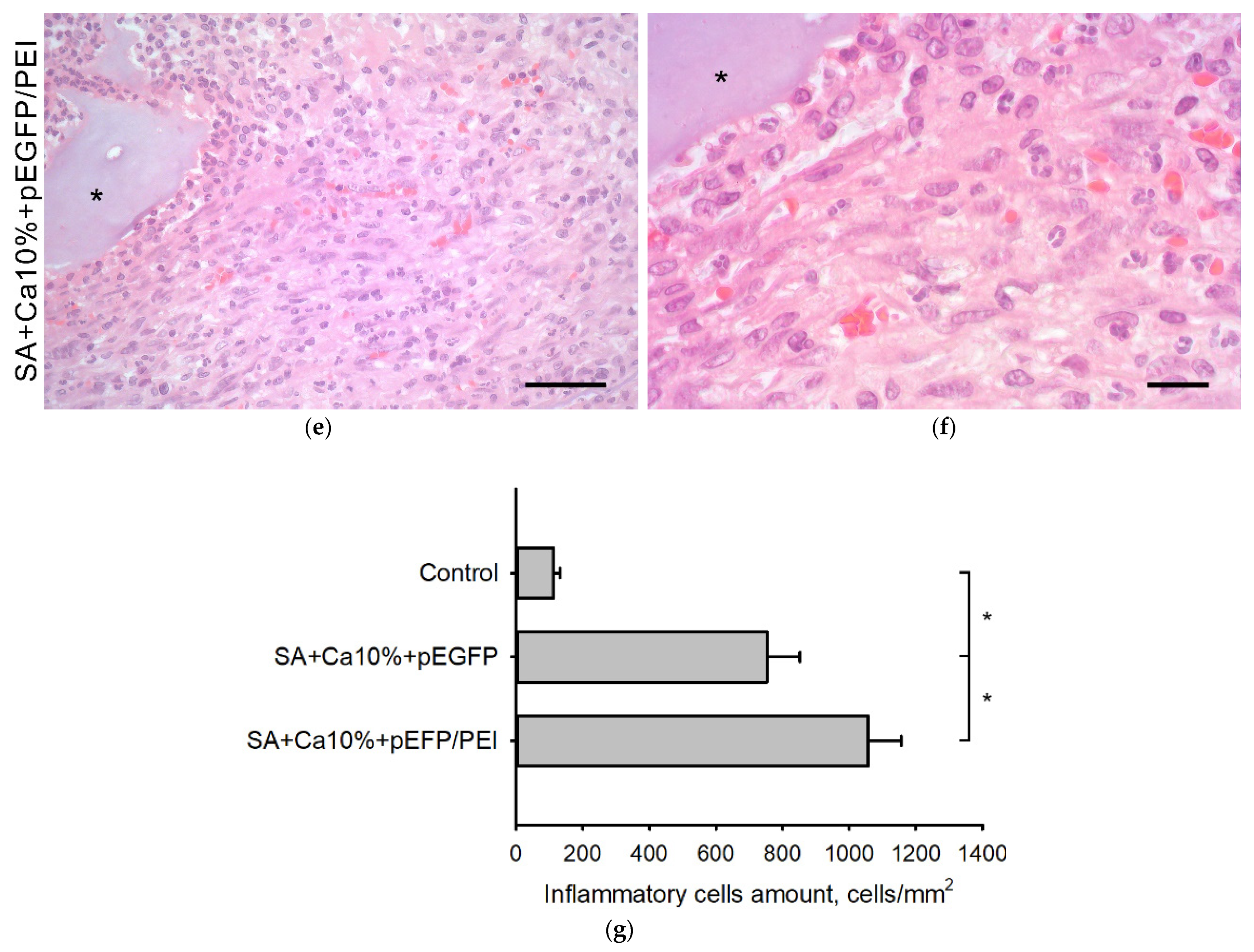
2.5. In Vivo Biocompatibility Assessment
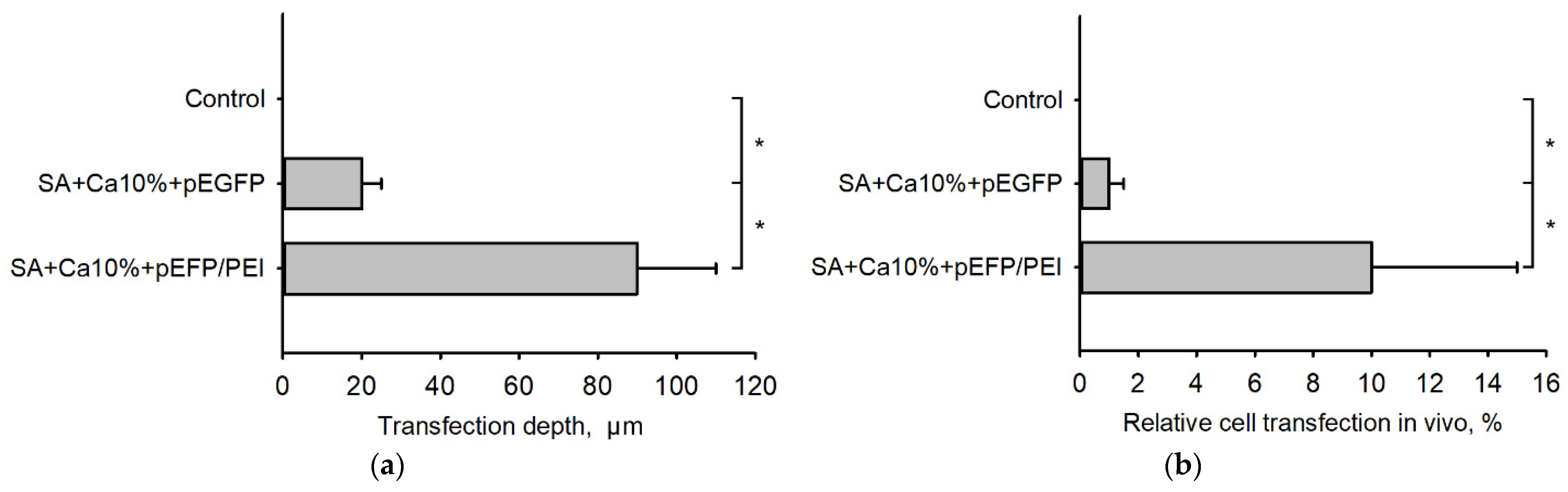
2.6. Transfection Efficiency In Vivo
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plasmid DNA
5.2. Plasmid DNA Delivery
5.3. 3D Cryoprinting
5.4. Cell Culture
5.5. MTT-Test
5.6. Cell Adhesion Study
5.7. Hydrogel Scaffold Bioresorption In Vitro
5.8. Structural Stability of the Genetic Constructs in Hydrogel Scaffolds
5.9. In Vivo Study
5.10. Histological and Immunohistochemical Assays
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D printed scaffolds for biomedical applications. Mater. Chem. Phys. 2020, 255. [Google Scholar] [CrossRef]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef]
- Komlev, V.S.; Popov, V.K.; Mironov, A.V.; Fedotov, A.Y.; Teterina, A.Y.; Smirnov, I.V.; Bozo, I.Y.; Rybko, V.A.; Deev, R.V. 3D printing of octacalcium phosphate bone substitutes. Front. Bioeng. Biotechnol. 2015, 3, 81. [Google Scholar] [CrossRef] [Green Version]
- Shende, P.; Trivedi, R. 3D Printed Bioconstructs: Regenerative Modulation for Genetic Expression. Stem Cell Rev. 2021, 17, 1239–1250. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Deev, R.V.; Smirnov, I.V.; Fedotov, A.Y.; Popov, V.K.; Mironov, A.V.; Mironova, O.A.; Gerasimenko, A.Y.; Komlev, V.S. 3D Printed Gene-activated Octacalcium Phosphate Implants for Large Bone Defects Engineering. Int. J. Bioprint. 2020, 6, 93–109. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Xin, H.; Yu, H.; Wang, H.; Ma, D.; Cao, X.; Wang, Y. MicroRNA-activated hydrogel scaffold generated by 3D printing accelerates bone regeneration. Bioact. Mater. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. Res. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modra, K.; Dai, S.; Zhang, H.; Shi, B.; Bi, J. Polycation-mediated gene delivery: Challenges and considerations for the process of plasmid DNA transfection. Eng. Life Sci. 2015, 15, 489–498. [Google Scholar] [CrossRef]
- Aggarwal, R.; Targhotra, M.; Kumar, B.; Sahoo, P.K.; Chauhan, M.K. Polyplex: A promising gene delivery system. Int. J. Pharm. Sci. Nanotech. 2019, 12, 4681–4686. [Google Scholar] [CrossRef]
