Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
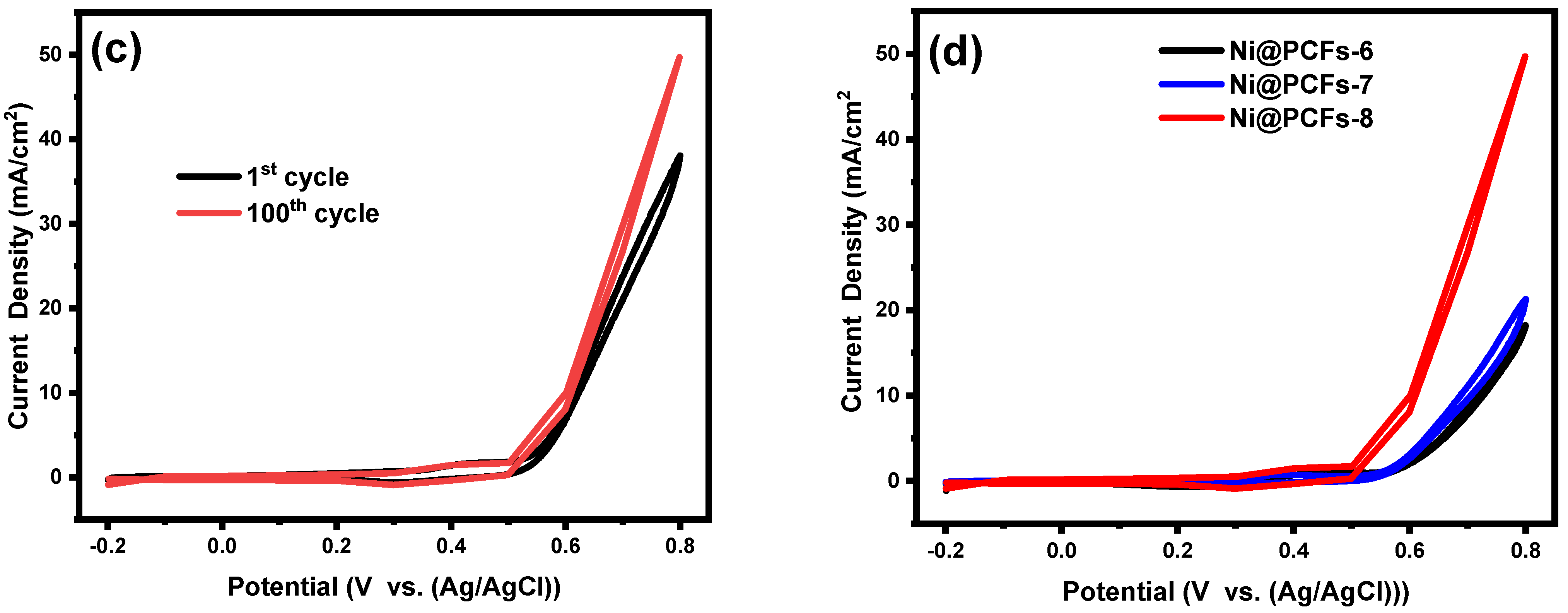
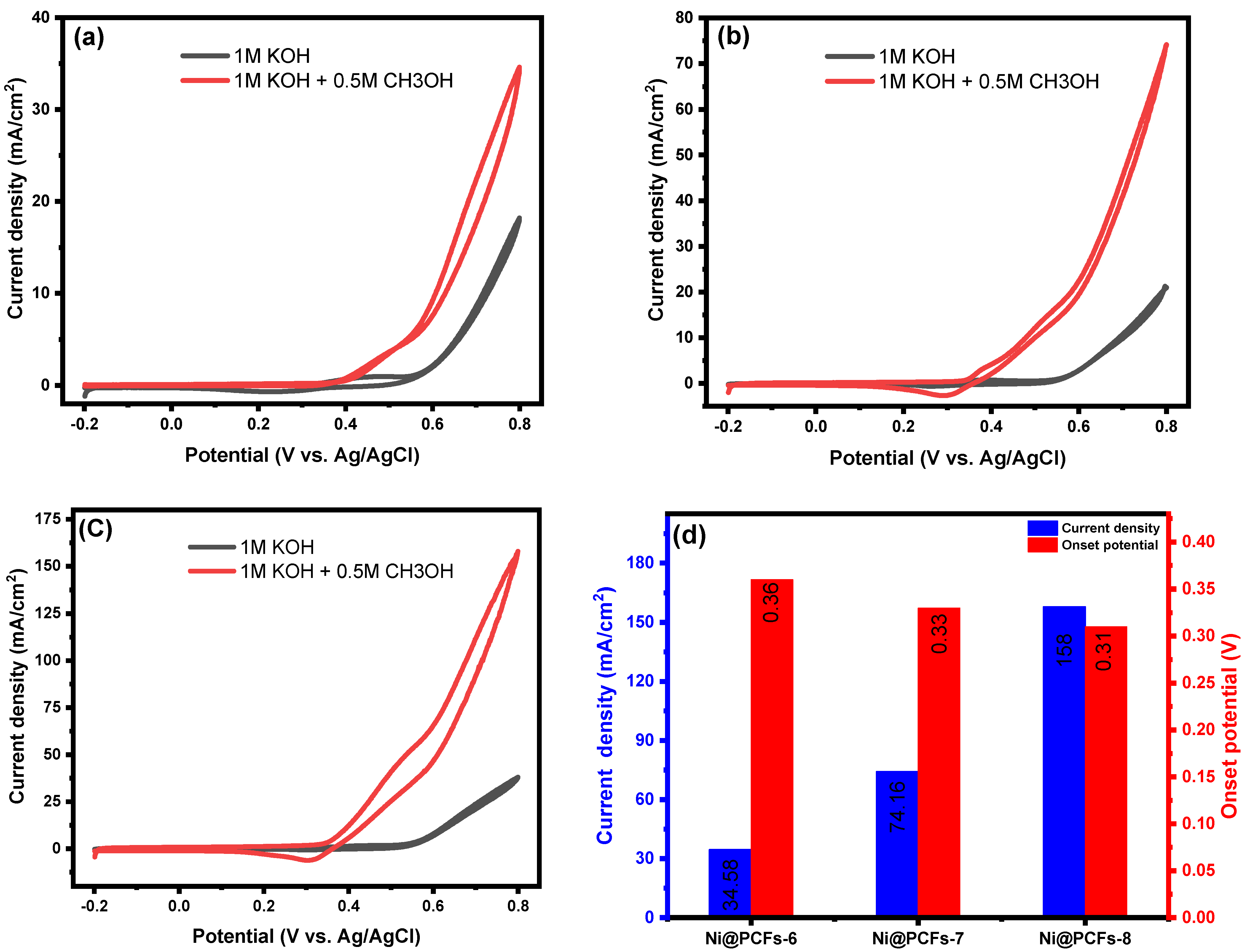
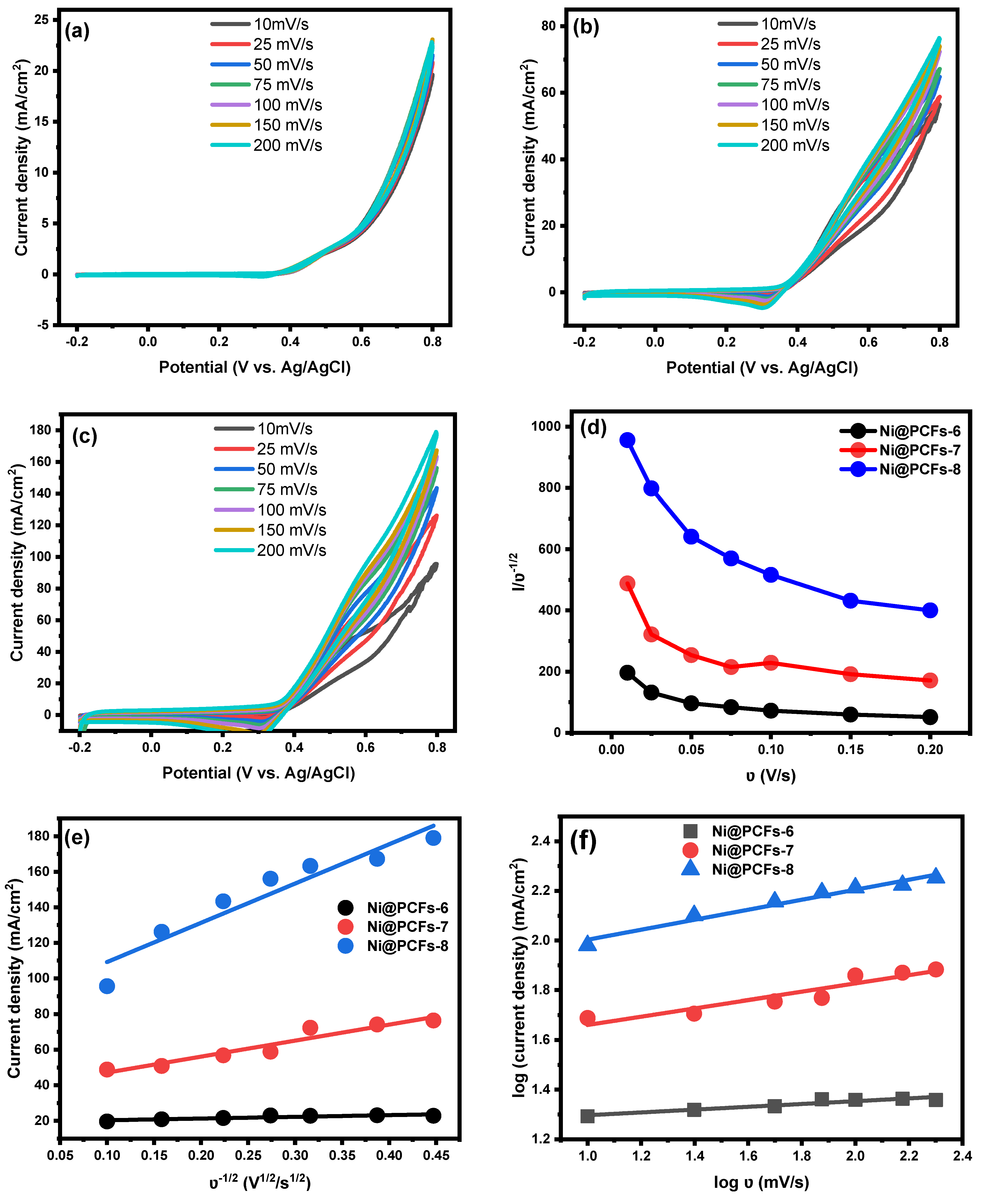
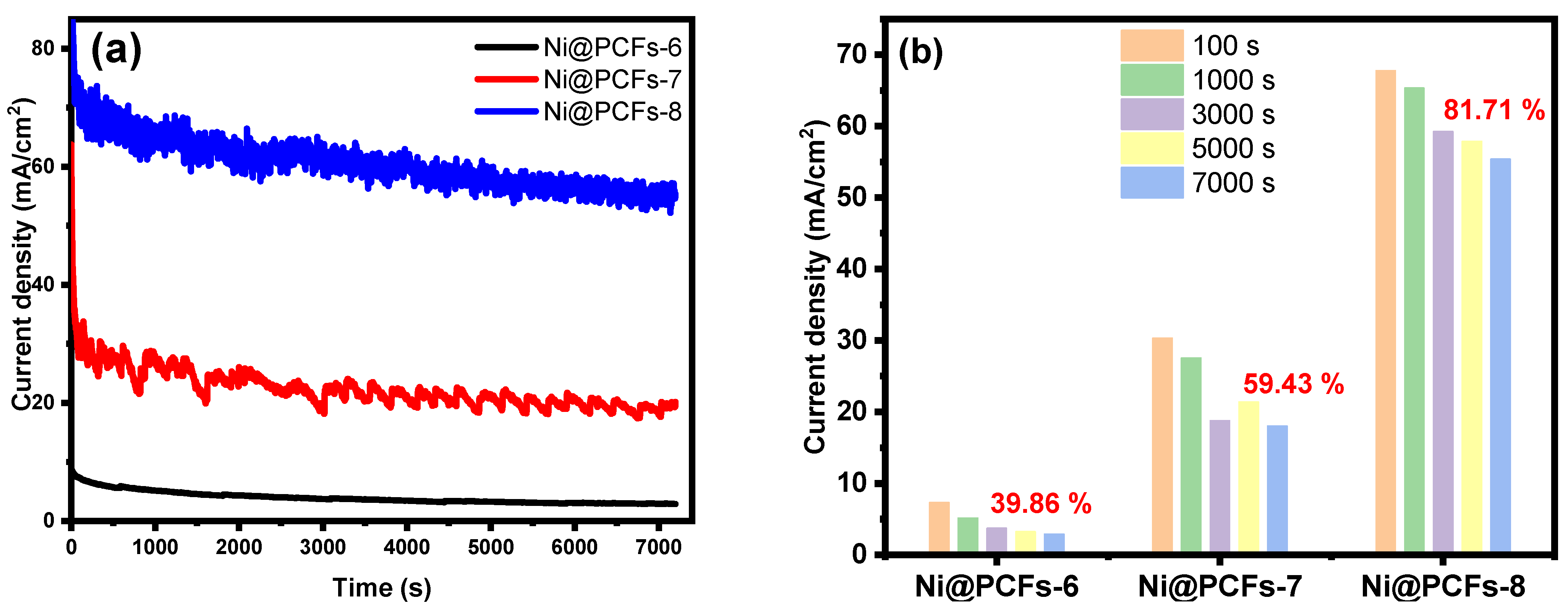
2.2. Electrocatalytic Activity of the Ni@PCFs Catalyst
3. Conclusions
4. Materials and Method
4.1. Materials
4.2. Synthesis of Hydrogel Nanocomposites
4.3. Synthesis of Ni@PCFs Catalyst
4.4. Catalyst Evaluation
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Seyboth, K.; Kadner, S.; Zwickel, T.; Eickemeier, P.; Hansen, G.; Schlömer, S.; von Stechow, C.; et al. Renewable Energy Sources and Climate Change Mitigation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Xia, Z.; Xu, X.; Zhang, X.; Li, H.; Wang, S.; Sun, G. Anodic engineering towards high-performance direct methanol fuel cells with non-precious-metal cathode catalysts. J. Mater. Chem. A 2020, 8, 1113–1119. [Google Scholar] [CrossRef]
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble Metal Based Electrocatalysts for Alcohol Oxidation Reactions in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. In-situ synthesis of Ni/N-doped CNFs-supported graphite disk as effective immobilized catalyst for methanol electrooxidation. Int. J. Hydrogen Energy 2015, 40, 14845–14856. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. Influence of Nitrogen doping on the Catalytic Activity of Ni-incorporated Carbon Nanofibers for Alkaline Direct Methanol Fuel Cells. Electrochim. Acta 2014, 142, 228–239. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel Hameed, R.M.; Al-Enizi, A.M.; Thamer, B.M.; Yousef, A.; El-Newehy, M.H. Decorated carbon nanofibers with mixed nickel−manganese carbides for methanol electro-oxidation in alkaline solution. Int. J. Hydrogen Energy 2021, 46, 6494–6512. [Google Scholar] [CrossRef]
- Huang, L.; Wei, M.; Hu, N.; Tsiakaras, P.; Kang Shen, P. Molybdenum-modified and vertex-reinforced quaternary hexapod nano-skeletons as efficient electrocatalysts for methanol oxidation and oxygen reduction reaction. Appl. Catal. B Environ. 2019, 258, 117974. [Google Scholar] [CrossRef]
- Chen, G.; Pan, Y.; Lu, T.; Wang, N.; Li, X. Highly catalytical performance of nanoporous copper for electro-oxidation of methanol in alkaline media. Mater. Chem. Phys. 2018, 218, 108–115. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Abdelkareem, M.A.; Kim, H.Y.; Barakat, N.A.M. Cobalt-incorporated, nitrogen-doped carbon nanofibers as effective non-precious catalyst for methanol electrooxidation in alkaline medium. Appl. Catal. A Gen. 2015, 498, 230–240. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Al-Deyab, S.S.; Kim, H.Y. Preparation of zero-valent Co/N-CNFs as an immobilized thin film onto graphite disc for methanol electrooxidation. Fibers Polym. 2017, 18, 696–705. [Google Scholar] [CrossRef]
- Wu, N.; Zhai, M.; Chen, F.; Zhang, X.; Guo, R.; Hu, T.; Ma, M. Nickel nanocrystal/nitrogen-doped carbon composites as efficient and carbon monoxide-resistant electrocatalysts for methanol oxidation reactions. Nanoscale 2020, 12, 21687–21694. [Google Scholar] [CrossRef]
- Dubale, A.A.; Zheng, Y.; Wang, H.; Hübner, R.; Li, Y.; Yang, J.; Zhang, J.; Sethi, N.K.; He, L.; Zheng, Z.; et al. High-Performance Bismuth-Doped Nickel Aerogel Electrocatalyst for the Methanol Oxidation Reaction. Angew. Chemie Int. Ed. 2020, 59, 13891–13899. [Google Scholar] [CrossRef]
- Sun, H.; Ye, Y.; Liu, J.; Tian, Z.; Cai, Y.; Li, P.; Liang, C. Pure Ni nanocrystallines anchored on rGO present ultrahigh electrocatalytic activity and stability in methanol oxidation. Chem. Commun. 2018, 54, 1563–1566. [Google Scholar] [CrossRef]
