A Brief Evaluation of Pore Structure Determination for Bioaerogels
Abstract
:1. Introduction
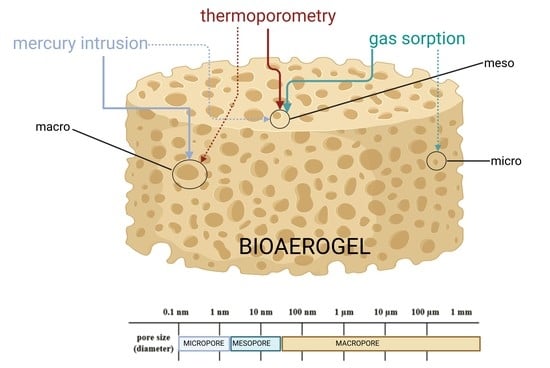
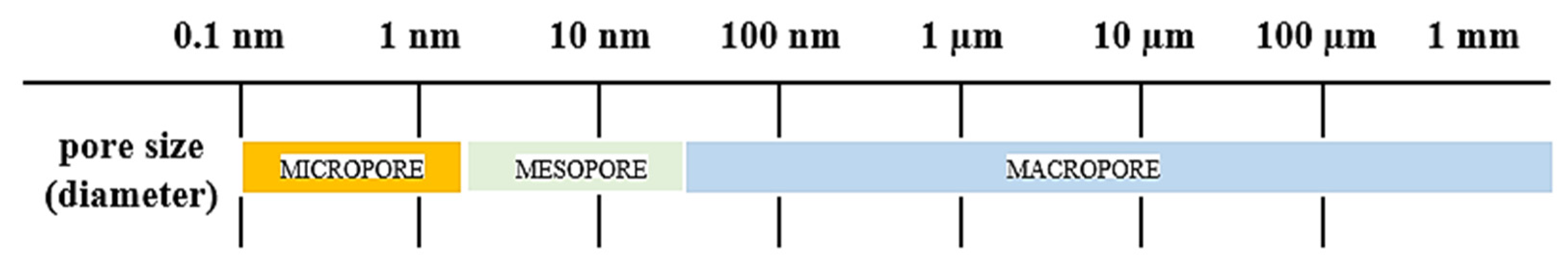
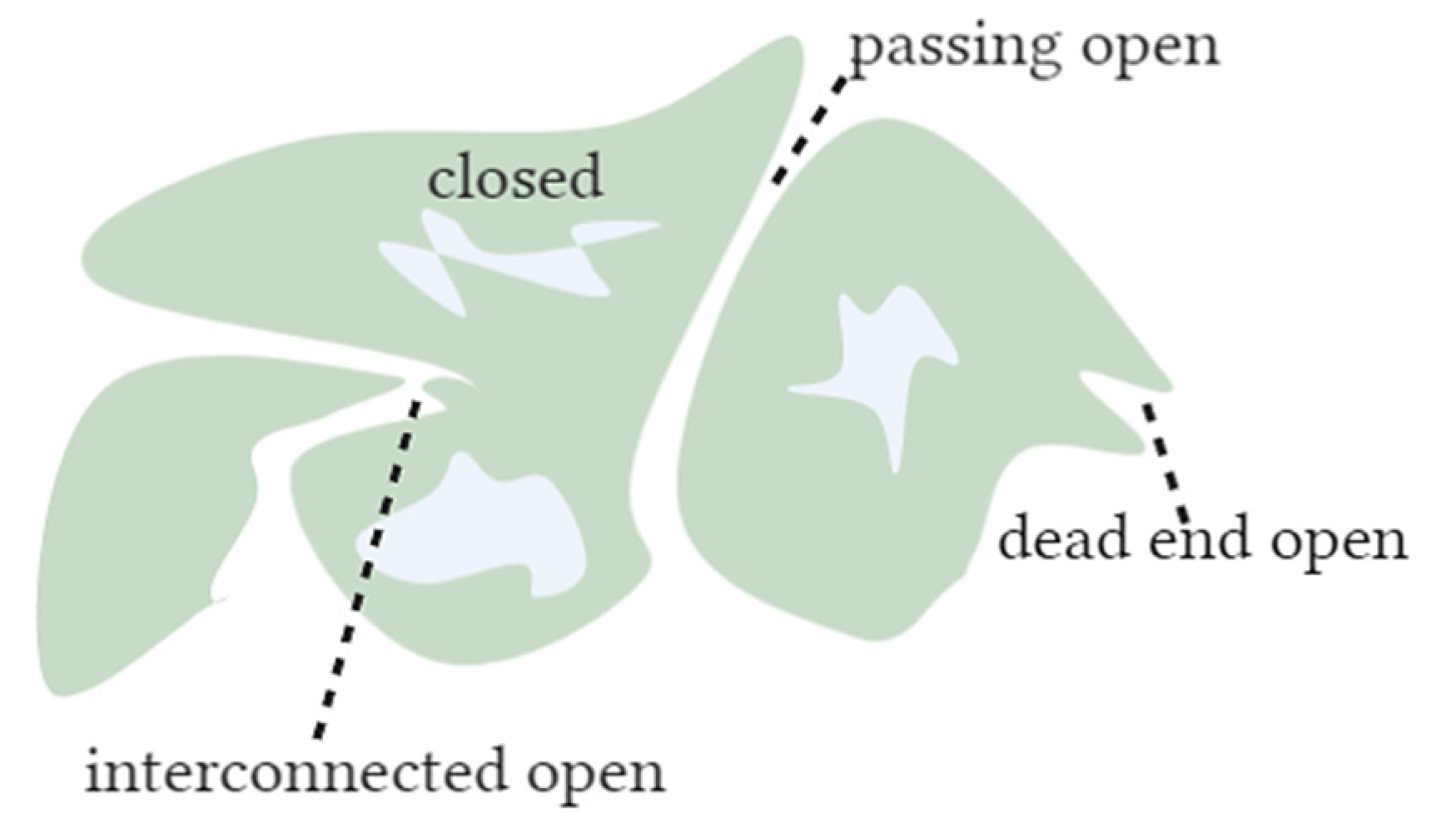
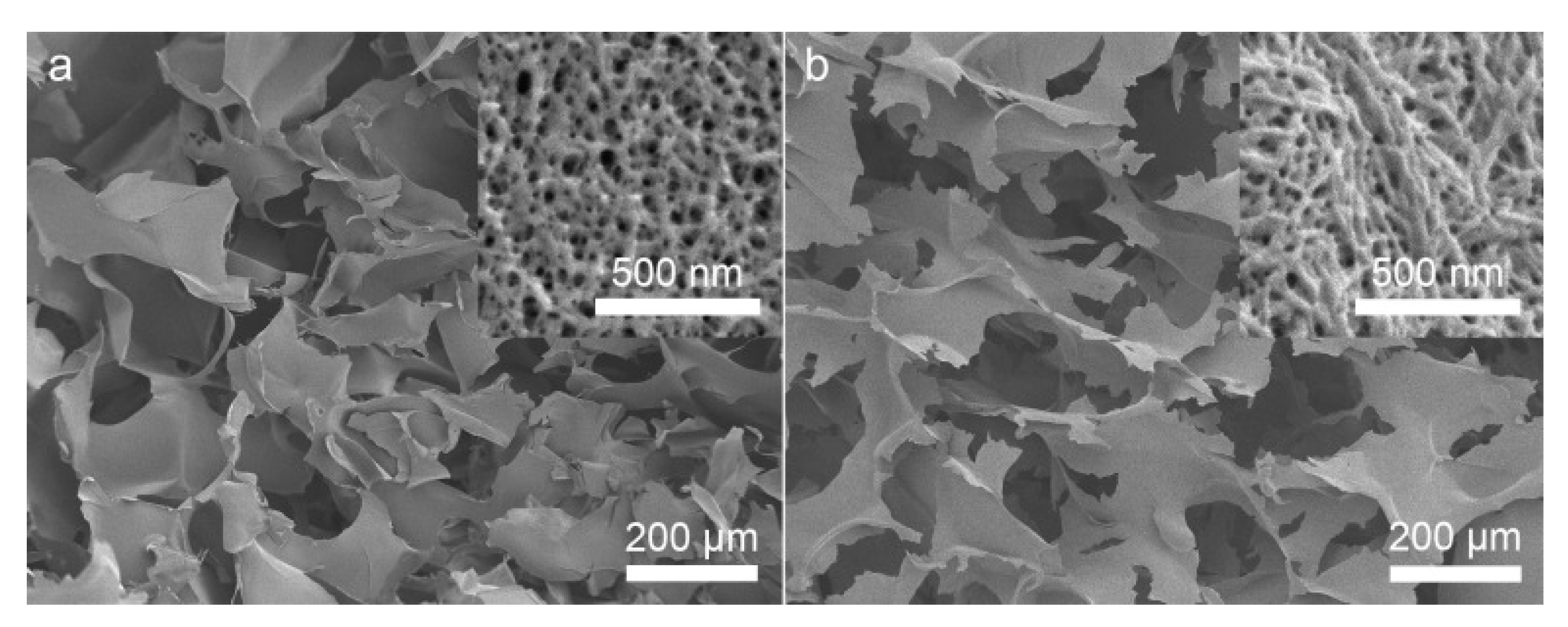
2. Pore Size Determination
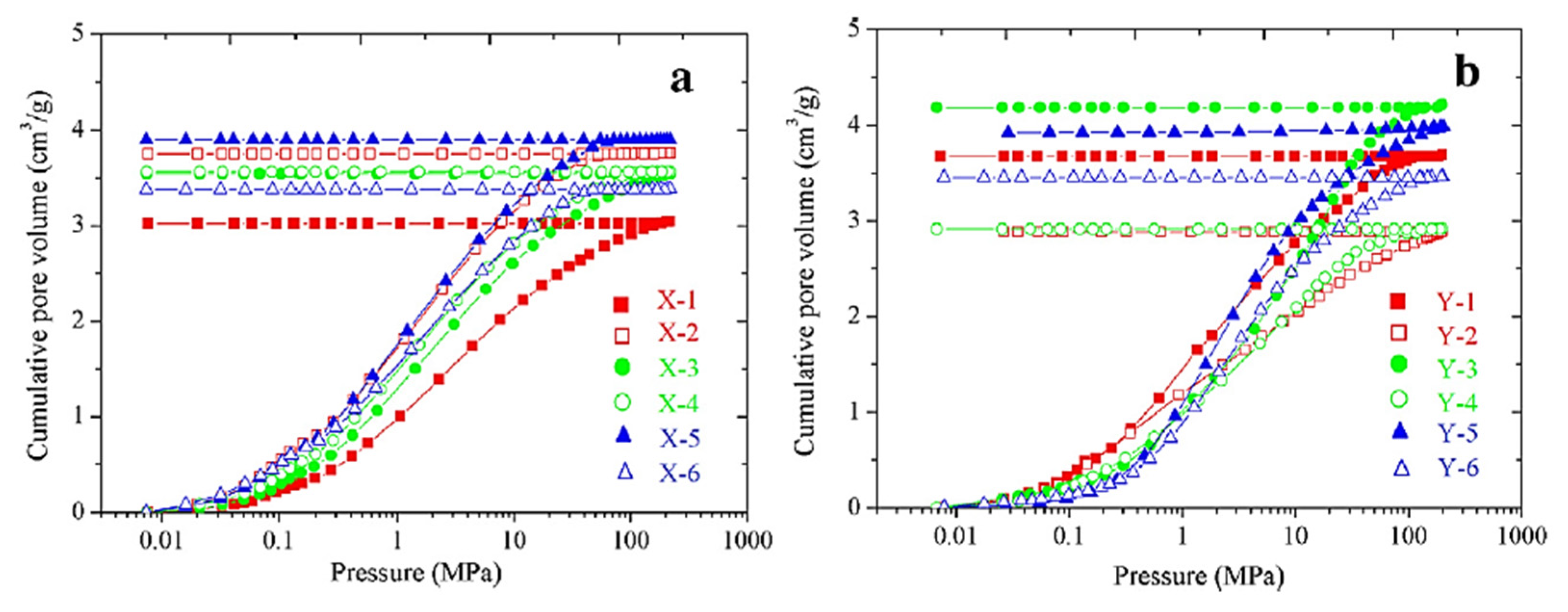
3. Gas Sorption
4. Mercury Porosimetry
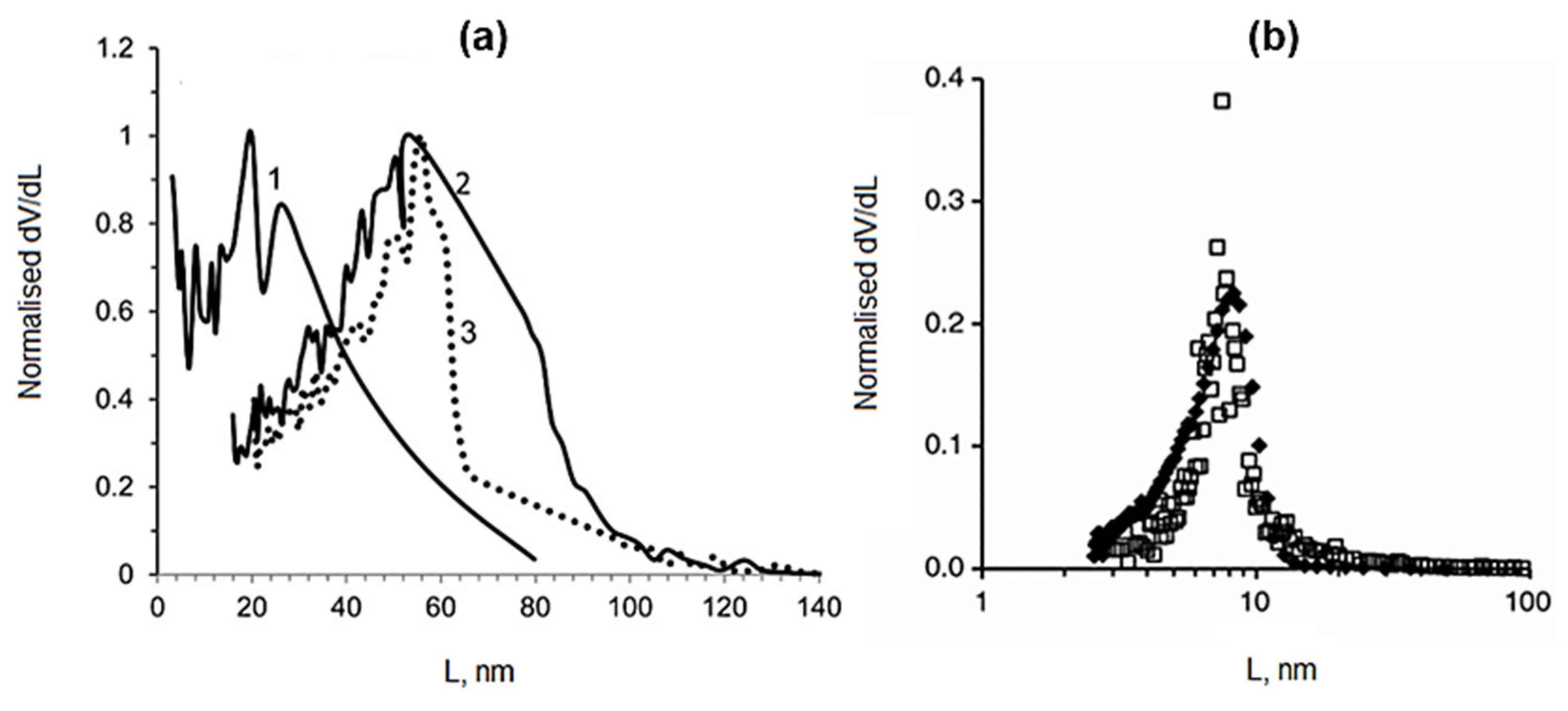
5. Thermoporometry
6. Other Characterization Method
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully Biomass-Based Mechanically Strong and Thermal Superinsulating Aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Pantić, M.; Novak, Z.; Knez, Ž. Supercritical Impregnation of Drugs and Supercritical Fluid Deposition of Metals into Aerogels. J. Mater. Sci. 2015, 50, 1–12. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Preparation and Characterization of Polysaccharide—Silica Hybrid Aerogels. Sci. Rep. 2019, 9, 16492. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramίrez, J. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Sing, K.S.W. IUPAC: Reporting Physisorption Data for Gas/Solid Systems with Special Reference Tot He Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Kaymak, H.; Brunner, S.; Koebel, M.M. Mechanical Properties of Monolithic Silica Aerogels Made from Polyethoxydisiloxanes. Microporous Mesoporous Mater. 2014, 183, 23–29. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Thermal Conductivity/Structure Correlations in Thermal Super-Insulating Pectin Aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of Micro- and Mesoporosity in SBA-15 Materials from Adsorption Data by the NLDFT Method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-Linked Cellulose Nanocrystal Aerogels as Viable Bone Tissue Scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. NLDFT Pore Size Distribution in Amorphous Microporous Materials. Langmuir 2017, 33, 11138–11145. [Google Scholar] [CrossRef] [Green Version]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched Solid Density Functional Theory Method for Characterization of Mesoporous Carbons by Nitrogen Adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Juhász, L.; Moldován, K.; Gurikov, P.; Liebner, F.; Fábián, I.; Kalmár, J.; Cserháti, C. False Morphology of Aerogels Caused by Gold Coating for SEM Imaging. Polymers 2021, 13, 588. [Google Scholar] [CrossRef]
- Kenvin, J.; Mitchell, S.; Sterling, M.; Warringham, R.; Keller, T.C.; Crivelli, P.; Jagiello, J.; Pérez-Ramírez, J. Quantifying the Complex Pore Architecture of Hierarchical Faujasite Zeolites and the Impact on Diffusion. Adv. Funct. Mater. 2016, 26, 5621–5630. [Google Scholar] [CrossRef]