- Remaut, K.; Lucas, B.; Raemdonck, K.; Braeckmans, K.; Demeester, J.; de Smedt, S.C. Protection of Oligonucleotides against enzymatic degradation by pegylated and nonpegylated branched polyethyleneimine. Biomacromolecules 2007, 8, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, Y.R.; Song, Y.F.; Yang, M.; Ye, J.; Zhou, G.; Yu, J.K. Scaffold-Based Gene Therapeutics for Osteochondral Tissue Engineering. Front. Pharmacol. 2019, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Yan, J.; Bryers, J.D. Scaffold-mediated delivery for non-viral mRNA vaccines. Gene Ther. 2018, 25, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.Z.; Acri, T.M.; Tingle, K.; Salem, A.K. Gene- and RNAi-Activated Scaffolds for Bone Tissue Engineering: Current Progress and Future Directions. Adv. Drug Deliv. Rev. 2021, 174, 613–627. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches—An Overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef]
- Yi Wee, C.; Yang, Z.; San Thian, E. Past, present and future development of microspheres for bone tissue regeneration: A review. Mater. Technol 2020, 36, 364–374. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, J.; Park, Y.D.; Hong, S.; Lee, J.J.; Ahn, C.B.; Choi, H.; Sun, K. Sodium Alginate Hydrogel-Based Bioprinting Using a Novel Multinozzle Bioprinting System. Artif. Organs 2011, 35, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Pecci, R.; Baiguera, S.; Ioppolo, P.; Bedini, R.; del Gaudio, C. 3D printed scaffolds with random microarchitecture for bone tissue engineering applications: Manufacturing and characterization. J. Mech. Behav. Biomed. Mater. 2020, 103, 103583. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, C.; Wei, H. Injectable Hydrogels as Three-Dimensional Network Reservoirs for Osteoporosis Treatment. Tissue Eng. Part B Rev. 2020, 27, 430–454. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Algebraistova, P.Y.; Komlev, V.S.; Mironova, O.A.; Popov, V.K. An Experimental Device for Studying the 3D Cryoprinting Processes. Instrum. Exp. Tech. 2020, 63, 890–892. [Google Scholar] [CrossRef]