- Javan, H.; Asghari, E.; Ashassi-Sorkhabi, H.; Moradi-Haghighi, M. Nickel nanoparticles decorated on carbon quantum dots as a novel non-platinum catalyst for methanol oxidation; a green, low-cost, electrochemically-synthesized electrocatalyst. Chem. Eng. Sci. 2020, 217, 115534. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q.; Hou, H.; Wu, Y.; Yu, W.; Ji, X.; Shao, L. Nickel nanoparticles supported on nitrogen-doped honeycomb-like carbon frameworks for effective methanol oxidation. RSC Adv. 2017, 7, 14152–14158. [Google Scholar] [CrossRef] [Green Version]
- Chalgin, A.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Wu, J. Effect of supporting materials on the electrocatalytic activity, stability and selectivity of noble metal-based catalysts for oxygen reduction and hydrogen evolution reactions. Prog. Nat. Sci. Mater. Int. 2020, 30, 289–297. [Google Scholar] [CrossRef]
- Hayden, B.E. Particle Size and Support Effects in Electrocatalysis. Acc. Chem. Res. 2013, 46, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, S.; McBreen, J. Effect of particle size on the electrocatalysis by carbon-supported Pt electrocatalysts: An in situ XAS investigation. J. Electroanal. Chem. 1998, 448, 163–171. [Google Scholar] [CrossRef]
