Advances in 3D Gel Printing for Enzyme Immobilization
Abstract
:1. Introduction
1.1. Conventional Enzyme Immobilization Methods
1.2. 3D Printing Basics
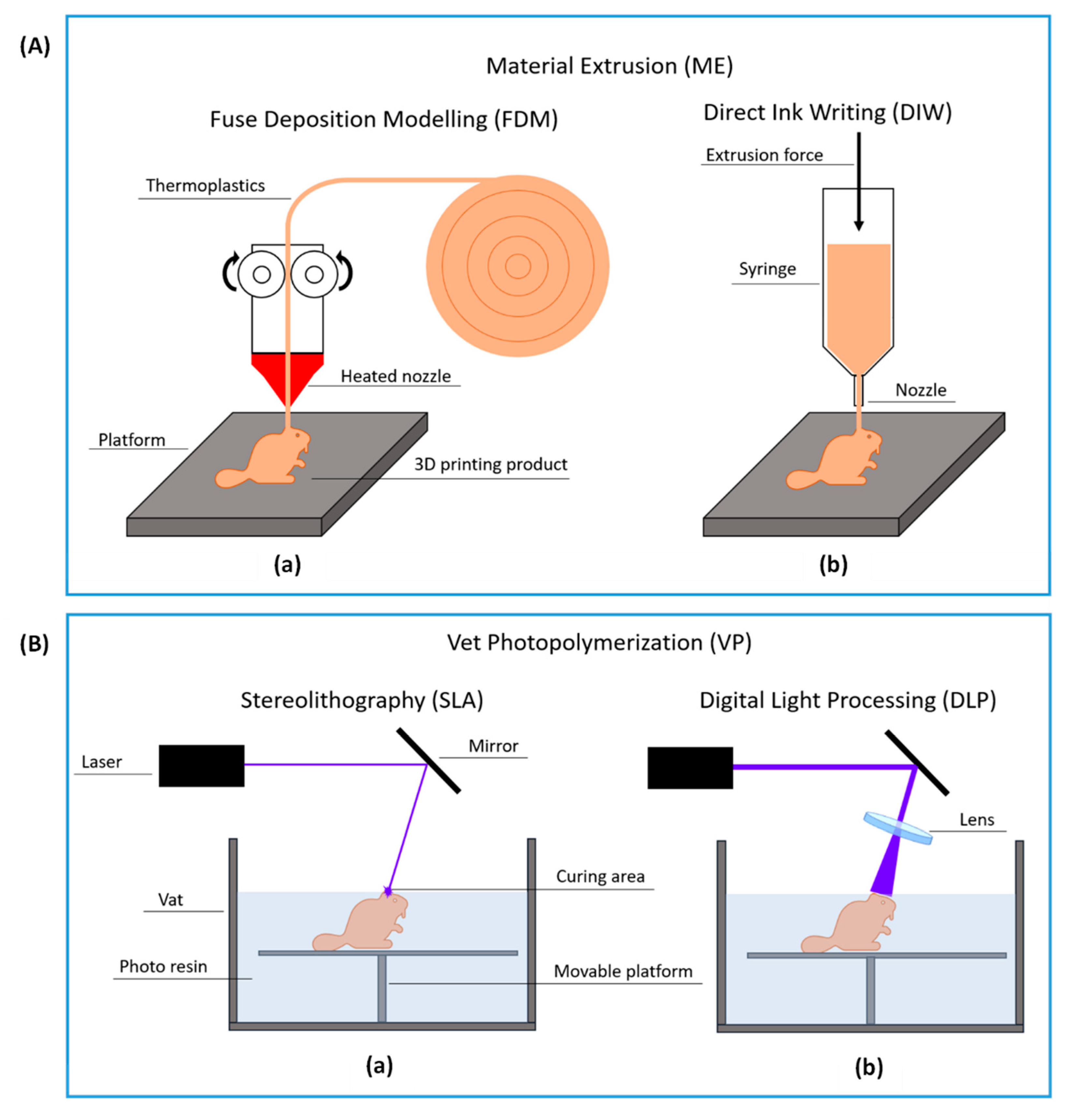
1.2.1. Material Extrusion
1.2.2. Vat Photopolymerization
2. Post-Printing Immobilization
2.1. Fused Deposition Modeling
2.2. Direct Ink Writing
2.3. Vat Photopolymerization with Digital Light Processing
2.4. Vat Photopolymerization by Stereolithography
3. Immobilization by Physical Entrapment during 3D Printing
3.1. Gel Printing
3.1.1. Gelation by Physical Crosslinking
3.1.2. Gelation by Photo-Crosslinking
3.1.3. Gelation by Enzymatic Crosslinking
3.2. Molten Matrix
3.3. Low Viscosity Resin
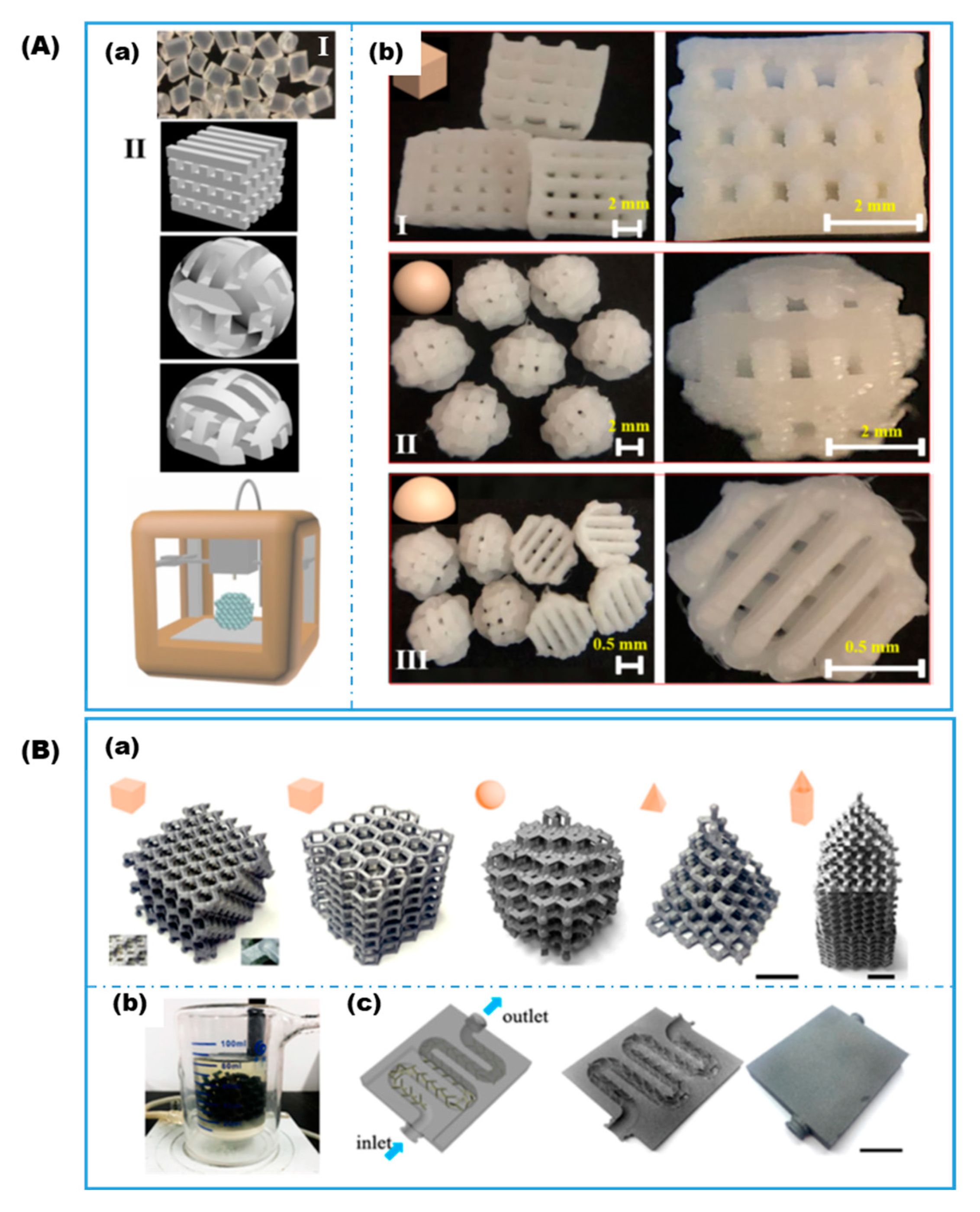
3.4. Novel Variations
4. Limitation of the Review
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schäfer, T.; Borchert, T.W.; Nielsen, V.S.; Skagerlind, P.; Gibson, K.; Wenger, K.; Hatzack, F.; Nilsson, L.D.; Salmon, S.; Pedersen, S.; et al. Industrial Enzymes. Adv. Biochem. Eng. Biotechnol. 2006, 105, 59–131. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527312320. [Google Scholar]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding Enzyme Immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Salmon, S.; House, A. Enzyme-Catalyzed Solvents for CO2 Separation. In Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 23–86. [Google Scholar]
- Soares, J.C.; Moreira, P.R.; Queiroga, A.C.; Morgado, J.; Malcata, F.X.; Pintado, M.E. Application of Immobilized Enzyme Technologies for the Textile Industry: A Review. Biocatal. Biotransform. 2011, 29, 223–237. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S. Organic–Inorganic Hybrid Nanoflowers: A Novel Host Platform for Immobilizing Biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Hu, H.; Xu, J.; Wang, Z.; Du, Y.; Cui, J.; Jia, S. Enhanced Enzymatic Performance of Immobilized Lipase on Metal Organic Frameworks with Superhydrophobic Coating for Biodiesel Production. J. Colloid Interface Sci. 2021, 602, 426–436. [Google Scholar] [CrossRef]
- Othman, A.; Karimi, A.; Andreescu, S. Functional Nanostructures for Enzyme Based Biosensors: Properties, Fabrication and Applications. J. Mater. Chem. B 2016, 4, 7178–7203. [Google Scholar] [CrossRef]
- Conte, M.P.; Sahoo, J.K.; Abul-Haija, Y.M.; Lau, K.H.A.; Ulijn, R.V. Biocatalytic Self-Assembly on Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3069–3075. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and Chitosan-Based Support Materials for Enzyme Immobilization and Biotechnological Applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan Activated with Divinyl Sulfone: A New Heterofunctional Support for Enzyme Immobilization. Application in the Immobilization of Lipase B from Candida Antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, Y.; Salmon, S. Carbonic Anhydrase Immobilized on Textile Structured Packing Using Chitosan Entrapment for CO2 Capture. ACS Sustain. Chem. Eng. 2022, 10, 7772–7785. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on Methods and Easy Separated Support Materials for Enzymes Immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Pose-Boirazian, T.; Martínez-Costas, J.; Eibes, G. 3D Printing: An Emerging Technology for Biocatalyst Immobilization. Macromol. Biosci. 2022, 2200110. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liao, Z.; Gao, B.; He, B. Emerging 3D Printing Strategies for Enzyme Immobilization: Materials, Methods, and Applications. ACS Omega 2022, 7, 11530–11543. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K.K. 3D Printing of Polymer Composites: Materials, Processes, and Applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Su, C.K. Review of 3D-Printed Functionalized Devices for Chemical and Biochemical Analysis. Anal. Chim. Acta 2021, 1158, 338348. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Saha, S.; Satapathy, B.K. Recent Advances in Polymer Scaffolds for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 342–408. [Google Scholar] [CrossRef]
- Schmieg, B.; Döbber, J.; Kirschhöfer, F.; Pohl, M.; Franzreb, M. Advantages of Hydrogel-Based 3D-Printed Enzyme Reactors and Their Limitations for Biocatalysis. Front. Bioeng. Biotechnol. 2019, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Su, C.K.; Yen, S.C.; Li, T.W.; Sun, Y.C. Enzyme-Immobilized 3D-Printed Reactors for Online Monitoring of Rat Brain Extracellular Glucose and Lactate. Anal. Chem. 2016, 88, 6265–6273. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Malla, S.; Rusling, J.F. Automated 3-D Printed Arrays to Evaluate Genotoxic Chemistry: E-Cigarettes and Water Samples. ACS Sens. 2017, 2, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Su, C.K.; Chen, J.C. One-Step Three-Dimensional Printing of Enzyme/Substrate–Incorporated Devices for Glucose Testing. Anal. Chim. Acta 2018, 1036, 133–140. [Google Scholar] [CrossRef]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In Situ Immobilization of Glucose Oxidase and Catalase in a Hybrid Interpenetrating Polymer Network by 3D Bioprinting and Its Application. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Schmieg, B.; Schimek, A.; Franzreb, M. Development and Performance of a 3D-Printable Poly(Ethylene Glycol) Diacrylate Hydrogel Suitable for Enzyme Entrapment and Long-Term Biocatalytic Applications. Eng. Life Sci. 2018, 18, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.W.; Khattak, W.A.; Ul-Islam, M.; Khan, S.; Park, J.K. Metabolic Engineering of Synthetic Cell-Free Systems: Strategies and Applications. Biochem. Eng. J. 2016, 105, 391–405. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Yoo, Y.J. Recent Progress in Nanobiocatalysis for Enzyme Immobilization and Its Application. Biotechnol. Bioprocess Eng. 2014, 19, 553–567. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization Strategies to Develop Enzymatic Biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of Protein Immobilization: Coupling Immobilization and Site-Directed Mutagenesis to Improve Biocatalyst or Biosensor Performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Peschke, T.; Skoupi, M.; Burgahn, T.; Gallus, S.; Ahmed, I.; Rabe, K.S.; Niemeyer, C.M. Self-Immobilizing Fusion Enzymes for Compartmentalized Biocatalysis. ACS Catal. 2017, 7, 7866–7872. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-Step Immobilization-Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Bilal, M.; Rizwan, K.; Gul, I.; Rasheed, T.; Iqbal, H.M.N. Smart Chemistry of Enzyme Immobilization Using Various Support Matrices—A Review. Int. J. Biol. Macromol. 2021, 190, 396–408. [Google Scholar] [CrossRef]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef] [PubMed]
- Rahim, T.N.A.T.; Abdullah, A.M.; Md Akil, H. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. 2019, 59, 589–624. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef]