- Taheri, P.; Lang, J.C.; Kenvin, J.; Kroll, P. Differential Hysteresis Scanning of Non-Templated Monomodal Amorphous Aerogels. Phys. Chem. Chem. Phys. 2021, 23, 5422–5430. [Google Scholar] [CrossRef]
- Robitzer, M.; Renzo, F.D.; Quignard, F. Natural Materials with High Surface Area. Physisorption Methods for the Characterization of the Texture and Surface of Polysaccharide Aerogels. Microporous Mesoporous Mater. 2011, 140, 9–16. [Google Scholar] [CrossRef]
- Robitzer, M.; Tourrette, A.; Horga, R.; Valentin, R.; Boissière, M.; Devoisselle, J.M.; Di Renzo, F.; Quignard, F. Nitrogen Sorption as a Tool for the Characterisation of Polysaccharide Aerogels. Carbohydr. Polym. 2011, 85, 44–53. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. PH Sensitive Mesoporous Materials for Immediate or Controlled Release of NSAID. Microporous Mesoporous Mater. 2016, 224, 190–200. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-Based Aerogels as Drug Carriers. J. Non-Cryst. Solids 2009, 355, 2472–2479. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of Polysaccharide Aerogels in Ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Pantić, M.; Knez, Ž.; Novak, Z. Supercritical Impregnation as a Feasible Technique for Entrapment of Fat-Soluble Vitamins into Alginate Aerogels. J. Non-Cryst. Solids 2016, 432 Pt B, 519–526. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, A.; Sanz-Moral, L.M.; Rueda, M.; Cocero, M.J. Impregnation of Medicinal Plant Phytochemical Compounds into Silica and Alginate Aerogels. J. Supercrit. Fluids. 2016, 116, 251–263. [Google Scholar] [CrossRef]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of Biodegradable Nanoporous Microspherical Aerogel Based on Alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent Alginate Aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Ž.; Novak, Z. Preparation of Multi-Membrane Alginate Aerogels Used for Drug Delivery. J. Supercrit. Fluids 2013, 79, 209–215. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as Drug Delivery Systems: In Vitro and in Vivo Evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- White, R.J.; Antonio, C.; Budarin, V.L.; Bergström, E.; Thomas-Oates, J.; Clark, J.H. Polysaccharide-Derived Carbons for Polar Analyte Separations. Adv. Funct. Mater. 2010, 20, 1834–1841. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Renzo, F.D. Aerogel Materials from Marine Polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Barley Beta-Glucan Aerogels via Supercritical CO2 Drying. Food Res. Int. 2012, 48, 442–448. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of Processing Parameters on Preparation of Carrageenan Aerogel Microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Abdellatif, F.H.H.; Abdellatif, M.M. Bio-Based i-Carrageenan Aerogels as Efficient Adsorbents for Heavy Metal Ions and Acid Dye from Aqueous Solution. Cellulose 2020, 27, 441–453. [Google Scholar] [CrossRef]
