Enhanced Development of Sweat Latent Fingerprints Based on Ag-Loaded CMCS/PVA Composite Hydrogel Film by Electron Beam Radiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Behaviors of CMCS/PVA Hydrogel Film at Different Conditions
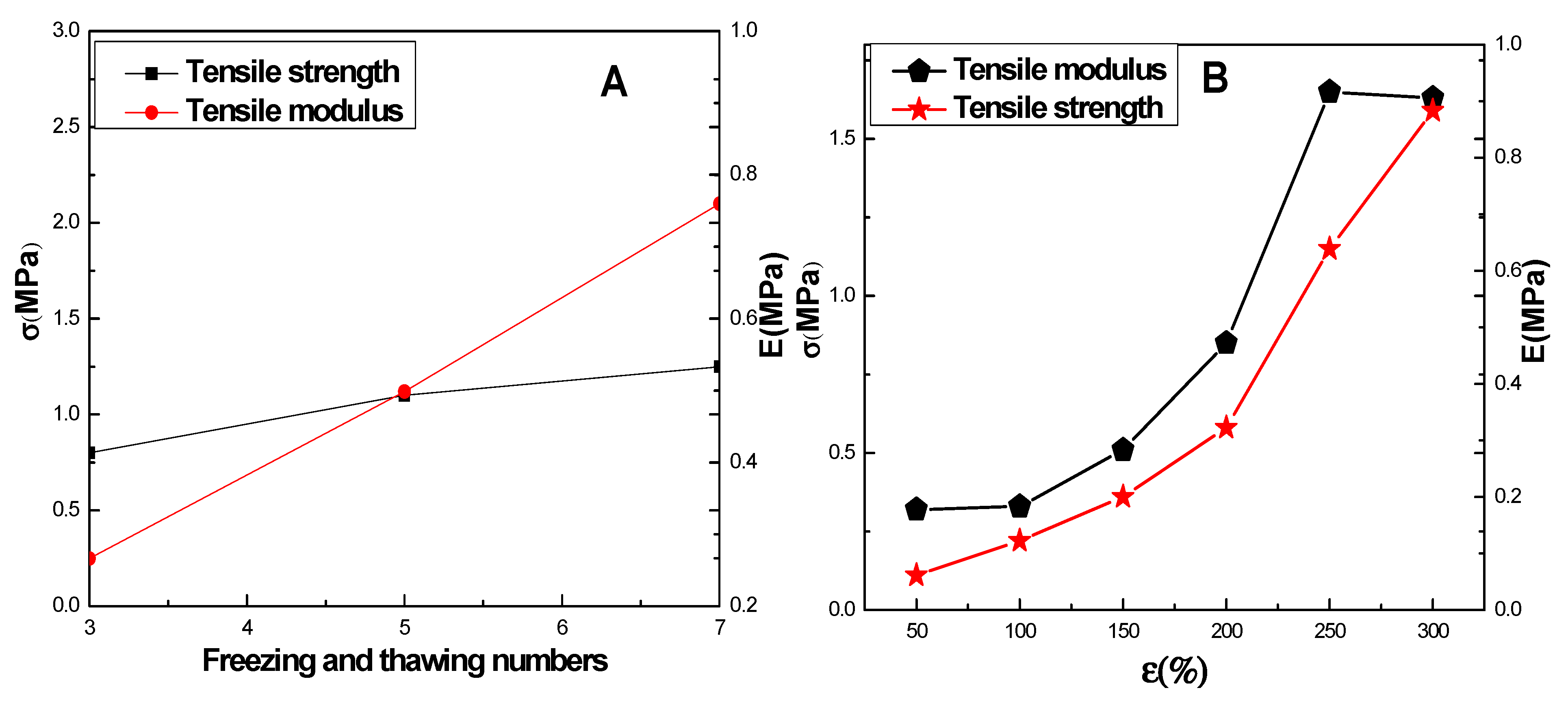
2.2. Mechanical Properties of CMCS/PVA Hydrogel Film
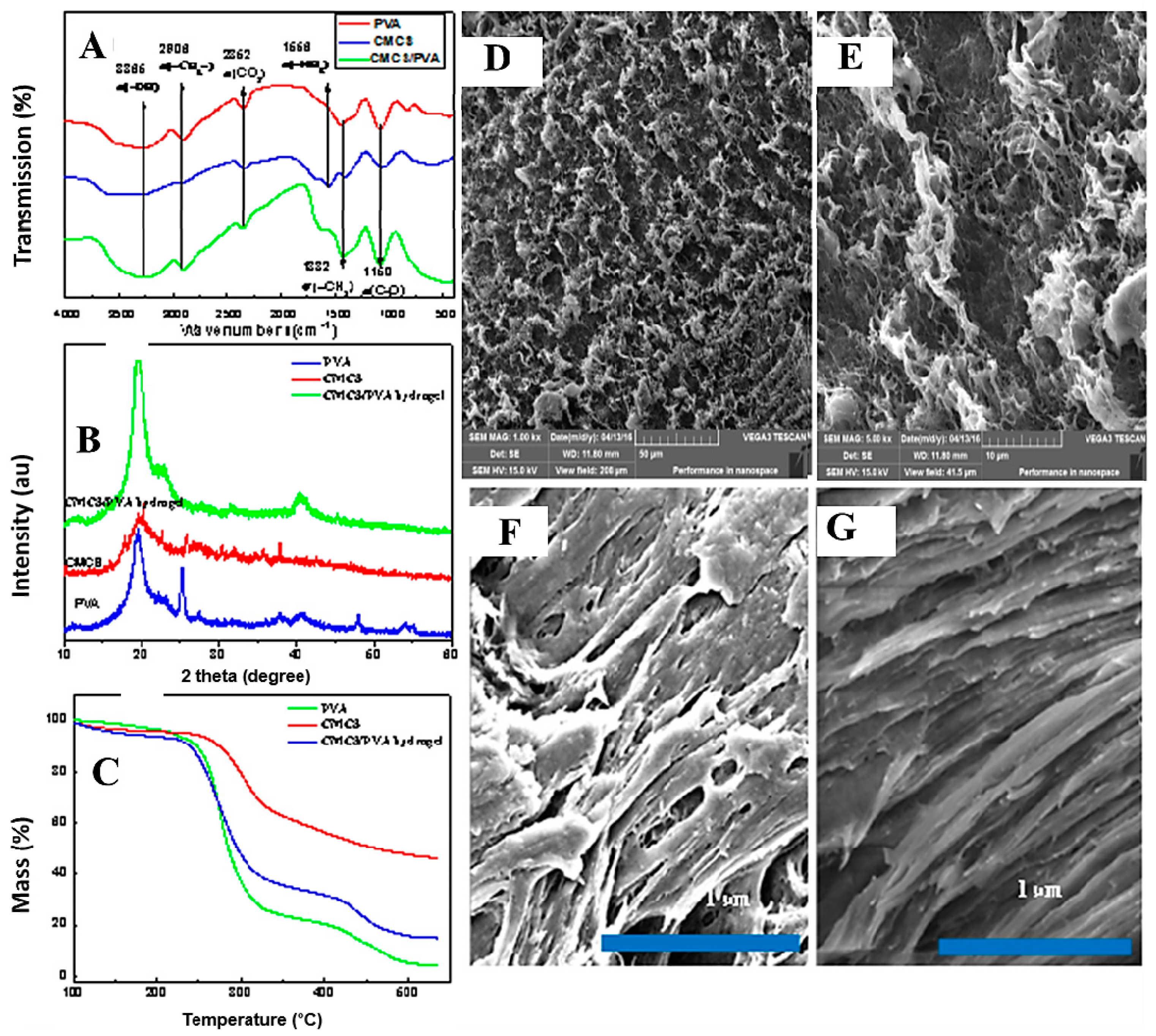
2.3. Microstructural Studies
2.4. TG Analysis
2.5. Morphology
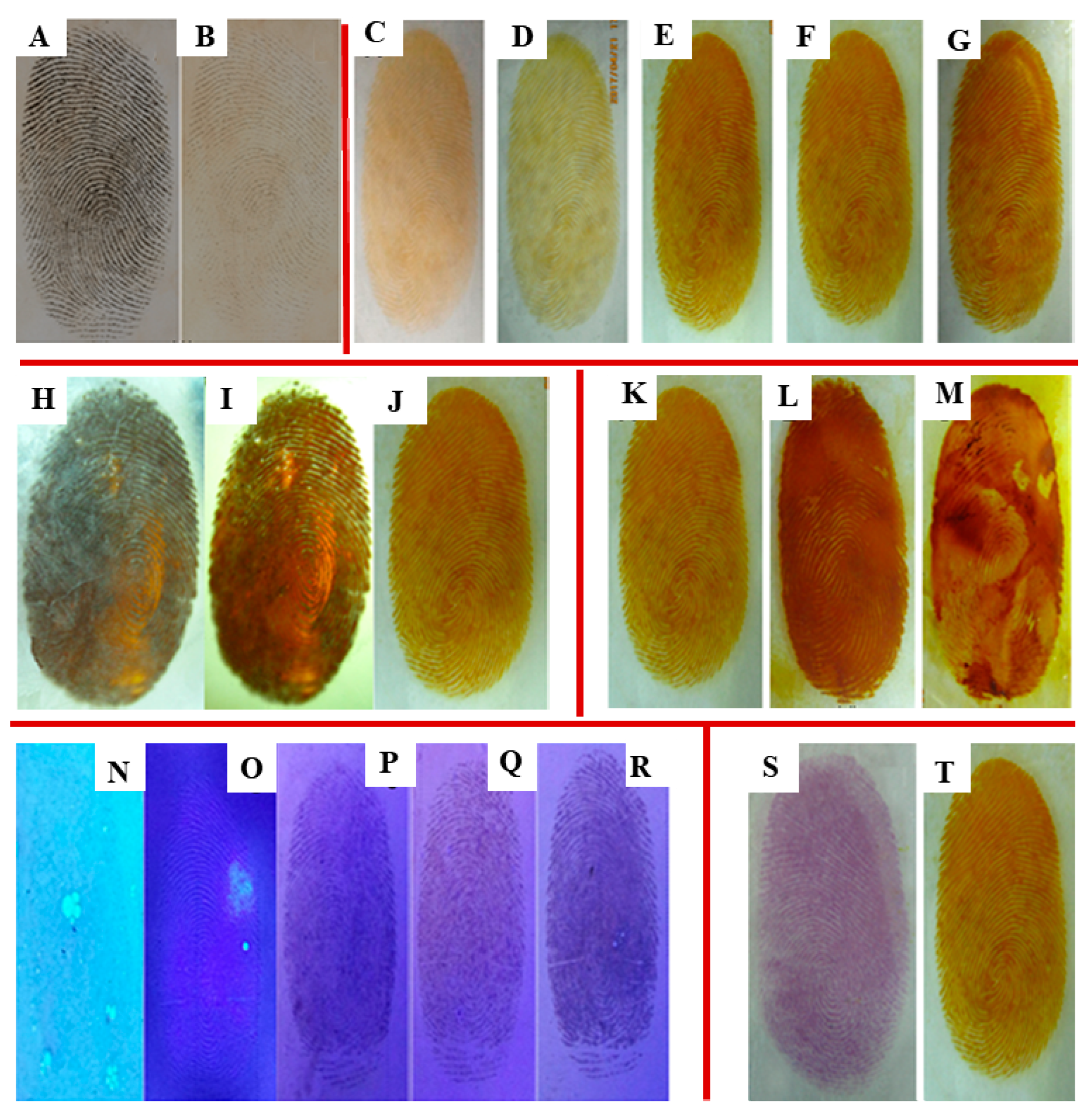
2.6. Effect of Varying UV Light Wavelengths
2.7. Influence of Irradiation Time
2.8. Relationship between Preparation Method and Fingerprint Development
2.9. Impact of Different Coating Modes
2.10. Behaviors of Fingerprint Development with Different Concentrations of Ag+ Ions
2.11. Comparison of Fingerprint Development with Different Developing Reagents
2.12. Evaluation of the Toxicity of Hydrogel Film by the Alamar Blue Method
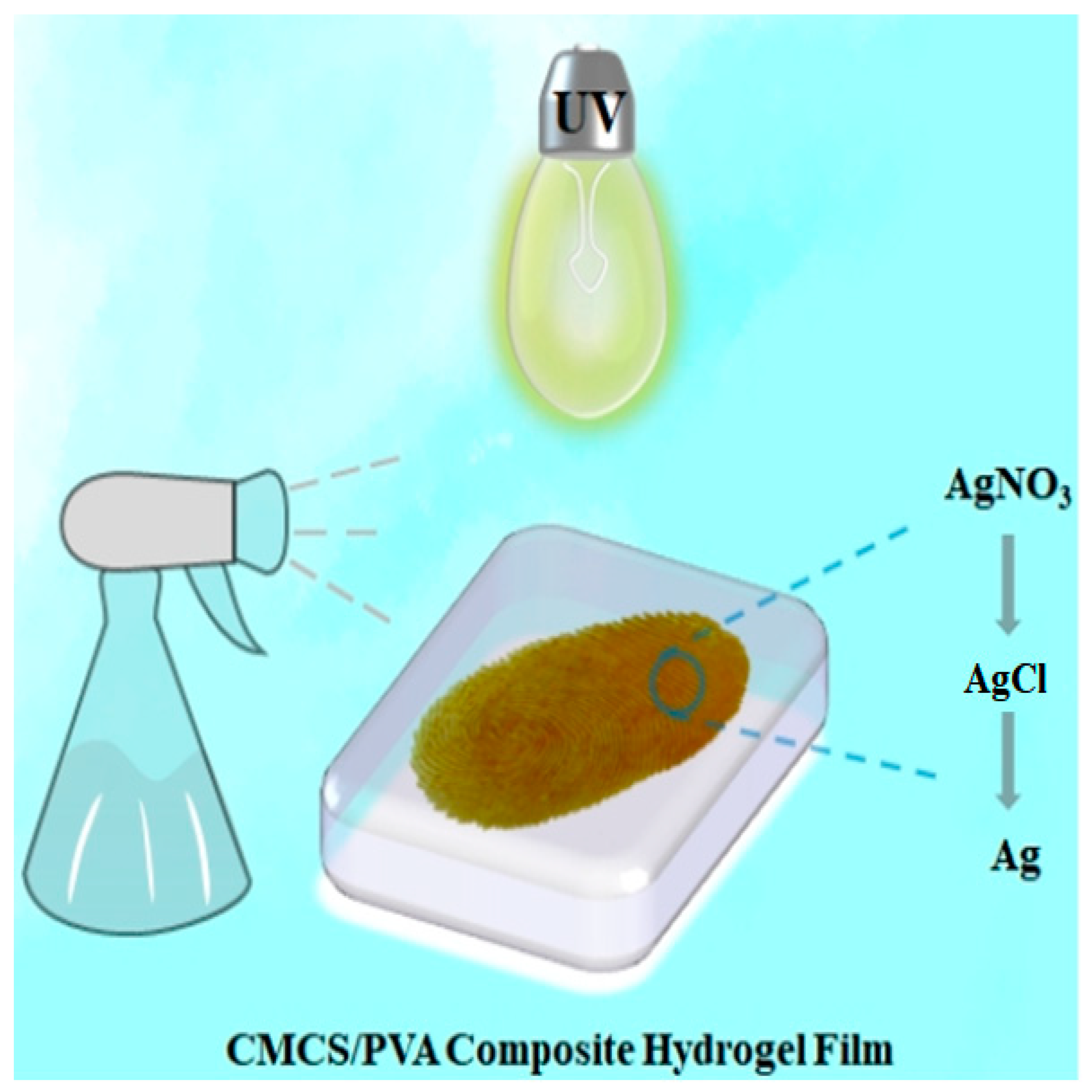
2.13. Mechanism of Fingerprint Development
3. Conclusions
4. Experimental
4.1. Materials
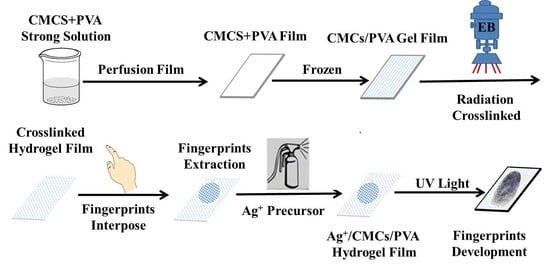
4.2. Preparation of CMCS/PVA Hydrogel Film by Electron Beam Radiation
4.3. Preparation of CMCS/PVA Hydrogel Sweat Latent Fingerprint Film