- Raymond, C.; Tom, R.; Perret, S.; Moussouami, P.; L’Abbé, D.; St-Laurent, G.; Durocher, Y. A simplified polyethylenimine-mediated transfection process for large-scale and high-throughput applications. Methods 2011, 55, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Pulix, M.; Lukashchuk, V.; Smith, D.C.; Dickson, A.J. Molecular characterization of HEK293 cells as emerging versatile cell factories. Curr. Opin. Biotechnol. 2021, 71, 18–24. [Google Scholar] [CrossRef]
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C 2016, 68, 904–918. [Google Scholar] [CrossRef]
- Honoré, I.; Grosse, S.; Frison, N.; Favatier, F.; Monsigny, M.; Fajac, I. Transcription of plasmid DNA: Influence of plasmid DNA/polyethylenimine complex formation. J. Control. Release 2005, 107, 537–546. [Google Scholar] [CrossRef]
- Nedorubova, I.A.; Bukharova, T.B.; Vasilyev, A.V.; Syachina, M.A.; Goldshtein, D.V.; Kulakov, A.A. Comparative study of BMP-2 gene delivery to Human adipose tissue-derived mesenchymal stem cells with Turbofect and Polyethylenimine. IOP Conf. Ser. Ear. Environ. Sci. 2021, 677, 042024. [Google Scholar] [CrossRef]
- Wegman, F.; Bijenhof, A.; Schuijff, L.; Öner, F.C.; Dhert, W.J.A.; Alblas, J. Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur. Cells Mater. 2011, 21, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, B.W.; Jung, Y.C.; Yoon, B.I.; Woo, H.M.; Kang, B.J. Application of alginate microbeads as a carrier of bone morphogenetic protein-2 for bone regeneration. J. Biomed. Mater. Res. Part. B 2018, 107, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304, 1800698. [Google Scholar] [CrossRef]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.G.; Bedzinski, R. Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes 2020, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, D.; Koffler, J.; Lynam, D.A.; Tuszynski, M.H.; Sakamoto, J.S. Characterizing the degradation of alginate hydrogel for use in multilumen scaffolds for spinal cord repair. J. Biomed. Mater. Res. A 2016, 104, 611–619. [Google Scholar] [CrossRef]