- Lopes, J.M.; Mustapa, A.N.; Pantić, M.; Bermejo, M.D.; Martín, Á.; Novak, Z.; Knez, Ž.; Cocero, M.J. Preparation of Cellulose Aerogels from Ionic Liquid Solutions for Supercritical Impregnation of Phytol. J. Supercrit. Fluids 2017, 130, 17–22. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Qiang, T.; Li, J. Synthesis of Superhydrophobic Ultralight Aerogels from Nanofibrillated Cellulose Isolated from Natural Reed for High-Performance Adsorbents. Appl. Phys. A 2016, 122, 686. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose Aerogels from Aqueous Alkali Hydroxide–Urea Solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose–Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chem. Int. Ed. 2012, 51, 2076–2079. [Google Scholar] [CrossRef]
- Carlsson, D.O.; Nyström, G.; Zhou, Q.; Berglund, L.A.; Nyholm, L.; Strømme, M. Electroactive Nanofibrillated Cellulose Aerogel Composites with Tunable Structural and Electrochemical Properties. J. Mater. Chem. 2012, 22, 19014–19024. [Google Scholar] [CrossRef]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldaña, M.D.A. Lupin Hull Cellulose Nanofiber Aerogel Preparation by Supercritical CO2 and Freeze Drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Liebner, F.; Haimer, E.; Potthast, A.; Loidl, D.; Tschegg, S.; Neouze, M.-A.; Wendland, M.; Rosenau, T. Cellulosic Aerogels as Ultra-Lightweight Materials. Part 2: Synthesis and Properties 2nd ICC 2007, Tokyo, Japan, October 25–29, 2007. Holzforschung 2008, 63, 3–11. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.; Yang, D.; She, X.; Yao, X.; Zhu, G.; Liu, Y.; Zhao, H.; Li, J. Fabrication of Mesoporous Lignocellulose Aerogels from Wood via Cyclic Liquid Nitrogen Freezing–Thawing in Ionic Liquid Solution. J. Mater. Chem. 2012, 22, 13548–13557. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Fischer, F.; Rigacci, A.; Pirard, R.; Berthon-Fabry, S.; Achard, P. Cellulose-Based Aerogels. Polymer 2006, 47, 7636–7645. [Google Scholar] [CrossRef]
- Hossen, M.R.; Talbot, M.W.; Kennard, R.; Bousfield, D.W.; Mason, M.D. A Comparative Study of Methods for Porosity Determination of Cellulose Based Porous Materials. Cellulose 2020, 27, 6849–6860. [Google Scholar] [CrossRef]
- Osorio, D.A.; Seifried, B.; Moquin, P.; Grandfield, K.; Cranston, E.D. Morphology of Cross-Linked Cellulose Nanocrystal Aerogels: Cryo-Templating versus Pressurized Gas Expansion Processing. J. Mater. Sci. 2018, 53, 9842–9860. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-Loaded Chitosan Aerogel Particles for Chronic Wound Applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y. Formation of Enhanced Gelatum Using Ethanol/Water Binary Medium for Fabricating Chitosan Aerogels with High Specific Surface Area. Chem. Eng. J. 2017, 309, 700–707. [Google Scholar] [CrossRef]