4.4. Development of ACP Hydrogel Sweat Latent Fingerprint Film
4.5. Gel Fraction
4.6. Swelling Behaviors
4.7. Characterization
4.8. Cell Toxicity Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonathan, S.; Simon, A. Cole. Suspect Identities: A History of Fingerprinting and Criminal Identification. Am. J. Ophthalmol. 2003, 108, 165–166. [Google Scholar]
- Lee, J.; Joullié, M.M. Novel design and approach to latent fingerprint detection on paper using a 1,2-indanedione-based bi-functional reagent. Tetrahedron Lett. 2015, 56, 3378–3381. [Google Scholar] [CrossRef] [Green Version]
- Badiye, A.; Kapoor, K. Efficacy of Robin powder blue for latent fingerprint development on various surfaces. Egypt. J. Forensic Sci. 2015, 5, 166–173. [Google Scholar] [CrossRef] [Green Version]
- D’Elia, V.; Materazzi, S.; Iuliano, G.; Niola, L. Evaluation and comparison of 1,2-indanedione and 1,8-diazafluoren-9-one solutions for the enhancement of latent fingerprints on porous surfaces. Forensic Sci. Int. 2015, 254, 205–214. [Google Scholar] [CrossRef]
- Rohatgi, R.; Kapoor, A.K. Development of latent fingerprints on wet non-porous surfaces with SPR based on basic fuchsin dye. Egypt. J. Forensic Sci. 2016, 6, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.K.; Kumari, H.; Kaur, R. A