New Carbamates and Ureas: Comparative Ability to Gel Organic Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the New Carbamates and New Ureas
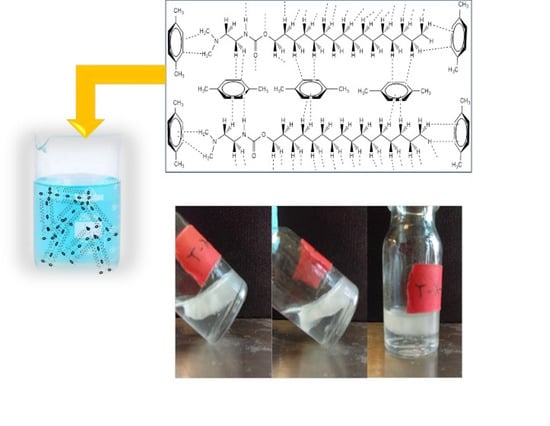
2.2. Gelation Test of New Carbamates and New Ureas
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of New Carbamates and Ureas
4.2.2. Characterization of New Carbamates and Ureas
4.2.3. Gelation Test
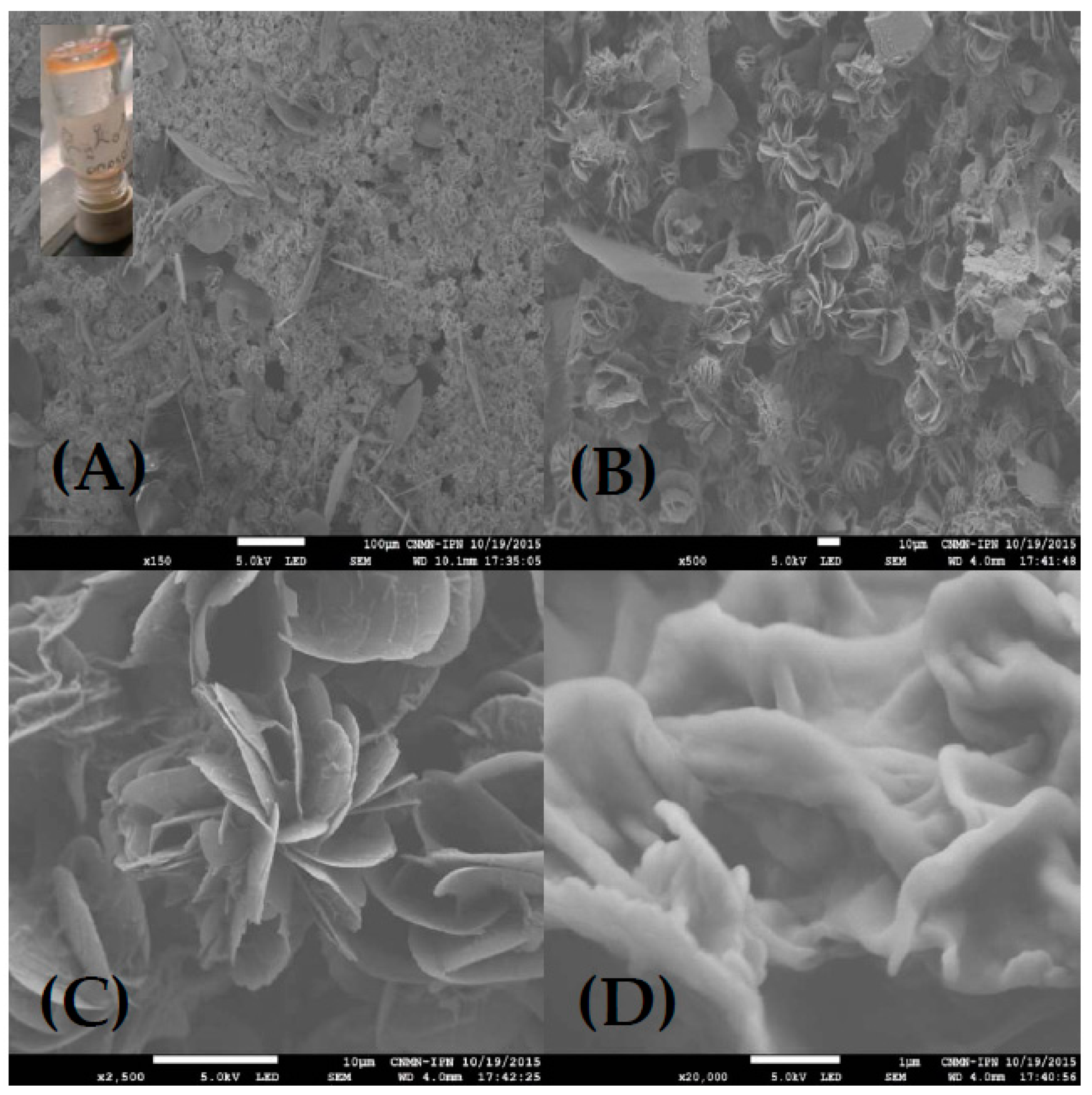
4.2.4. Scanning Electron Microscopy
4.2.5. Capture of Solvents in Wastewater Test
4.2.6. Rheological Analysis
4.2.7. DFT Results of NMR Chemical Shift and IR Spectra
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohar, M.; Das, T. Phenylalanine-based low-molecular-weight gelator for the removal of metal ions and dyes from wastewater. Soft Mater. 2019, 17, 328–341. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Krishnan-Ghosh, Y. First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Commun. 2001, 2, 185–186. [Google Scholar] [CrossRef]
- Baker, B.C.; Higgins, C.L.; Ravishankar, D.; Colquhoun, H.M.; Stevens, G.C.; Greco, F.; Greenland, B.W.; Hayes, W. Multifunctional, Biocompatible, Non-peptidic Hydrogels: From Water Purification to Drug Delivery. Chem. Select. 2016, 1, 1641–1649. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Zhang, Y.; Wang, L.; Xu, B. Small molecule hydrogels based on a class of antiinflammatory agents. Chem. Commun. 2004, 4, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.A.; Escuder, B.; Miravet, J.F. Supramolecular hydrogels for enzymatically triggered self-immolative drug delivery. Tetrahedron 2010, 66, 2614–2618. [Google Scholar] [CrossRef]
- Marlow, M.; Al-Ameedee, M.; Smith, T.; Wheeler, S.; Stocks, M.J. Linifanib-a multi-targeted receptor tyrosine kinase inhibitor and a low molecular weight gelator. Chem. Commun. 2015, 51, 6384–6387. [Google Scholar] [CrossRef] [Green Version]
- Sukegawa, H.; Nishimura, T.; Yoshio, M.; Kajiyama, S.; Kato, T. One-dimensional supramolecular hybrids: Self-assembled nanofibrous materials based on a sugar gelator and calcite developed along an unusual axis. Cryst. Eng. Comm. 2017, 19, 1580–1584. [Google Scholar] [CrossRef]
- Liao, S.W.; Rawson, J.; Omori, K.; Ishiyama, K.; Mozhdehi, D.; Oancea, A.R.; Ito, T.; Guan, Z.; Mullen, Y. Maintaining functional islets through encapsulation in an injectable saccharide-peptide hydrogel. Biomaterials 2013, 34, 3984–3991. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng.—Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Chemical Review-Hydrogel. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Vemula, P.K.; Aslam, U.; Mallia, V.A.; John, G. In Situ synthesis of gold nanoparticles using molecular gels and liquid crystals from vitamin-C amphiphiles. Chem. Mater. 2007, 19, 138–140. [Google Scholar] [CrossRef]
- Kumar, P.; Kadam, M.M.; Gaikar, V.G. Low molecular weight organogels and their application in the synthesis of CdS nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 15374–15385. [Google Scholar] [CrossRef]