- Takeshita, S.; Akasaka, S.; Yoda, S. Structural and Acoustic Properties of Transparent Chitosan Aerogel. Mater. Lett. 2019, 254, 258–261. [Google Scholar] [CrossRef]
- Chang, X.; Chen, D.; Jiao, X. Chitosan-Based Aerogels with High Adsorption Performance. J. Phys. Chem. B 2008, 112, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, A.; Tkalec, G.; Knez, Ž.; Novak, Z. Characterisation of Biodegradable Pectin Aerogels and Their Potential Use as Drug Carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Budarin, V.L.; Clark, J.H. Pectin-Derived Porous Materials. Chem.-Eur. J. 2010, 16, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of Biocompatible Magnetic Pectin Aerogel Monoliths and Microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast Production of High-Methoxyl Pectin Aerogels for Enhancing the Bioavailability of Low-Soluble Drugs. J. Supercrit. Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
- Horvat, G.; Xhanari, K.; Finšgar, M.; Gradišnik, L.; Maver, U.; Knez, Ž.; Novak, Z. Novel Ethanol-Induced Pectin–Xanthan Aerogel Coatings for Orthopedic Applications. Carbohydr. Polym. 2017, 166, 365–376. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Formation of Nanoporous Aerogels from Wheat Starch. Carbohydr. Polym. 2016, 147, 125–132. [Google Scholar] [CrossRef]
- Goimil, L.; Braga, M.E.M.; Dias, A.M.A.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; de Sousa, H.C.; García-González, C.A. Supercritical Processing of Starch Aerogels and Aerogel-Loaded Poly(ε-Caprolactone) Scaffolds for Sustained Release of Ketoprofen for Bone Regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. ScCO2-Foamed Silk Fibroin Aerogel/Poly(ε-Caprolactone) Scaffolds Containing Dexamethasone for Bone Regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk Fibroin Aerogels: Potential Scaffolds for Tissue Engineering Applications. Biomed. Mater. Bristol Engl. 2015, 10, 035002. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of Novel Whey Protein-Based Aerogels as Drug Carriers for Life Science Applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of Alginate-Based Aerogel for Nonsteroidal Anti-Inflammatory Drugs Controlled Delivery Systems Using Prilling and Supercritical-Assisted Drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Escudero, R.R.; Robitzer, M.; Di Renzo, F.; Quignard, F. Alginate Aerogels as Adsorbents of Polar Molecules from Liquid Hydrocarbons: Hexanol as Probe Molecule. Carbohydr. Polym. 2009, 75, 52–57. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose Aerogels Prepared from an Aqueous Zinc Chloride Salt Hydrate Melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M.M. Strong, Thermally Superinsulating Biopolymer–Silica Aerogel Hybrids by Cogelation of Silicic Acid with Pectin. Angew. Chem. Int. Ed. 2015, 54, 14282–14286. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Tuning Structure and Properties of Pectin Aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin Hydrogels, Aerogels, Cryogels and Xerogels: Influence of Drying on Structural and Release Properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Enhancing Chloride Corrosion Resistance of Precast Reinforced Concrete by Carbonation Curing. ACI Mater. J. 2019, 116, 10. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Laroche, C.; Pérez-Pellitero, J.; Coasne, B. Characterization of Hierarchical Zeolites: Combining Adsorption/Intrusion, Electron Microscopy, Diffraction and Spectroscopic Techniques. Microporous Mesoporous Mater. 2019, 287, 167–176. [Google Scholar] [CrossRef]
- Jiang, G.; Fan, M.; Shen, X.; Gordon Harris, H.; Kenarsari, S.D. The Buckling Deformation and Mechanical Properties of Aerogels Prepared with Polyethoxydisiloxane. Microporous Mesoporous Mater. 2015, 202, 183–188. [Google Scholar] [CrossRef]
- Majling, J.; Komarneni, S.; Fajnor, V.S. Mercury Porosimeter as a Means to Measure Mechanical Properties of Aerogels. J. Porous Mater. 1995, 1, 91–95. [Google Scholar] [CrossRef]
- Pirard, R.; Rigacci, A.; Maréchal, J.C.; Quenard, D.; Chevalier, B.; Achard, P.; Pirard, J.P. Characterization of Hyperporous Polyurethane-Based Gels by Non-Intrusive Mercury Porosimetry. Polymer 2003, 44, 4881–4887. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from Cellulose–Ionic Liquid Solutions: Preparation, Properties and Comparison with Cellulose–NaOH and Cellulose–NMMO Routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Pirard, R.; Blacher, S.; Brouers, F.; Pirard, J.P. Interpretation of Mercury Porosimetry Applied to Aerogels. J. Mater. Res. 1995, 10, 2114–2119. [Google Scholar] [CrossRef]
- Seraji, M.M.; Ghafoorian, N.S.; Bahramian, A.R. Investigation of Microstructure and Mechanical Properties of Novolac/Silica and C/SiO2/SiC Aerogels Using Mercury Porosimetry Method. J. Non-Cryst. Solids 2016, 435, 1–7. [Google Scholar] [CrossRef]
- Scherer, G.W.; Smith, D.M.; Stein, D. Deformation of Aerogels during Characterization. J. Non-Cryst. Solids 1995, 186, 309–315. [Google Scholar] [CrossRef]