- Peris, E.; Okafor, O.; Kulcinskaja, E.; Goodridge, R.; Luis, S.V.; Garcia-Verdugo, E.; O’Reilly, E.; Sans, V. Tuneable 3D Printed Bioreactors for Transaminations under Continuous-Flow. Green Chem. 2017, 19, 5345–5349. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Lin, X.; Wang, Y.; Gong, X. Fabrication of Paper-Based Enzyme Immobilized Microarray by 3D-Printing Technique for Screening α-Glucosidase Inhibitors in Mulberry Leaves and Lotus Leaves. Chin. Med. 2019, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Mittmann, E.; Wenger, L.; Hubbuch, J.; Engqvist, M.K.M.; Niemeyer, C.M.; Rabe, K.S. 3D-Printed Phenacrylate Decarboxylase Flow Reactors for the Chemoenzymatic Synthesis of 4-Hydroxystilbene. Chem. A Eur. J. 2019, 25, 15998–16001. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.M.; Silva, P.R.L.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A.A. 3D-Printed Graphene/Polylactic Acid Electrode for Bioanalysis: Biosensing of Glucose and Simultaneous Determination of Uric Acid and Nitrite in Biological Fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Covalently Modified Enzymatic 3D-Printed Bioelectrode. Microchim. Acta 2021, 188, 374. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Lv, K.; Kumissay, L.; Wu, B.; Chu, J.; He, B. Specific Immobilization of Lipase on Functionalized 3D Printing Scaffolds via Enhanced Hydrophobic Interaction for Efficient Resolution of Racemic 1-Indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. [Google Scholar] [CrossRef]
- Tan, J.J.Y.; Lee, C.P.; Hashimoto, M. Preheating of Gelatin Improves Its Printability with Transglutaminase in Direct Ink Writing 3D Printing. Int. J. Bioprint. 2020, 6, 118–129. [Google Scholar] [CrossRef]
- Wenger, L.; Radtke, C.P.; Göpper, J.; Wörner, M.; Hubbuch, J. 3D-Printable and Enzymatically Active Composite Materials Based on Hydrogel-Filled High Internal Phase Emulsions. Front. Bioeng. Biotechnol. 2020, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, S.; Chu, Y.; Yuan, B.; Tao, X.; Hu, X.; Wang, Y. An Injectable Bioink with Rapid Prototyping in the Air and In-Situ Mild Polymerization for 3D Bioprinting. Biofabrication 2021, 13, 045026. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pose-Boirazian, T.; Eibes, G.; McCoubrey, L.E.; Martínez-Costas, J.; Gaisford, S.; Goyanes, A.; Basit, A.W. A Customizable 3D Printed Device for Enzymatic Removal of Drugs in Water. Water Res. 2022, 208, 117861. [Google Scholar] [CrossRef]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. 3D–4D Printed Objects: New Bioactive Material Opportunities. Micromachines 2017, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Chai, M.; Razavi Bazaz, S.; Daiyan, R.; Razmjou, A.; Ebrahimi Warkiani, M.; Amal, R.; Chen, V. Biocatalytic Micromixer Coated with Enzyme-MOF Thin Film for CO2 Conversion to Formic Acid. Chem. Eng. J. 2021, 426, 130856. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Manzanares-Palenzuela, C.L.; Hermanova, S.; Sofer, Z.; Pumera, M. Proteinase-Sculptured 3D-Printed Graphene/Polylactic Acid Electrodes as Potential Biosensing Platforms: Towards Enzymatic Modeling of 3D-Printed Structures. Nanoscale 2019, 11, 12124–12131. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Muñoz, J.; Redondo, E.; Pumera, M. Chiral 3D-Printed Bioelectrodes. Adv. Funct. Mater. 2021, 31, 2010608. [Google Scholar] [CrossRef]