- Fitch, K.R.; Goodwin, A.P. Mechanochemical reaction cascade for sensitive detection of covalent bond breakage in hydrogels. Chem. Mater. 2014, 26, 6771–6776. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Oyanagi, A.; Miyawaki, A.; Tomioka, K. Enhancement of self-assembly and gelation ability of N,N’-didodecanoyl ethylenediamine organogelator by terminal functionalization. Tetrahedron Lett. 2016, 57, 5889–5892. [Google Scholar] [CrossRef]
- Luo, X.; Li, Z.; Xiao, W.; Wang, Q.; Zhong, J. Self-assembled organogels formed by monochain derivatives of ethylenediamine. J. Colloid Interface Sci. 2009, 336, 803–807. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M. Vicinal solvent effect on supramolecular gelation: Alcohol controlled topochemical reaction and the toruloid nanostructure. Chem.—A Eur. J. 2014, 20, 10110–10116. [Google Scholar] [CrossRef]
- Demir-Ordu, Ö.; Şimşir, H.; Alper, K. Synthesis of bis[N-(p-aryl)-carbamoyloxy]alkanes as new low-molecular weight organogelators. Tetrahedron 2015, 71, 1529–1539. [Google Scholar] [CrossRef]
- Bacsik, Z.; Zhang, P.; Hedin, N. Ammonium-carbamate-rich organogels for the preparation of amorphous calcium carbonates. Minerals 2017, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Lascialfari, L.; Pescitelli, G.; Brandi, A.; Mannini, M.; Berti, D.; Cicchi, S. Urea vs. carbamate groups: A comparative study in a chiral C 2 symmetric organogelator. Soft Matter 2015, 11, 8333–8341. [Google Scholar] [CrossRef]
- Hou, X.; Butz, J.; Chen, J.; Wang, Z.D.; Zhao, J.X.; Shiu, T.; Chu, Q.R. Low molecular weight organogelators derived from threefold symmetric tricarbamates. Tetrahedron Lett. 2017, 58, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Sone, E.D.; Stupp, S.I. Bioinspired Magnetite Mineralization of Peptide À Amphiphile Nanofibers. Chem. Mater. 2011, 23, 2005–2007. [Google Scholar] [CrossRef]
- Mathiselvam, M.; Loganathan, D.; Varghese, B. Synthesis and characterization of thiourea- and urea-linked glycolipids as low-molecular-weight hydrogelators. RSC Adv. 2013, 3, 14528–14542. [Google Scholar] [CrossRef]
- Goyal, N.; Cheuk, S.; Wang, G. Synthesis and characterization of d-glucosamine-derived low molecular weight gelators. Tetrahedron 2010, 66, 5962–5971. [Google Scholar] [CrossRef]
- Wang, G.; Goyal, N.; Mangunuru, H.P.R.; Yang, H.; Cheuk, S.; Reddy, P.V.N. Preparation and self-assembly study of amphiphilic and bispolar diacetylene-containing glycolipids. J. Org. Chem. 2015, 80, 733–743. [Google Scholar] [CrossRef]
- Minakuchi, N.; Hoe, K.; Yamaki, D.; Ten-No, S.; Nakashima, K.; Goto, M.; Mizuhata, M.; Maruyama, T. Versatile supramolecular gelators that can harden water, organic solvents and ionic liquids. Langmuir 2012, 28, 9259–9266. [Google Scholar] [CrossRef] [PubMed]
- Nanofibers, C. Nanotube Formation from Renewable Resources. Adv. Mater. 2001, 13, 715–718. [Google Scholar]