- Maloney, T.C. Thermoporosimetry of Hard (Silica) and Soft (Cellulosic) Materials by Isothermal Step Melting. J. Therm. Anal. Calorim. 2015, 121, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, H. Alginate/Pectin Aerogel Microspheres for Controlled Release of Proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Gavillon, R.; Budtova, T. Aerocellulose: New Highly Porous Cellulose Prepared from Cellulose−NaOH Aqueous Solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Díez-Municio, M.; Montilla, A.; Herrero, M.; Olano, A.; Ibáñez, E. Supercritical CO2 Impregnation of Lactulose on Chitosan: A Comparison between Scaffolds and Microspheres Form. J. Supercrit. Fluids 2011, 57, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, M.; Kondor, A.; Plappert, S.; Mitterer, C.; Opietnik, M.; Potthast, A.; Rosenau, T. Surface Properties and Porosity of Highly Porous, Nanostructured Cellulose II Particles. Cellulose 2017, 24, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Landry, M.R. Thermoporometry by Differential Scanning Calorimetry: Experimental Considerations and Applications. Thermochim. Acta 2005, 433, 27–50. [Google Scholar] [CrossRef]
- Veselá, P.; Riikonen, J.; Nissinen, T.; Lehto, V.-P.; Slovák, V. Optimisation of Thermoporometry Measurements to Evaluate Mesoporous Organic and Carbon Xero-, Cryo- and Aerogels. Thermochim. Acta 2015, 621, 81–89. [Google Scholar] [CrossRef]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of Selected Solvent Systems on the Pore and Solid Structure of Cellulose Aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [Green Version]
- Riikonen, J.; Salonen, J.; Lehto, V.-P. Utilising Thermoporometry to Obtain New Insights into Nanostructured Materials. J. Therm. Anal. Calorim. 2011, 105, 811–821. [Google Scholar] [CrossRef]
- Dessources, A.H.; Hartmann, S.; Baba, M.; Huesing, N.; Nedelec, J.M. Multiscale Characterization of Hierarchically Organized Porous Hybrid Materials. J. Mater. Chem. 2012, 22, 2713–2720. [Google Scholar] [CrossRef] [Green Version]
- Iza, M.; Woerly, S.; Danumah, C.; Kaliaguine, S.; Bousmina, M. Determination of Pore Size Distribution for Mesoporous Materials and Polymeric Gels by Means of DSC Measurements: Thermoporometry. Polymer 2000, 41, 5885–5893. [Google Scholar] [CrossRef]
- Zelenková, G.; Zelenka, T.; Slovák, V. Thermoporometry of Porous Carbon: The Effect of the Carbon Surface Chemistry on the Thickness of Non-Freezable Pore Water Layer (Delta Layer). Microporous Mesoporous Mater. 2021, 326, 111358. [Google Scholar] [CrossRef]
- Barbieri, O.; Ehrburger-Dolle, F.; Rieker, T.P.; Pajonk, G.M.; Pinto, N.; Venkateswara Rao, A. Small-Angle X-Ray Scattering of a New Series of Organic Aerogels. J. Non-Cryst. Solids 2001, 285, 109–115. [Google Scholar] [CrossRef]
- Scherer, G.W. Characterization of Aerogels. Adv. Colloid Interface Sci. 1998, 76–77, 321–339. [Google Scholar] [CrossRef]
- van Bommel, M.J.; den Engelsen, C.W.; van Miltenburg, J.C. A Thermoporometry Study of Fumed Silica/Aerogel Composites. J. Porous Mater. 1997, 4, 143–150. [Google Scholar] [CrossRef]