- Hoover, R.L.; Folgerf, R.; Haering, W.A.; Waref, B.R.; Karnovsky, M.J. Adhesion of leukocytes to endothelium: Roles of divalent cations, surface charge, chemotactic agents and substrate. J. Cell Sci. 1980, 45, 73–86. [Google Scholar] [CrossRef]
- Mori, M.; Asahi, R.; Yamamoto, Y.; Mashiko, T.; Yoshizumi, K.; Saito, N.; Shirado, T.; Wu, Y.; Yoshimura, K. Sodium Alginate as a Potential Therapeutic Filler: An In Vivo Study in Rats. Mar. Drugs 2020, 18, 520. [Google Scholar] [CrossRef]
- Aescht, E.; Büchl-Zimmermann, S.; Burmester, A.; Dänhardt-Pfeiffer, S.; Desel, C.; Hamers, C.; Jach, G.; Kässens, M.; Makovitzky, J.; Mulisch, M.; et al. Romeis Mikroskopische Technik; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]





| Hydrodynamic Diameter, nm | Zeta-Potential, mV | |
|---|---|---|
| pEGFP | 420 ± 70 | −17 ± 6 |
| TF | 700 ± 100 | +3.0 ± 0.2 |
| PEI | 310 ± 110 | +6.0 ± 1.0 |
| pEGFP/TF | 140 ± 20 | +1.1 ± 0.5 |
| pEGFP/PEI | 90 ± 20 | +23 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khvorostina, M.A.; Mironov, A.V.; Nedorubova, I.A.; Bukharova, T.B.; Vasilyev, A.V.; Goldshtein, D.V.; Komlev, V.S.; Popov, V.K. 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels 2022, 8, 421. https://doi.org/10.3390/gels8070421
Khvorostina MA, Mironov AV, Nedorubova IA, Bukharova TB, Vasilyev AV, Goldshtein DV, Komlev VS, Popov VK. 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels. 2022; 8(7):421. https://doi.org/10.3390/gels8070421
Chicago/Turabian StyleKhvorostina, Maria A., Anton V. Mironov, Irina A. Nedorubova, Tatiana B. Bukharova, Andrey V. Vasilyev, Dmitry V. Goldshtein, Vladimir S. Komlev, and Vladimir K. Popov. 2022. "3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds" Gels 8, no. 7: 421. https://doi.org/10.3390/gels8070421
APA StyleKhvorostina, M. A., Mironov, A. V., Nedorubova, I. A., Bukharova, T. B., Vasilyev, A. V., Goldshtein, D. V., Komlev, V. S., & Popov, V. K. (2022). 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels, 8(7), 421. https://doi.org/10.3390/gels8070421