- Kamiya, S.; Minamikawa, H.; Jung, J.H.; Yang, B.; Masuda, M.; Shimizu, T. Molecular Structure of Glucopyranosylamide Lipid and Nanotube Morphology. Langmuir 2005, 21, 743–750. [Google Scholar] [CrossRef]
- Ressouche, E.; Pensec, S.; Isare, B.; Ducouret, G.; Bouteiller, L. Rational Design of Urea-Based Two-Component Organogelators. ACS Macro Lett. 2016, 5, 244–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.A.; Edkins, R.M.; Cameron, G.J.; Colgin, N.; Fucke, K.; Ridgeway, S.; Crawford, A.G.; Marder, T.B.; Beeby, A.; Cobb, S.L.; et al. Blending gelators to tune gel structure and probe anion-induced disassembly. Chem.—A Eur. J. 2014, 20, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Tsuge, A.; Fujiwara, T.; Yakeya, D.; Kawasaki, H.; Moriguchi, T.; Araki, K. Organogelators based on metacyclophane skeleton having urea units in the bridge. Tetrahedron 2015, 71, 9429–9432. [Google Scholar] [CrossRef]
- Yamanaka, M. Development of C 3 -Symmetric Tris- Urea Low-Molecular-Weight Gelators. Chem. Rec. 2016, 16, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, S.J.; Croisetu, C.M.; Stuart, M.C.A.; Feringa, B.L. Reversible gel-sol photoswitching with an overcrowded alkene-based bis-urea supergelator. Chem. Sci. 2016, 7, 4341–4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.U.; Schollmeyer, D.; Brehmer, M.; Zentel, R. Simple chiral urea gelators, (R)- and (S)-2-heptylurea: Their gelling ability enhanced by chirality. J. Colloid Interface Sci. 2011, 357, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Fages, F.; Vögtle, F.; Zinic, M. Systematic design of amide- and urea-type gelators with tailored properties. Top. Curr. Chem. 2005, 256, 77–131. [Google Scholar]
- Hardy, J.G.; Hirst, A.R.; Ashworth, I.; Brennan, C.; Smith, D.K. Exploring molecular recognition pathways within a family of gelators with different hydrogen bonding motifs. Tetrahedron 2007, 63, 7397–7406. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhang, Y.; Ramström, O. Gelation-driven Dynamic Systemic Resolution: In situ Generation and Self-Selection of an Organogelator. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.M.; Edwards, V.; Smith, D.K. Self-organisation effects in dynamic nanoscale gels self-assembled from simple mixtures of commercially available molecular-scale components. Chem. Sci. 2013, 4, 671–676. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Miyamoto, M.; Watanabe, H.; Oono, M.; Tanaka, A. Alkylhydrazide derivatives as new organogelators and their potential ability to gel electrolytes. Bull. Chem. Soc. Jpn. 2013, 86, 671–673. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wang, H.; Bai, B.; Qu, S.; Song, J.; Ran, X.; Zhang, Y.; Li, M. Organogels from unsymmetrical π -conjugated 1,3,4-oxadiazole derivatives. New J. Chem. 2013, 37, 1454–1460. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable cationic antibacterial amphiphiles: Synthesis, mechanism of action, and cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Guo, M.; Cao, X.; Meijer, E.W.; Dankers, P.Y.W. Core-Shell Capsules Based on Supramolecular Hydrogels Show Shell-Related Erosion and Release Due to Confinement. Macromol. Biosci. 2013, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ohkawabata, S.; Kanemaru, M.; Kuawahara, S.Y.; Yamamoto, K.; Kadokawa, J.I. Synthesis of 6-O-hexadecyl-and 6-O-octylsucroses and their self-assembling properties under aqueous conditions. J. Carbohydr. Chem. 2012, 31, 659–672. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1443. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and use. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, R.; Zhang, Y.S.; Fitriyani, S.; Liu, J.H. Click chemistry-assisted, bis-cholesteryl-appended, isosorbide-based, dual-responsive organogelators and their self-assemblies. Soft Matter 2016, 12, 5214–5223. [Google Scholar] [CrossRef] [PubMed]
- Curcio, P.; Allix, F.; Pickaert, G.; Jamart-grøgoire, B.A. Favorable narrow, δh Hansen-parameter domain for gelation of low- molecular-weight amino acid derivatives. Chem.—Eur. J. 2011, 17, 13603–13612. [Google Scholar] [CrossRef]
- Zhu, G.; Dordick, J.S. Solvent effect on organogel formation by low molecular weight molecules. Chem. Mater. 2006, 18, 5988–5995. [Google Scholar] [CrossRef]
- Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar]
- Wang, R.; Geiger, C.; Chen, L.; Swanson, B.; Whitten, D.G. Direct observation of sol—Gel conversion: The role of the solvent in organogel formation. J. Am. Chem. Soc. 2000, 122, 2399–2400. [Google Scholar] [CrossRef]
- Sakurai, K.; Jeong, Y.; Koumoto, K.; Friggeri, A.; Gronwald, O.; Sakurai, S.; Okamoto, S.; Inoue, K. Supramolecular structure of a sugar-appended organogelator explored with synchrotron X-ray small-angle scattering. Langmuir 2003, 19, 8211–8217. [Google Scholar] [CrossRef]
- Keller, A. Aspects of polymer gels. Faraday Discuss. 1995, 101, 1–49. [Google Scholar] [CrossRef]
- Hoek, E. Introductory lecture. In Proceedings of the 6th ISRM Congress of the International Society for Rock Mechanics, Montreal, QC, Canada, 30 August–3 September; 1987; Volume 1987, pp. 1357–1362. [Google Scholar]
- Yang, L.; Adam, C.; Cockroft, S.L. Quantifying solvophobic effects in nonpolar cohesive interactions. J. Am. Chem. Soc. 2015, 137, 10084–10087. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. n-Alkanes gel n-alkanes (and many other organic liquids). Langmuir 2000, 16, 352–355. [Google Scholar] [CrossRef]
- Seiffert, S.; Sprakel, J. Physical chemistry of supramolecular polymer networks. Chem. Soc. Rev. 2012, 41, 909–930. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hawke, L.G.D.; Goldansaz, H.; Van Ruymbeke, E. Dynamics of entangled linear supramolecular chains with sticky side groups: Influence of hindered fluctuations. Macromolecules 2015, 48, 7300–7310. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, Q.; Zhang, L.; Shen, Z.; Jiang, L.; Liu, M. Self-assembly of hierarchical chiral nanostructures based on metal-benzimidazole interactions: Chiral nanofibers, nanotubes, and microtubular flowers. Small 2016, 12, 4743–4752. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2014, 104, 1201–1217. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Recommended Health-Based Limits in Occupational Exposure to Selected Organic Solvents; WHO: Geneva, Switzerland, 1981; pp. 1–84. [Google Scholar]
- Fedorov, A.V.; Cable, J.R.; Carey, J.R.; Zwier, S. Infrared spectroscopy of H-bonded bridges stretched across the cis-amide group: II. Ammonia and mixed ammonia/water bridges. J. Phys. Chem. A 2001, 105, 8162–8175. [Google Scholar] [CrossRef]
- Xue, P.; Lu, R.; Li, D.; Jin, M.; Tan, C.; Bao, C.; Wang, Z.; Zhao, Y. Novel CuS nanofibers using organogel as a template: Controlled by binding sites. Langmuir 2004, 20, 11234–11239. [Google Scholar] [CrossRef]
- Aggeli, A.; Nyrkova, I.A.; Bell, M.; Harding, R.; Carrick, L.; McLeish, T.C.B.; Semenov, A.N.; Boden, N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide β-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-K.; Zhang, C.; He, X.-N.; Wang, P.-Y. Effects of alkyl chain lengths on 12-hydroxystearic acid derivatives based supramolecular organogels. Colloids Surf. A 2021, 616, 126319. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Valiev, R.R.; Li, Q.; Li, C.; Xie, Y.; Ågren, H. Computational study of aromaticity, 1H NMR spectra and intermolecular interactions of twisted thia-norhexaphyrin and its multiply annulated polypyrrolic derivatives. Phys. Chem. Chem. Phys. 2019, 21, 25334–25343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piana, F.; Case, D.H.; Ramalhete, S.M.; Pileio, G.; Facciotti, M.; Day, G.M.; Khimyak, Y.Z.; Angulo, J.; Brown, R.C.D.; Gale, P.A. Substituent interference on supramolecular assembly in urea gelators: Synthesis, structure prediction and NMR. Soft Matter 2016, 12, 4034–4043. [Google Scholar] [CrossRef] [Green Version]
- Yesiltepe, Y.; Nuñez, J.R.; Colby, S.M.; Thomas, D.G.; Borkum, M.I.; Reardon, P.N.; Washton, N.M.; Metz, T.O.; Teeguarden, J.G.; Govind, N.; et al. An automated framework for NMR chemical shift calculations of small organic molecules. J. Cheminform. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Martínez-Mejía, G.; Vázquez-Torres, N.A.; Castell-Rodríguez, A.; del Río, J.M.; Corea, M.; Jiménez-Juárez, R. Synthesis of new chitosan-glutaraldehyde scaffolds for tissue engineering using Schiff reactions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123658. [Google Scholar] [CrossRef]
- De Godoi, K.R.R.; Basso, R.C.; Ming, C.C.; da Silva, V.M.; da Cunha, R.L.; Barrera-Arellano, D.; Ribeiro, A.P.B. Physicochemicaland rheological properties of soybean organogels: Interactions between different structuring agents. Food Res. Int. 2019, 124, 108475. [Google Scholar] [CrossRef]