newtechnique for visualization of latent fingerprints on various surfaces using powder from turmeric: A rhizomatous herbaceous plant (Curcuma longa). Egypt. J. Forensic Sci. 2011, 1, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. pH/thermo-responsive poly (N-isopropylacrylamide-co-maleic acid) hydrogel with a sensor and an actuator for biomedical applications. Polymer 2017, 110, 177–186. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Ma, J.; Vancso, G.J. Thermo-responsive semi-IPN hydrogel microfibers from continuous fluidic processing with high elasticity and fast actuation. ACS Appl. Mater. Int. 2017, 9, 901–908. [Google Scholar] [CrossRef]
- Bonetti, L.; Fiorati, A.; D’ Agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart Methylcellulose Hydrogels for pH-Triggered Delivery of Silver Nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Wang, Y.; Zhang, G.; Shao, Y.; Jia, H.; Liu, D. Remote controlling DNA hydrogel by magnetic field. ACS Appl. Mater. Int. 2017, 9, 1995–2000. [Google Scholar] [CrossRef]
- Jia, X.; Wang, K.; Wang, J.; Hu, Y.; Shen, L.; Zhu, J. Full-color photonic hydrogels for pH and ionic strength sensing. Eur. Polym. J. 2016, 83, 60–66. [Google Scholar] [CrossRef]