- Rewatkar, P.; Goel, S. 3D Printed Bioelectrodes for Enzymatic Biofuel Cell: Simple, Rapid, Optimized and Enhanced Approach. IEEE Trans. Nanobiosci. 2020, 19, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jayapiriya, U.S.; Goel, S. Surface Modified 3D Printed Carbon Bioelectrodes for Glucose/O2 Enzymatic Biofuel Cell: Comparison and Optimization. Sustain. Energy Technol. Assess. 2020, 42, 100811. [Google Scholar] [CrossRef]
- Gkantzou, E.; Skonta, A.; Tsakni, A.; Polydera, A.; Moschovas, D.; Spyrou, K.; Avgeropoulos, A.; Gournis, D.; Houhoula, D.; Stamatis, H. 3D Printed PLA Enzyme Microreactors: Characterization and Application for the Modification of Bioactive Compounds. J. Biotechnol. 2022, 350, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A Versatile Approach for Enzyme Immobilization Using Chemically Modified 3D-Printed Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- Dong, Y.; Min, X.; Kim, W.S. A 3-D-Printed Integrated PCB-Based Electrochemical Sensor System. IEEE Sens. J. 2018, 18, 2959–2966. [Google Scholar] [CrossRef]
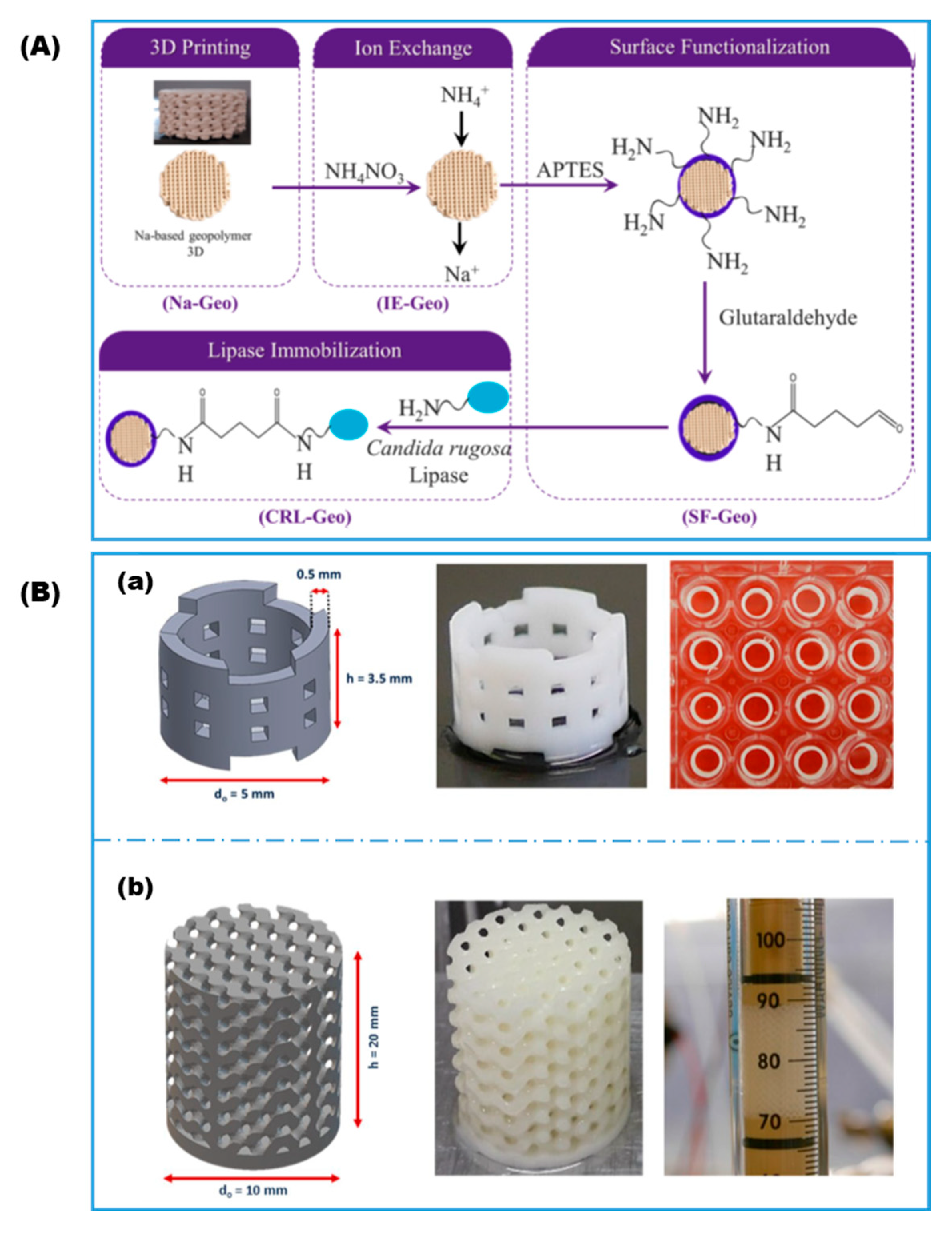
- dos Santos, L.K.; Botti, R.F.; de Mello Innocentini, M.D.; Marques, R.F.C.; Colombo, P.; de Paula, A.V.; Flumignan, D.L. 3D Printed Geopolymer: An Efficient Support for Immobilization of Candida Rugosa Lipase. Chem. Eng. J. 2021, 414, 128843. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous Monitoring of Diabetes with an Integrated Microneedle Biosensing Device through 3D Printing. Microsyst. Nanoeng. 2021, 7, 75. [Google Scholar] [CrossRef]
- Dimartino, S.; Galindo-Rodriguez, G.R.; Simon, U.; Conti, M.; Sarwar, M.S.; Athi Narayanan, S.M.; Jiang, Q.; Christofi, N. Flexible Material Formulations for 3D Printing of Ordered Porous Beds with Applications in Bioprocess Engineering. Bioresour. Bioprocess. 2022, 9, 20. [Google Scholar] [CrossRef]
- Singh, R.; Souillard, G.; Chassat, L.; Gao, Y.; Mulet, X.; Doherty, C.M. Fabricating Bioactive 3D Metal–Organic Framework Devices. Adv. Sustain. Syst. 2020, 4, 2000059. [Google Scholar] [CrossRef]
- Valotta, A.; Maier, M.C.; Soritz, S.; Pauritsch, M.; Koenig, M.; Brouczek, D.; Schwentenwein, M.; Gruber-Woelfler, H. 3D Printed Ceramics as Solid Supports for Enzyme Immobilization: An Automated DoE Approach for Applications in Continuous Flow. J. Flow Chem. 2021, 11, 675–689. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Biegert, E.; Wohlgemuth, J.; Wagner, H.; Franzreb, M. A 3D-Printed Modular Reactor Setup Including Temperature and PH Control for the Compartmentalized Implementation of Enzyme Cascades. Eng. Life Sci. 2016, 16, 560–567. [Google Scholar] [CrossRef]
- Shen, J.; Nada, A.A.; Abou-Zeid, N.Y.; Hudson, S.M. Synthesis of Chitosan Iodoacetamides via Carbodiimide Coupling Reaction: Effect of Degree of Substitution on the Hemostatic Properties. Carbohydr. Polym. 2020, 229, 115522. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-Organic Frameworks with Different Dimensionalities: An Ideal Host Platform for Enzyme@MOF Composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Maier, M.; Radtke, C.P.; Hubbuch, J.; Niemeyer, C.M.; Rabe, K.S. On-Demand Production of Flow-Reactor Cartridges by 3D Printing of Thermostable Enzymes. Angew. Chem. Int. Ed. 2018, 57, 5539–5543. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, W.; Liu, M.; Wu, Q.; Cheng, L.; Wang, Q. Viscosity-Controlled Printing of Supramolecular-Polymeric Hydrogels: Via Dual-Enzyme Catalysis. J. Mater. Chem. B 2016, 4, 6302–6306. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, M.; Liao, C.; Wu, Q.; Liu, M.; Zhang, Y.; Wu, C.; Cheng, L.; Wang, Q. Printable Hybrid Hydrogel by Dual Enzymatic Polymerization with Superactivity. Chem. Sci. 2016, 7, 2748–2752. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shen, X.; Zheng, Z.; Li, M.; Zhu, X.; Cao, H.; Cui, C. Immobilization of Laccase by 3D Bioprinting and Its Application in the Biodegradation of Phenolic Compounds. Int. J. Biol. Macromol. 2020, 164, 518–525. [Google Scholar] [CrossRef]
- Jiang, W.; Pei, R.; Zhou, S.F. 3D-Printed Xylanase within Biocompatible Polymers as Excellent Catalyst for Lignocellulose Degradation. Chem. Eng. J. 2020, 400, 125920. [Google Scholar] [CrossRef]
- Pei, R.; Jiang, W.; Fu, X.; Tian, L.; Zhou, S.F. 3D-Printed Aldo-Keto Reductase within Biocompatible Polymers as Catalyst for Chiral Drug Intermediate. Chem. Eng. J. 2022, 429, 132293. [Google Scholar] [CrossRef]
- Yang, C.; Zheng, Z.; Younis, M.R.; Dong, C.; Chen, Y.; Lei, S.; Zhang, D.Y.; Wu, J.; Wu, X.; Lin, J.; et al. 3D Printed Enzyme-Functionalized Scaffold Facilitates Diabetic Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2101372. [Google Scholar] [CrossRef]
- Zhou, M.; Lee, B.H.; Tan, Y.J.; Tan, L.P. Microbial Transglutaminase Induced Controlled Crosslinking of Gelatin Methacryloyl to Tailor Rheological Properties for 3D Printing. Biofabrication 2019, 11, 025011. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liang, X.; Zhang, P.; Lin, S.; Cai, C.; Yu, Z.; Liu, J. Bioinspired 3D Printing of Functional Materials by Harnessing Enzyme-Induced Biomineralization. Adv. Funct. Mater. 2022, 2113262. [Google Scholar] [CrossRef]
- Heichel, D.L.; Tumbic, J.A.; Boch, M.E.; Ma, A.W.K.; Burke, K.A. Silk Fibroin Reactive Inks for 3D Printing Crypt-like Structures. Biomed. Mater. 2020, 15, 055037. [Google Scholar] [CrossRef]
- Shen, S.; Shen, J.; Shen, H.; Wu, C.; Chen, P.; Wang, Q. Dual-Enzyme Crosslinking and Post-Polymerization for Printing of Polysaccharide-Polymer Hydrogel. Front. Chem. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, H.; Delporte, C.; Bernaerts, K.V.; Alimoradi, H.; Nie, L.; Podstawczyk, D.; Tam, K.C.; Shavandi, A. Synergistic Complexation of Phenol Functionalized Polymer Induced in Situ Microfiber Formation for 3D Printing of Marine-Based Hydrogels. Green Chem. 2022, 24, 2409–2422. [Google Scholar] [CrossRef]
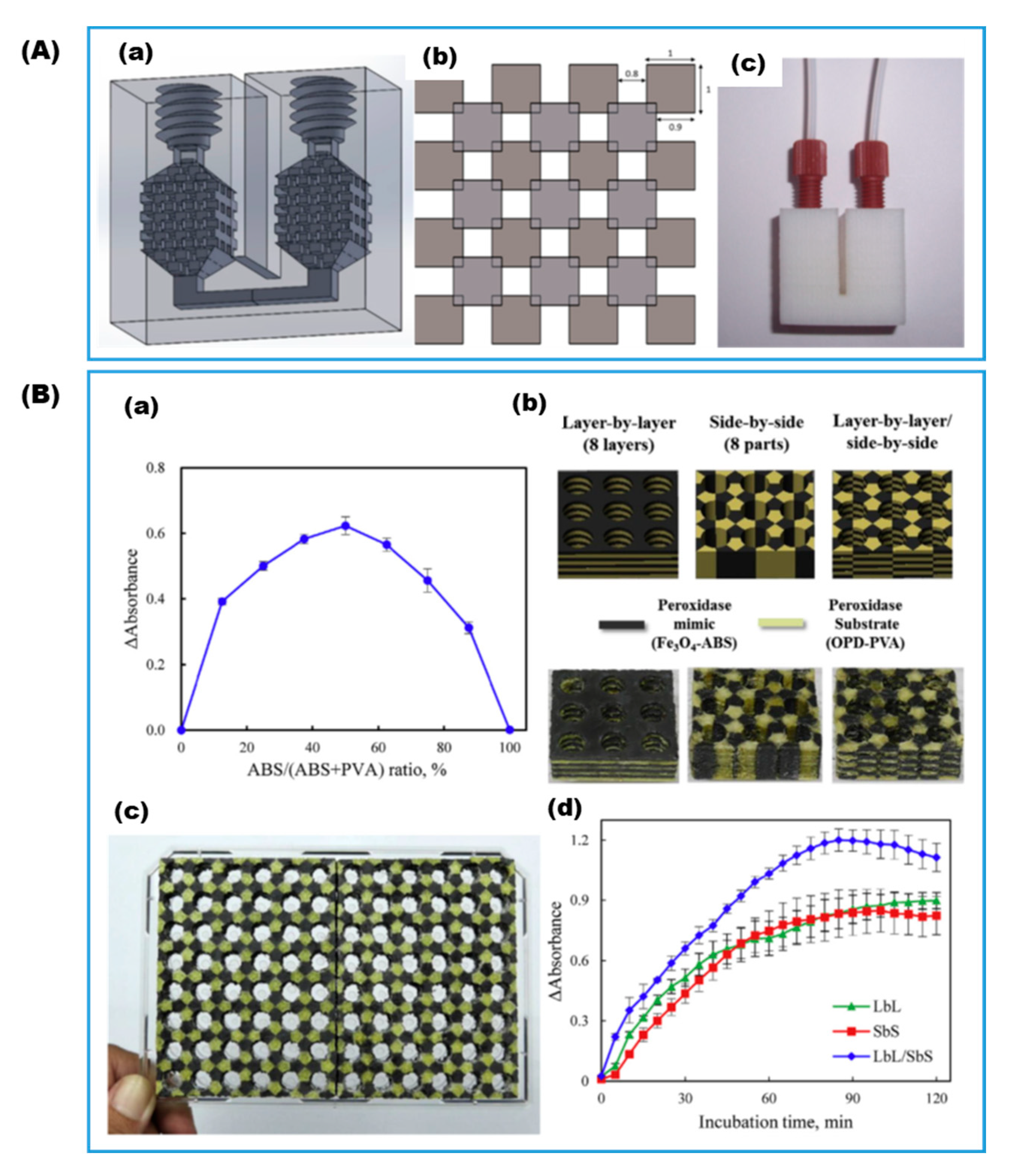
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. Adding Biomolecular Recognition Capability to 3D Printed Objects. Anal. Chem. 2016, 88, 10767–10772. [Google Scholar] [CrossRef] [PubMed]
- Devillard, C.D.; Mandon, C.A.; Lambert, S.A.; Blum, L.J.; Marquette, C.A. Bioinspired Multi-Activities 4D Printing Objects: A New Approach Toward Complex Tissue Engineering. Biotechnol. J. 2018, 13, 1800098. [Google Scholar] [CrossRef]
- Steier, A.; Schmieg, B.; Irtel von Brenndorff, Y.; Meier, M.; Nirschl, H.; Franzreb, M.; Lahann, J. Enzyme Scaffolds with Hierarchically Defined Properties via 3D Jet Writing. Macromol. Biosci. 2020, 20, 2000154. [Google Scholar] [CrossRef]
- Zhang, W.H.; Day, G.J.; Zampetakis, I.; Carrabba, M.; Zhang, Z.; Carter, B.M.; Govan, N.; Jackson, C.; Chen, M.; Perriman, A.W. Three-Dimensional Printable Enzymatically Active Plastics. ACS Appl. Polym. Mater. 2021, 3, 6070–6077. [Google Scholar] [CrossRef]