- Woignier, T.; Quinson, J.F.; Pauthe, M.; Repellin-Lacroix, M.; Phalippou, J. Evolution of the Porous Volume during the Aerogel-Glass Transformation. J. Phys. IV 1992, 2, C2-123–C2-126. [Google Scholar] [CrossRef] [Green Version]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of Bacterial Cellulose Aerogels with Biocompatible Polymers. Carbohydr. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Lázár, I.; Forgács, A.; Horváth, A.; Király, G.; Nagy, G.; Len, A.; Dudás, Z.; Papp, V.; Balogh, Z.; Moldován, K.; et al. Mechanism of Hydration of Biocompatible Silica-Casein Aerogels Probed by NMR and SANS Reveal Backbone Rigidity. Appl. Surf. Sci. 2020, 531, 147232. [Google Scholar] [CrossRef]
- Mincov, I.; Petkov, M.P.; Tsou, P.; Troev, T. Porosity Characterization of Aerogels Using Positron Annihilation Lifetime Spectroscopy. J. Non-Cryst. Solids 2004, 350, 253–258. [Google Scholar] [CrossRef]
- Petrov, O.V.; Furó, I. NMR Cryoporometry: Principles, Applications and Potential. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 54, 97–122. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Bányai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin Content Governs Hydration Induced Structural Changes in Silica-Gelatin Hybrid Aerogels—Implications in Drug Delivery. Acta Biomater. 2020, 105, 131–145. [Google Scholar] [CrossRef] [PubMed]


| Bioaerogel | Method | Pore Size (nm) | Specific Surface Area, m2/g | Reference |
|---|---|---|---|---|
| Agar | DFT | 35 | 320 | [21] |
| 36 | 320 | [22] | ||
| Alginate | BJH | 11.1–13.1 | 261–437 | [23] |
| 11.7 | 150–300 | [24] | ||
| 14 | 147 | [25] | ||
| 10.5–10.9 | 400–430 | [26] | ||
| 25.5 | 125.9 | [27] | ||
| 10–15 | 394–590 | [28] | ||
| 14–28 | 376–575 | [29] | ||
| 8.8–20 | 402–419 | [30] | ||
| 30.5 | 359.5 | [31] | ||
| Alginic acid | BJH | 25.1 | 320 | [32] |
| DFT | 40 | 375 | [21] | |
| DFT | 20 | 390 | [22] | |
| Ca-alginate | BJH | 38 | 570 | [33] |
| DFT | 28 | 495 | [21] | |
| DFT | 37 | 570 | [22] | |
| Cu-alginate | DFT | 30 | 680 | [21] |
| Alginate/pectin | BJH | 10.4–14.2 | 299–417 | [23] |
| Barely beta-glucan | BJH | 2.7–2.8 | 160–167 | [34] |
| Carrageenan | BJH | 7.4–16.5 | 34–174 | [35] |
| BJH | 1.2–2.7 | 128.8–385.5 | [36] | |
| BJH | 19 | 200 | [33] | |
| DFT | 22 | 230 | [21] | |
| DFT | 18 | 200 | [22] | |
| Cellulose | BJH | 7.9–34 | 154–434 | [37] |
| 1–106 | 55.2 | [38] | ||
| 7.9–39.4 | 260–485 | [39] | ||
| 19 | 356 | [40] | ||
| 40–90 | 147–246 | [41] | ||
| 7.1–11.7 | 72–115 | [42] | ||
| 8.9–11.7 | 55–310 | [43] | ||
| 10.5–28.9 | 2.0–80.7 | [44] | ||
| 8 | 250 | [45] | ||
| 17–30 | 140–250 | [46] | ||
| 2–200 | / | [47] | ||
| Cellulose nanocrystal | DFT | 6–11 | 130–190 | [15] |