- Chen, J.; Gao, L.X.; Han, X.; Chen, T.; Luo, J.; Liu, K.; Zhang, W. Preparation and electro-response of chitosan-g-poly (acrylic acid) hydrogel elastomers with interpenetrating network. Mater. Chem. Phys. 2016, 169, 105–112. [Google Scholar] [CrossRef]
- Weingarten, A.S.; Kazantsev, R.V.; Palmer, L.C.; McClendon, M.; Koltonow, A.R.; Samuel, A.P.S.; Kiebala, D.J.; Wasielewski, M.R.; Stupp, S.I. Self-assembling hydrogel scaffolds for photocatalytic hydrgen production. Nat. Chem. 2014, 6, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.S.; Qin, J.T.; Han, Y.; Du, J.F.; Dong, Z.B.; Sun, S.F.; Liu, Y. Controlled Preparation and Highly Photocatalytic Activity of Portable MCC-g-GMA@TiO2 Photocatalyst by Pre-radiation Grafting-Embedding Method. Appl. Catal. B Environ. 2017, 218, 101–110. [Google Scholar] [CrossRef]
- Paladini, F.; Meikle, S.T.; Cooper, I.R.; Lacey, J.; Perugini, V.; Santin, M. Silver-doped self-assembling di-phenylalanine hydrogels as wound dressing biomaterials. J. Mater. Sci. Mater. Med. 2013, 24, 2461–2472. [Google Scholar] [CrossRef]
- Park, K.; Choi, H.; Kang, K.; Shin, M.; Son, D. Soft Stretchable Conductive Carboxymethylcellulose Hydrogels for Wearable Sensors. Gels 2022, 8, 92. [Google Scholar] [CrossRef]