- Greene, A.F.; Vaidya, A.; Collet, C.; Wade, K.R.; Patel, M.; Gaugler, M.; West, M.; Petcu, M.; Parker, K. 3D-Printed Enzyme-Embedded Plastics. Biomacromolecules 2021, 22, 1999–2009. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Gelation Mechanism of Agarose. Carbohydr. Res. 1988, 180, 277–284. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-Regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Schmieg, B.; Nguyen, M.; Franzreb, M. Simulative Minimization of Mass Transfer Limitations Within Hydrogel-Based 3D-Printed Enzyme Carriers. Front. Bioeng. Biotechnol. 2020, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, B.; Qiao, B.; Jiang, T.; DelRe, C.; Obadia, M.M.; Nguyen, T.D.; Smith, A.A.A.; Hall, A.; Sit, I.; Crosby, M.G.; et al. Random Heteropolymers Preserve Protein Function in Foreign Environments. Science 2018, 359, 1239–1243. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; DelRe, C.; Kang, P.; Hall, A.; Arnold, D.; Jayapurna, I.; Ma, L.; Michalek, M.; Ritchie, R.O.; Xu, T. Conductive Ink with Circular Life Cycle for Printed Electronics. Adv. Mater. 2022, e2202177. [Google Scholar] [CrossRef]
- Spano, M.B.; Tran, B.H.; Majumdar, S.; Weiss, G.A. 3D-Printed Labware for High-Throughput Immobilization of Enzymes. J. Org. Chem. 2020, 85, 8480–8488. [Google Scholar] [CrossRef]
- Rainer, T.; Egger, A.-S.; Zeindl, R.; Tollinger, M.; Kwiatkowski, M.; Müller, T. 3D-Printed High-Pressure-Resistant Immobilized Enzyme Microreactor (ΜIMER) for Protein Analysis. Anal. Chem. 2022, 94, 8580–8587. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [Green Version]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G.; Samsom, R.A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, 2110054. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.; Modica, J.A.; Roll, J.; Perkovich, P.; Mrksich, M. An Immobilized Enzyme Reactor for Spatiotemporal Control over Reaction Products. Small 2018, 14, 1800923. [Google Scholar] [CrossRef] [PubMed]
- Narupai, B.; Smith, P.T.; Nelson, A. 4D Printing of Multi-Stimuli Responsive Protein-Based Hydrogels for Autonomous Shape Transformations. Adv. Funct. Mater. 2021, 31, 2011012. [Google Scholar] [CrossRef]
- Tasoglu, S.; Folch, A. Editorial for the Special Issue on 3D Printed Microfluidic Devices. Micromachines 2018, 9, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed, Dual Crosslinked and Sterile Aerogel Scaffolds for Bone Tissue Engineering. Polymers 2022, 14, 1211. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The Use of Bacterial Polysaccharides in Bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef]


| Method | Free Enzyme | Conventional Immobilization | 3D Printed Immobilization | |
|---|---|---|---|---|
| Post-Printing | Entrapment | |||
| Advantages | Ready-to-use; high selectivity; easily mixed; high mass transfer; mild operational conditions | High stability; reusable; some continuous flow geometries; large variety of material options; constant innovation in the field | High stability; reusable; continuous flow; customizable complex structure; scalable and low material waste; applicable for all 3D printing methods; wide variety of printable materials | High resolution; precise enzyme placement; high stability; reusable; continuous flow; customizable complex structure; scalable and low material waste; single step fabrication; mild immobilization conditions; versatile for multi-enzyme assemblies or cascades |