| 3.9–11.1 | 190–320 | [48] | ||
| Chitosan Chitosan (α-chitin) Chitosan (β-chitin) | BJH BJH BJH BJH BJH DFT DFT DFT DFT | 11 | 330 | [33] |
| 12.6–15 | 257–479 | [49] | ||
| 50–120 | 973 | [50] | ||
| 21.3–43.6 | 737–872 | [51] | ||
| 3.29–11.13 | 66–845 | [52] | ||
| 11 | 330 | [21] | ||
| 12 | 330 | [22] | ||
| 22 | 210 | [21] | ||
| 25 | 150 | [22] | ||
| 18 | 560 | [21] | ||
| β-Chitin | DFT | 20 | 560 | [22] |
| Guar | BJH | 15 | 111 | [25] |
| Pectin | BJH | 7.2–26.3 | 143–593 | [53] [23] [2] [54] [55] [56] [23] [25] |
| 13.1–16.8 | 272–407 | |||
| 11 | 230–270 | |||
| 7.3–28.8 | 174–485 | |||
| 17–22 | 247–284 | |||
| 15.6–17.1 | 354–386, 272–437 | |||
| 11.1–13.117, 19 | 510, 384 | |||
| Pectin/xanthan | BJH | 6.8 | 175–289 | [57] |
| Starch | BJH | 9.4 | 217 | [31] |
| Potato starch | 7.2 | 72.5 | [24] | |
| Eurylon7 starch | 1.9 | 90.3 | [24] | |
| Wheat starch | 19–26 | 20.2–59.7 | [58] | |
| PCL-starch | 14.1–15.1 | 1.3–1.7 | [59] | |
| Xanthan | BJH | 20 | 363 | [25] |
| Silk fibroin | BJH | 18–24 | 336–432 | [60] |
| 11–17 | 260–308 | [61] | ||
| Whey protein | BJH | 12.3–27.4 | 310–447 | [62] |
| Bioaerogel | Method | Pore Size (nm) | Reference |
|---|---|---|---|
| Alginate | SEM | 200 | [63] |
| Alginic acid | SEM | 11.4 * | [64] |
| Ca-alginate | SEM | 5.8 * | [64] |
| Ba-alginate | 7.4 * | ||
| Co-alginate | 6.6 * | ||
| Cu-alginate | 4.4 * | ||
| Ni-alginate | 6 * | ||
| Cellulose Cellulose nanocrystal | SEM | 50–1000 | [45] |
| 5–100 | [47] | ||
| 125–250 | [65] | ||
| 50–200 | [66] | ||
| 7 um | [48] | ||
| Chitosan | SEM | >10 | [67] |
| 10–50 | [68] |
| Bioaerogel | Pore Size (µm) | Reference |
|---|---|---|
| Alginate—pectin | 0.183–1.081 | [82] |
| Citrus pectin | 0.019–0.046 | [2] |
| Cellulose | 0.002–0.050 | [46] |
| 0.012–0.025 | [46] | |
| 0.7–47.5 | [83] | |
| 0.01–25 | [47] | |
| Chitosan | 56.8–57.04 | [84] |
| PCL-starch aerogel | 0.38–1.87 | [59] |
| Method | CL-TBAF | CL-EMIm | CL-NMMO | CL-CTO |
|---|---|---|---|---|
| Pore size TPM (nm) | 36/101 | 62 | 96 | 84 |
| Modal pore size BJH (nm) | 34.5 | 34.4 | 32.1 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels 2022, 8, 438. https://doi.org/10.3390/gels8070438
Horvat G, Pantić M, Knez Ž, Novak Z. A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels. 2022; 8(7):438. https://doi.org/10.3390/gels8070438
Chicago/Turabian StyleHorvat, Gabrijela, Milica Pantić, Željko Knez, and Zoran Novak. 2022. "A Brief Evaluation of Pore Structure Determination for Bioaerogels" Gels 8, no. 7: 438. https://doi.org/10.3390/gels8070438
APA StyleHorvat, G., Pantić, M., Knez, Ž., & Novak, Z. (2022). A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels, 8(7), 438. https://doi.org/10.3390/gels8070438