- Gebeyehu, E.K.; Sui, X.; Adamu, B.F.; Beyene, K.A.; Tadesse, M.G. Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary. Gels 2022, 8, 140. [Google Scholar] [CrossRef]
- Bjugstad, K.B.; Redmond, D.E.J.; Lampe, K.J.; Kern, D.S.; Mahoney, M.J. Biocompatibility of PEG-based hydrogels in primate brain. Cell Transplant. 2008, 17, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Saroia, J.; Wang, Y.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 15. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release 2014, 186, 54–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Yi, M. UV-syntheses and properties of responsive hydrogels containing N, N-dimethylaminoethyl methacrylate. Acta Polym. Sin. 2001, 80, 215–218. [Google Scholar]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Lad, U.; Kale, G.M.; Bryaskova, R. Glucose Oxidase Encapsulated Polyvinyl Alcohol-Silica Hybrid Films for an Electrochemical Glucose Sensing Electrode. Anal. Chem. 2013, 85, 6349–6355. [Google Scholar] [CrossRef]
- Varaprasad, K.; Mohan, Y.M.; Ravindra, S.; Reddy, N.N.; Vimala, K.; Monika, K.; Sreedhar, B.; Raju, K.M. Hydrogel-silver nanoparticle composites: A new generation of antimicrobials. J. Appl. Polym. Sci. 2010, 115, 1199–1207. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Guan, S.; Dong, A.; Cao, Y.; Chen, C. A novel Ag/AgO/carboxymethyl chitosan bacteriostatic hydrogel for drug delivery. Mater. Res. Express 2020, 7, 085403. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Francis, S. Designing sterile biocompatible moxifloxacinloaded trgacanth-PVA-alginate wound dressing by radiation crosslinking method. Wound Med. 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Mozalewska, W.; Czechowska-Biskup, R.; KOlejnik, A.; Wach, R.A.; Ulański, P.; Rosiak, J.M. Chitosan-containing hydrogel wound dressings prepared by radiation technique. Radiat. Phys. Chem. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Bala, R. Radiation formation of psyllium cross-linked poly (hydroxyethylmethacrylate)-co-poly (acrylamide) based sterile hydrogels for drug delivery applications. Polym. Sci. Ser. A 2017, 59, 1–13. [Google Scholar] [CrossRef]
- Bayramgil, N.P. Synthesis, characterization and drug release behavior of poly(1-vinyl1,2,4-triazole) hydrogels prepared by gamma irradiation. Colloids Surf. B Biointerfaces 2012, 97, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yanagawaa, F.; Mizutanib, T.; Sugiuraa, S.; Takagia, T.; Sumarua, K.; Kanamoria, T. Partially