| Challenges | Low stability; frequent replenishment; non-recyclable | Difficult to form complex structures; may require separate recycling steps; mass transfer limits; harsh chemicals; limited mechanical properties | Harsh chemicals for surface modification; time-consuming multi-step processes; difficult for enzyme cascade | Limited material types; limited mechanical properties; mass transfer limits |
| Future development | Protein engineering for stabilization and surface functionalization | More sustainable materials; milder chemical reactions; more efficient geometries | More sustainable materials; milder chemical reactions; combine advancements across conventional enzyme immobilization and 3D printing fields | New bioink materials; new mechanical reinforcement methods; highly controlled geometries; ultra-high (nano-scale) resolution |
| 3D Printing Technique | Immobilization Mechanism | Surface Materials | Reagents | Enzymes | Application | Name, Year, and Ref. |
|---|---|---|---|---|---|---|
| FDM | Covalent attachment; drop-casting | ABS | GA | GOx; LOx | Biosensing | Su and Sun, 2016, 2018 [20,22] |
| Covalent attachment | Nylon | GA | ω-transaminase | Biocatalytic reactors | Sans, 2017 [37] | |
| Physical adsorption; covalent attachment | Graphene/PLA; Carbon black/PLA | EDC/NHS | ALP; HRP; GOx; L-amino acid oxidase | Biosensing | Pumera, 2019–2021 [41,50,51,52] | |
| Drop-casting | Graphene/PLA | GA | GOx | Biosensing | Muñoz, 2020 [40] | |
| Physical adsorption; covalent attachment | Carbon/PLA | EDC/NHS | GOx; Laccase | Biofuel cell | Goel, 2020 [53,54] | |
| Physical adsorption | PLA | Aniline silane | Lipase | Biocatalytic reactors | Chu and He, 2021 [42] | |
| Covalent attachment | PLA | Chitosan coating with GA | Laccase | Biocatalytic reactors | Stamatis, 2022 [55] | |
| Covalent attachment; physical adsorption | Carbon-fiber reinforced PLA | Various silane coupling agents; GA | PGA; protease; glycosidase; lipase | Biocatalytic reactors | Gao and He, 2019 [56] | |
| DIW | Drop-casting | Nafion | BSA | Lactate oxidase | Biosensing | Kim, 2018 [57] |
| Covalent attachment | Na-based geopolymer | Aminosilane; GA | Lipase | Biocatalytic reactors | dos Santos, 2021 [58] | |
| DLP | Drop-casting | Gold | GA | GOx | Biosensing | Yang and Cui, 2021 [59] |
| Covalent attachment | Poly(2-carboxyethyl acrylate) | EDC | Trypsin | Biocatalytic reactor | Dimartino, 2022 [60] | |
| Encapsulation in ZIF grown on surface | ABS; BSA | ZIF precursor | OpdA | Wastewater remediation; Industrial biocatalysis | Doherty, 2020 [61] | |
| SLA | Covalent attachment | Ceramic | Aminosilane; GA or EDC/NHS | Phenolic acid Decarboxylase | Biocatalytic reactors | Valotta and Gruber-Woelfler, 2021 [62] |
| Encapsulation in ZIF grown on surface | PD/PEI | ZIF precursor | CA; FDH | Biocatalytic reactors | Razmjou, 2021 [48] | |
| Polyjet | Covalent attachment | Poly(acrylic acid) | EDC | GOx; HRP | Biocatalytic reactors | Franzreb, 2016 [63] |
| 3D Printing Technique | Materials | Gelation Mechanism | Enzymes | Application | Name, Year, and Ref. |
|---|---|---|---|---|---|
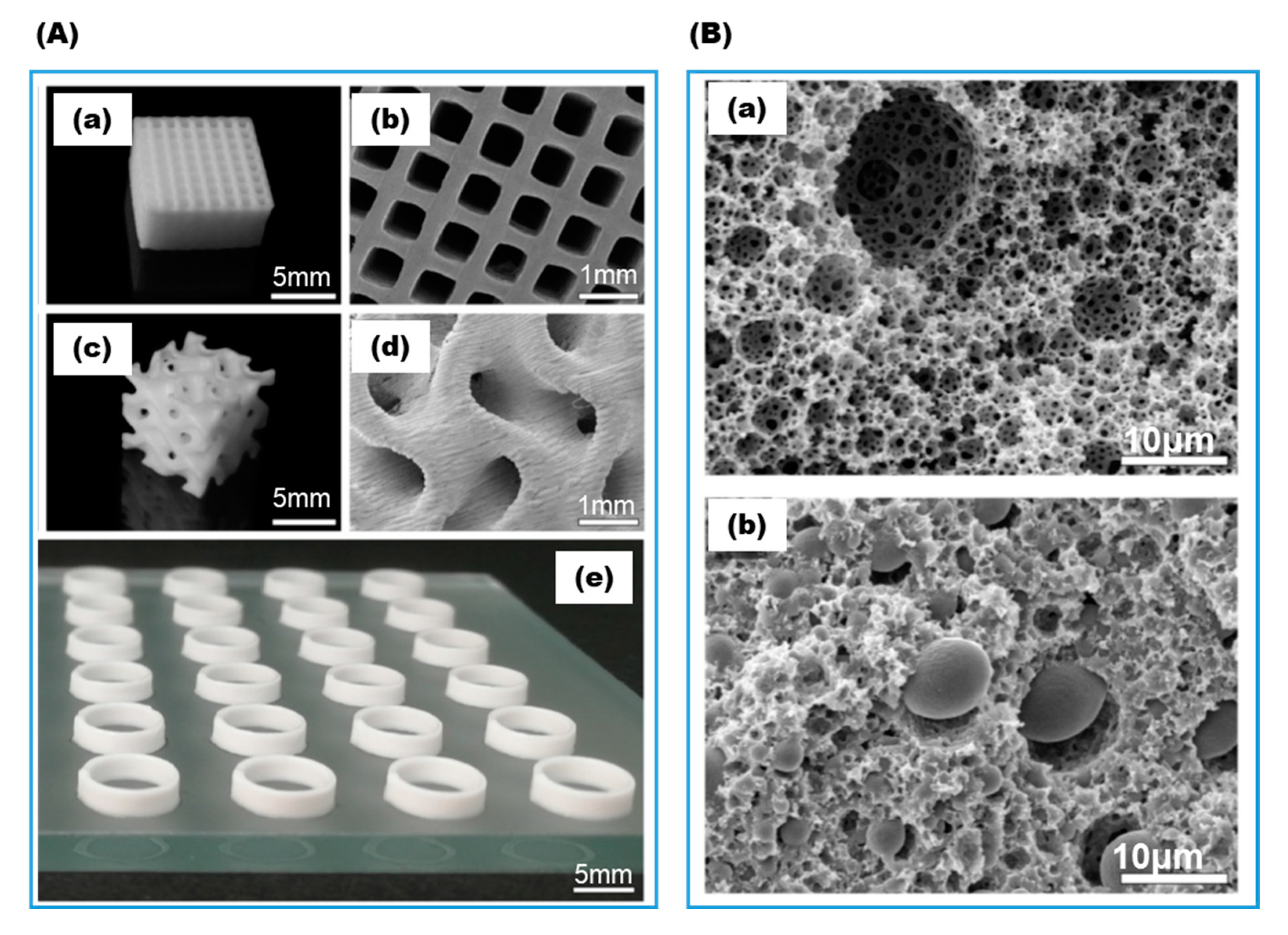