- Gökçe, E.H.; Yurdasiper, A.; Korkmaz, E.; Özer, Ö. A novel preparation method for organogels: High-speed homogenization and micro-irradiation. AAPS PharmSciTech. 2013, 14, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Ojeda-Serna, I.E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Cháirez-Ramírez, M.H.; Rosas-Flores, W.; Pérez-Martínez, J.D.; Moreno-Jiménez, M.R.; González-Laredo, R.F. Water-in-oil organogel based emulsions as a tool for increasing bioaccessibility and cell permeability of poorly water-soluble nutraceuticals. Food Res. Int. 2019, 120, 415–424. [Google Scholar] [CrossRef]
- Michler, G.H. Preparation of Surfaces in Electron Microscopy of Polymers; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Aston, R.; Sewell, K.; Klein, T.; Lawrie, G.; Grondahl, L. Evaluation of the impact of freezing preparation techniques on the characterization of alginate hydrogels by cryo-SEM. Eur. Polym. J. 2016, 82, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]







| Carbamate Compound | Urea Compound | |||||||
|---|---|---|---|---|---|---|---|---|
| Mixture | Solvent (g) | Water (g) | 3d (g) | Removal Efficiency (%) | Solvent (g) | Water (g) | 5d (g) | Removal Efficiency (%) |
| D-W | 0.2353 | 2.8036 | 0.0159 | 0.0 | 0.23 | 2.0783 | 0.0208 | 0.0 |
| T-W | 0.4884 | 2.3495 | 0.0201 | 28.00 | 0.4124 | 2.3160 | 0.1706 | 80.18 |
| X-W | 0.7671 | 3.2431 | 0.0252 | 82.53 | 0.7317 | 3.1273 | 0.0150 | 92.72 |
| T-X-W | 0.8819 | 1.2958 | 0.0258 | 58.95 | 0.0923 | 1.2584 | 0.0215 | 90.72 |
| D-T-X-W | 1.3522 | 1.0093 | 0.0256 | 62.40 | 1.3614 | 1.0147 | 0.0124 | 73.12 |
| Carbamate Compound 3d | Urea Compound 5d | |||
|---|---|---|---|---|
| Mixture | Gravimetry Removal Efficiency (%) | FT-IR Removal Efficiency (%) | Gravimetry Removal Efficiency (%) | FT-IR Removal Efficiency (%) |
| T-W | 28.00 | 19.30 | 80.18 | 80.70 |
| X-W | 82.53 | 79.3 | 92.72 | 89.80 |
| T-X-W | 58.95 | 58.7 | 90.72 | 90.40 |
| D-T-X-W | 62.40 | 64.90 | 73.12 | 65.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Mejía, G.; Bermeo-Solórzano, B.A.; González, S.; del Río, J.M.; Corea, M.; Jiménez-Juárez, R. New Carbamates and Ureas: Comparative Ability to Gel Organic Solvents. Gels 2022, 8, 440. https://doi.org/10.3390/gels8070440
Martínez-Mejía G, Bermeo-Solórzano BA, González S, del Río JM, Corea M, Jiménez-Juárez R. New Carbamates and Ureas: Comparative Ability to Gel Organic Solvents. Gels. 2022; 8(7):440. https://doi.org/10.3390/gels8070440
Chicago/Turabian StyleMartínez-Mejía, Gabriela, Brenda Afrodita Bermeo-Solórzano, Silvia González, José Manuel del Río, Mónica Corea, and Rogelio Jiménez-Juárez. 2022. "New Carbamates and Ureas: Comparative Ability to Gel Organic Solvents" Gels 8, no. 7: 440. https://doi.org/10.3390/gels8070440
APA StyleMartínez-Mejía, G., Bermeo-Solórzano, B. A., González, S., del Río, J. M., Corea, M., & Jiménez-Juárez, R. (2022). New Carbamates and Ureas: Comparative Ability to Gel Organic Solvents. Gels, 8(7), 440. https://doi.org/10.3390/gels8070440