photodegradable hybrid hydrogels with elasticity tunable by light irradiation. Colloids Surf. B Biointerfaces 2015, 126, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Cai, H.H.; Han, Y.; Huang, H.T.; Qin, J.T.; Song, Z.Y.; Sun, S.F.; Liu, Y. Photosensitive antibacterial and cytotoxicity performances of a TiO2/carboxymethyl chitosan/poly(vinyl alcohol) nanocomposite hydrogel by in situradiation construction. J. Appl. Polym. Sci. 2016, 133, 11450. [Google Scholar] [CrossRef]
- Croxton, R.; Kent, T.; Littlewood, A.; Smith, M. An Evaluation of Inkjet Printed Amino Acid Fingerprint Test Targets for Ninhydrin Process Monitoring—And Some Observations. Forensic Sci. Int. 2021, 321, 110741. [Google Scholar] [CrossRef]
- Jelly, R.; Patton, E.L.T.; Lennard, C.; Lewis, S.W.; Lim, K.F. The detection of latent fingermarks on porous surfaces using amino acid sensitive reagents: A review. Anal. Chim. Acta 2009, 652, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
- O’ Neill, M.E. The Development of Latent Fingerprints on Paper. J. Crim. Law Criminol. 1937, 28, 432. [Google Scholar]

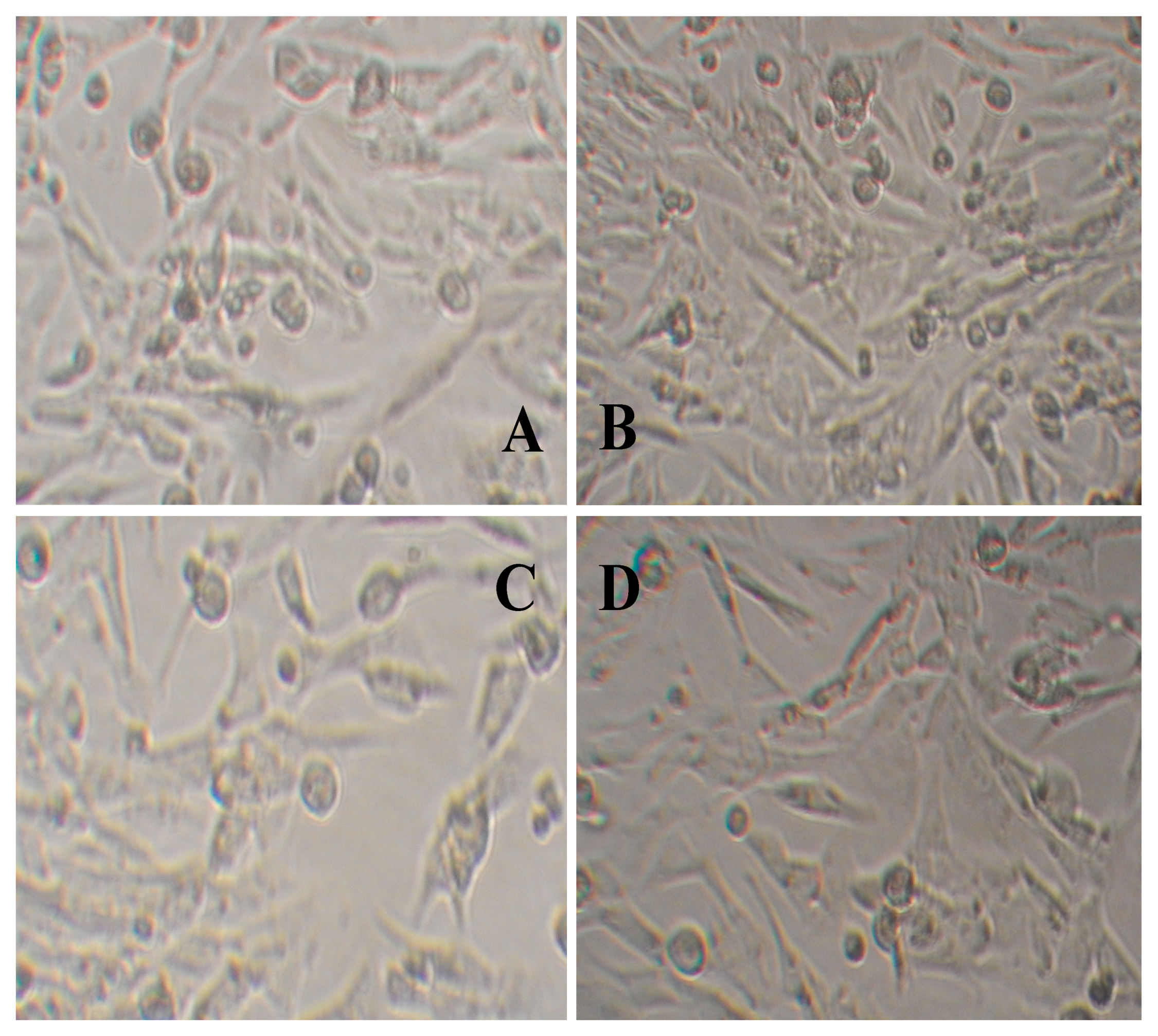
| Sample | Alamar Blue Reduction Ratio (%) | ||