| PEG-DA hydrogel; Colloidal clay and branched polysaccharide as viscosity enhancer | Photo-crosslinking | ADH; BFD; β-Gal | Biocatalytic reactor | Franzreb, 2018, 2019 [19,24] | |
| Thermoreversible agarose-based hydrogel | Temperature-induced | Esterase; ADH; decarboxylase | Biocatalytic reactor cartridge | Rabe, 2018, 2019 [39,66] | |
| PAA/PEG-DA hydrogel spheres enclosed in hydrophobic acrylates matrix | Photo-crosslinking | β-Gal | Biocatalytic reactors; rapid screening of immobilization formulation | Hubbuch, 2020 [44] | |
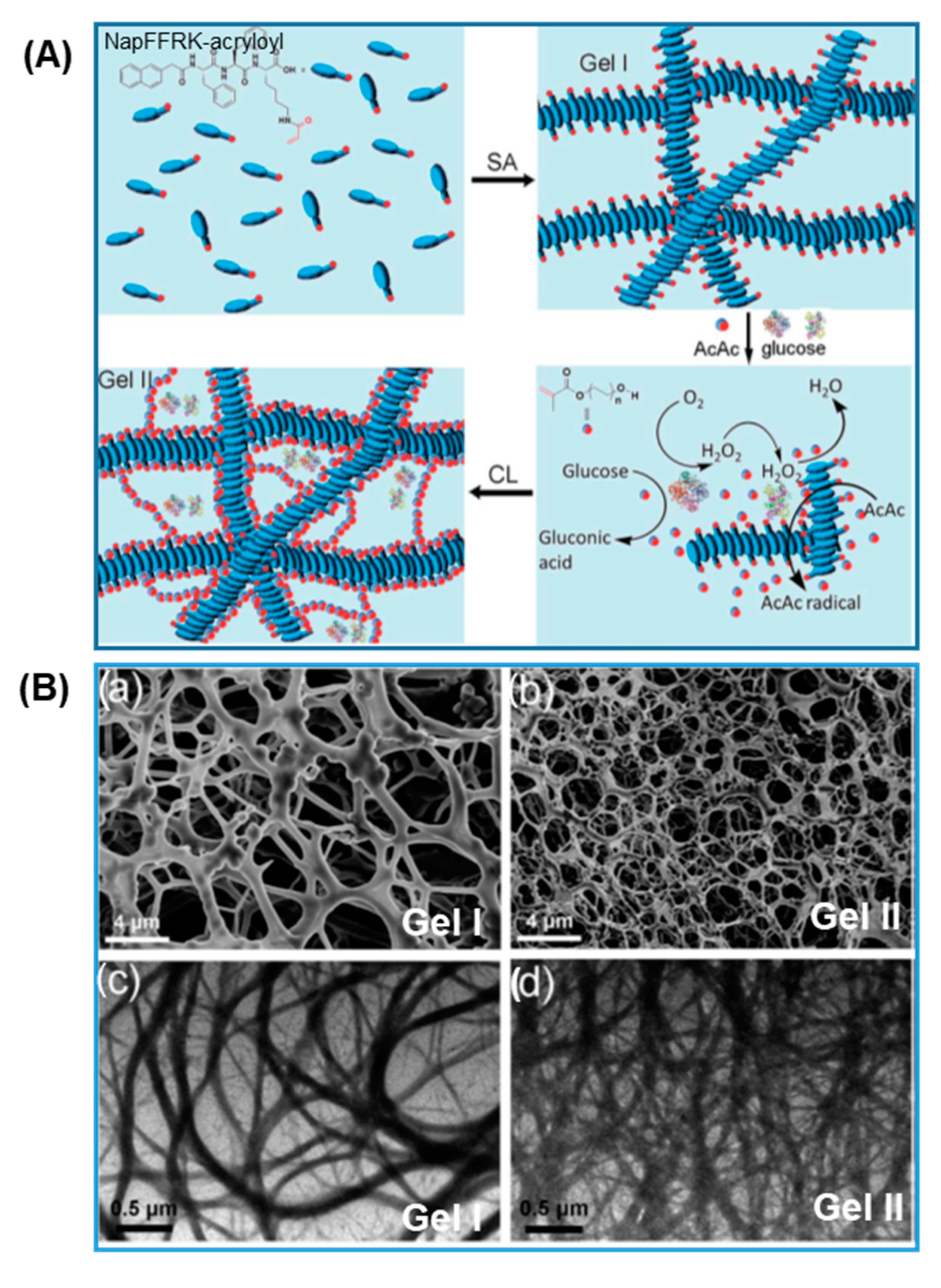
| NapFFRK-acryloyl and PEG-MA hydrogel | Enzyme-triggered pH change and radical polymerization | GOx; HRP | 3D cell culture; tissue engineering | Wang, 2016 [67,68] | |
| SA/PAm/hydroxyapatite hybrid interpenetrating polymer network (HIPN) hydrogel | Electrostatic; chain entanglement | GOx; CAT; Laccase | Biocatalytic reactors; wastewater remediation | Cui and Cao, 2019, 2020 [23,69] | |
| DIW | SA hydrogel | Electrostatic | Xylanase; Aldo-keto reductase | Biocatalytic reactors | Jiang and Zhou, 2020, 2022 [70,71] |
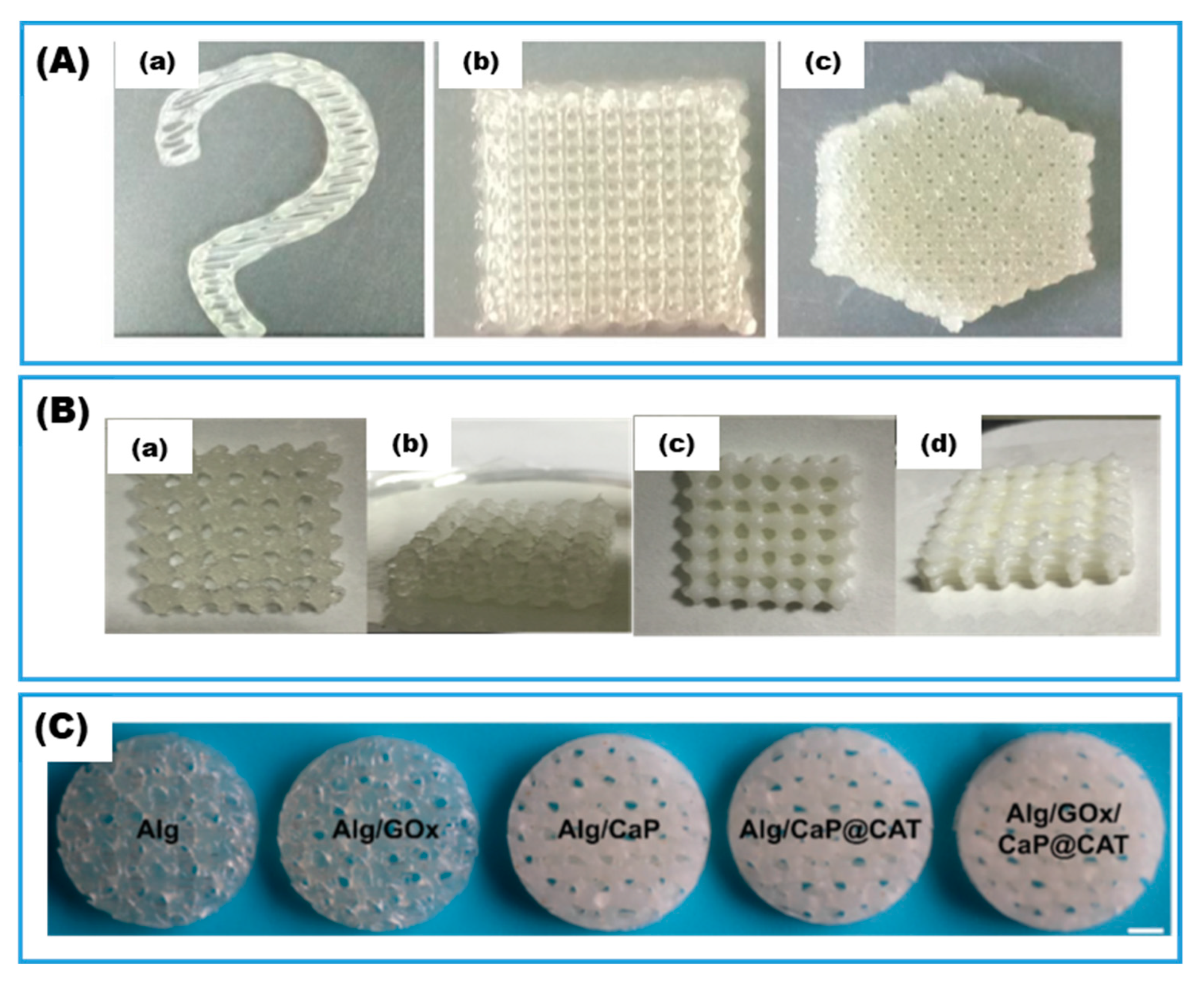
| SA hydrogel reinforced by calcium phosphate nanosheets | Electrostatic | GOx; CAT | Bone tissue engineering | Wang and Huang, 2021 [72] | |
| Gelatin-based hydrogel | Enzymatic crosslinking | TGase | Bioprinting; food printing | Hashimoto, 2020 [43] | |
| Gelatin-based hydrogel/bacterial cellulose | Enzymatic crosslinking | TGase | Bioprinting; tissue engineering | Hu and Wang, 2021 [45] | |
| Gelatin methacryloyl (GelMA) hydrogel | Enzymatic crosslinking; photo-crosslinking | TGase | Bioprinting; tissue engineering | Lee and Tan, 2019 [73] | |
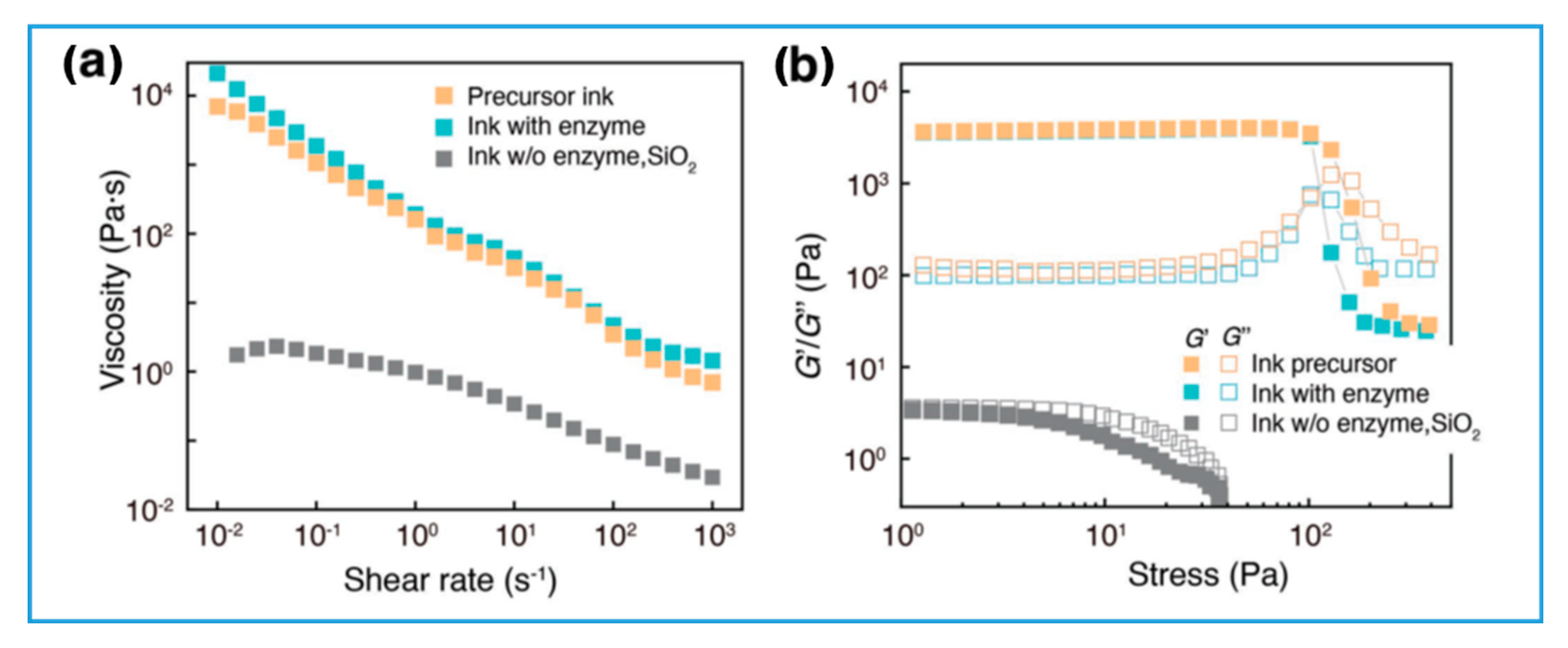
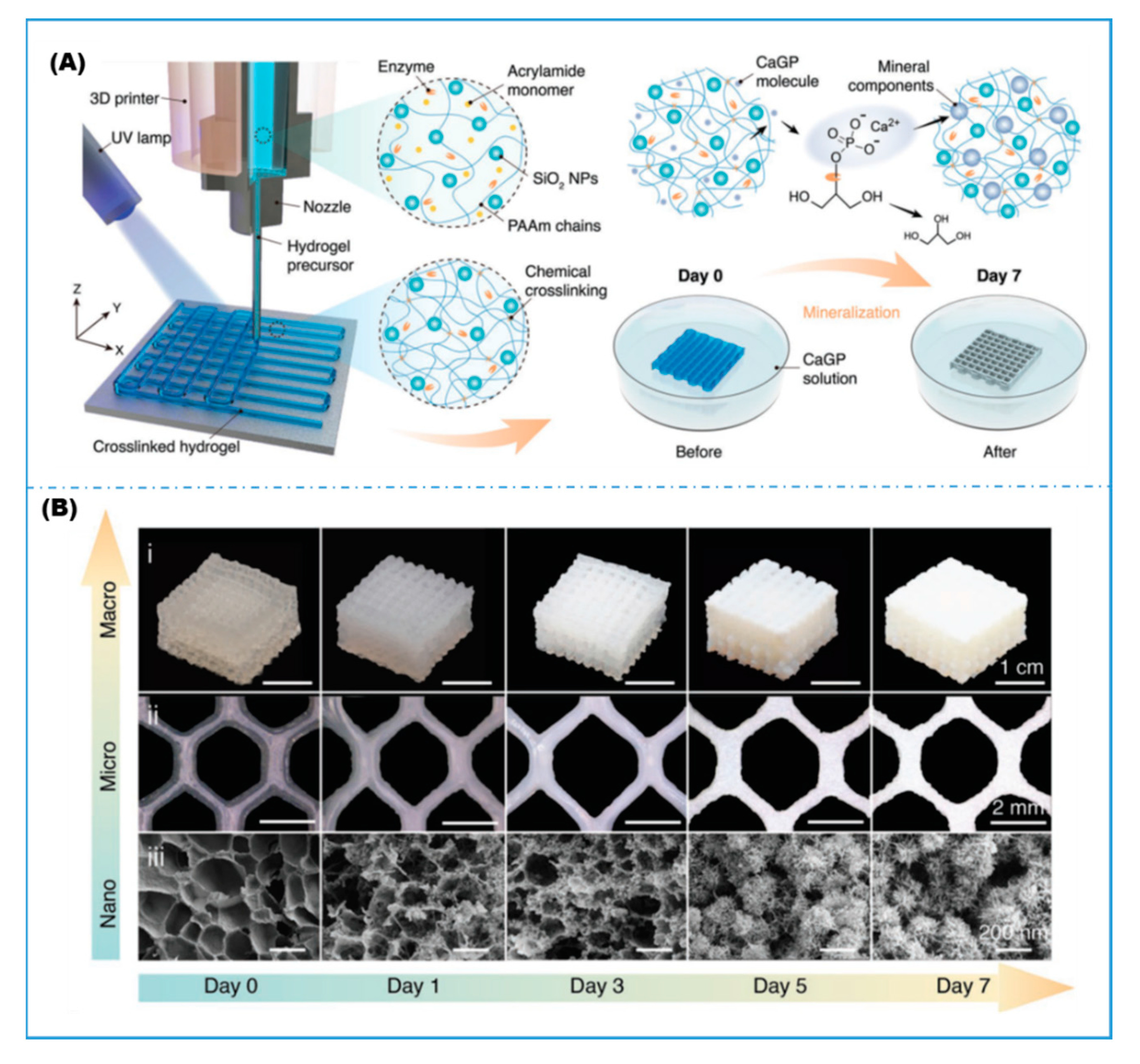
| PAm hydrogel/fused silica nanoparticles | Photo-crosslinking | ALP | Biomineralization; biomedical | Liu, 2022 [74] | |
| Silk fibroin hydrogel | Enzymatic crosslinking (dityrosine bond) | HRP | Bioprinting; tissue engineering | Burke, 2020 [75] | |
| Polysaccharide derivative/PAm hydrogel | Enzymatic crosslinking (phenolic); enzyme-initiated radical polymerization | GOx; HRP | Bioprinting; tissue engineering | Wang, 2020 [76] | |
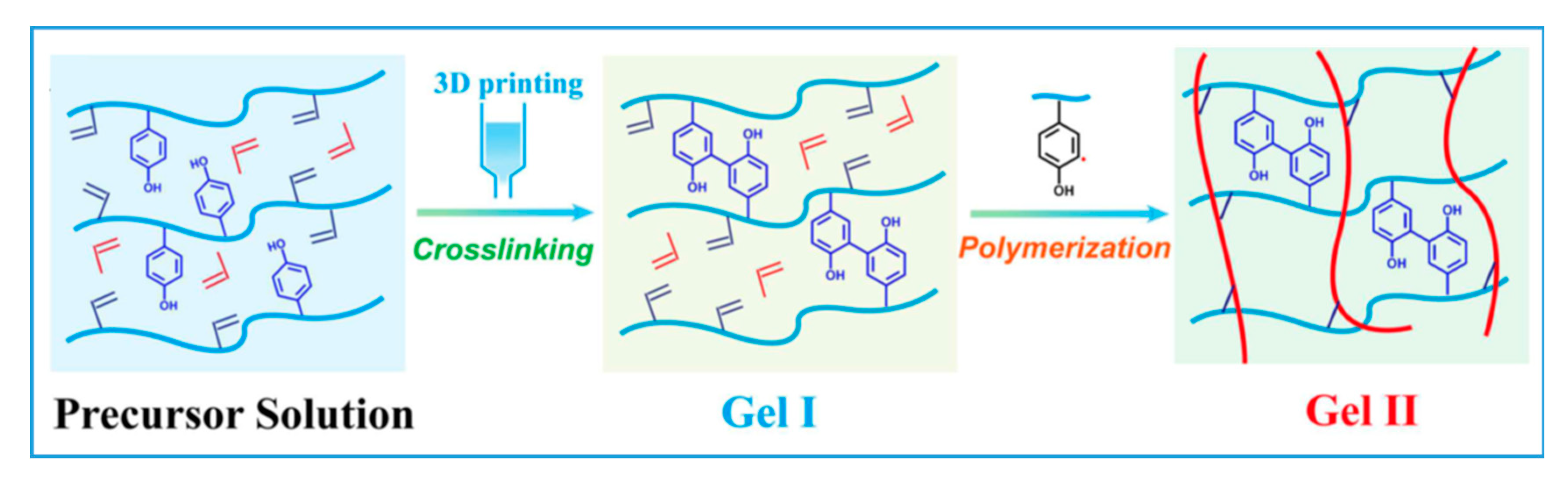
| Phenol functionalized chitosan and alginate hydrogel | Electrostatic; enzymatic crosslinking (Phenolic) | HRP | Bioprinting; tissue engineering | Shavandi, 2022 [77] | |
| DLP | PEG-DA hydrogel | Photo-crosslinking | GOx/HRP; ALP; thrombin | Biosensing; tissue engineering; biomineralization | Marquette, 2016, 2017 [47,78,79] |
| SLA | PEG-DA hydrogel | Photo-crosslinking | Laccase | Water remediation | Goyanes and Basit, 2022 [46] |
| 3DJW | PEG-DA hydrogel/PAA | Photo-crosslinking | β-Gal | Biocatalytic reactors | Lahann, 2020 [80] |
| MEW | PCL | N/A (Solidification) | PTE; sfGFP | Self- decontaminating surfaces; fully biodegradable fluorescent plastic | Perriman, 2021 [81] |
| FDM | PCL | N/A (Solidification) | Lipase | Biodegradable plastics | Greene, 2021 [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels 2022, 8, 460. https://doi.org/10.3390/gels8080460
Shen J, Zhang S, Fang X, Salmon S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels. 2022; 8(8):460. https://doi.org/10.3390/gels8080460
Chicago/Turabian StyleShen, Jialong, Sen Zhang, Xiaomeng Fang, and Sonja Salmon. 2022. "Advances in 3D Gel Printing for Enzyme Immobilization" Gels 8, no. 8: 460. https://doi.org/10.3390/gels8080460