|---|---|---|---|
| 0.1 g/mL | 0.2 g/mL | 0.4 g/mL | |
| Control | 76.12 ± 5.22 | 75.91 ± 5.38 | 75.52 ± 5.36 |
| CMCS | 76.09 ± 2.35 | 73.96 ± 3.52 | 72.86 ± 1.75 |
| PVA | 75.18 ± 2.74 | 73.93 ± 4.01 | 72.06 ± 1.55 |
| CMCS/PVA hydrogel | 74.38 ± 3.54 | 72.99 ± 4.77 | 70.28 ± 3.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, Y.; Zhao, Y.; Liu, D.; Rao, L.; Wang, Z.; Fu, L.; Wang, Y.; Yang, X.; Li, Y.; et al. Enhanced Development of Sweat Latent Fingerprints Based on Ag-Loaded CMCS/PVA Composite Hydrogel Film by Electron Beam Radiation. Gels 2022, 8, 446. https://doi.org/10.3390/gels8070446
Yang J, Wang Y, Zhao Y, Liu D, Rao L, Wang Z, Fu L, Wang Y, Yang X, Li Y, et al. Enhanced Development of Sweat Latent Fingerprints Based on Ag-Loaded CMCS/PVA Composite Hydrogel Film by Electron Beam Radiation. Gels. 2022; 8(7):446. https://doi.org/10.3390/gels8070446
Chicago/Turabian StyleYang, Jinyu, Yayang Wang, Yuan Zhao, Dongliang Liu, Lu Rao, Zhijun Wang, Lili Fu, Yifan Wang, Xiaojie Yang, Yuesheng Li, and et al. 2022. "Enhanced Development of Sweat Latent Fingerprints Based on Ag-Loaded CMCS/PVA Composite Hydrogel Film by Electron Beam Radiation" Gels 8, no. 7: 446. https://doi.org/10.3390/gels8070446