- Chen, S.; Min, X.; Zhao, Y.; Wu, X.; Zhang, D.; Hou, X.; Wu, X.; Liu, Y.; Huang, Z.; Abdelkader, A.M.; et al. Nickel Quantum Dots Anchored in Biomass-Derived Nitrogen-Doped Carbon as Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. Interfaces 2022, 9, 2102014. [Google Scholar] [CrossRef]
- Ye, X.R.; Lin, Y.; Wang, C.; Engelhard, M.H.; Wang, Y.; Wai, C.M. Supercritical fluid synthesis and characterization of catalytic metal nanoparticles on carbon nanotubes. J. Mater. Chem. 2004, 14, 908–913. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 1–6. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Richardson, Y.; Motuzas, J.; Julbe, A.; Volle, G.; Blin, J. Catalytic investigation of in situ generated Ni metal nanoparticles for tar conversion during biomass pyrolysis. J. Phys. Chem. C 2013, 117, 23812–23831. [Google Scholar] [CrossRef]
- Eibner, S.; Broust, F.; Blin, J.; Julbe, A. Catalytic effect of metal nitrate salts during pyrolysis of impregnated biomass. J. Anal. Appl. Pyrolysis 2015, 113, 143–152. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, F.; Lang, Q.; Liu, T.; Peng, N.; Liu, Z. Facile one-pot synthesis of iron nanoparticles immobilized into the porous hydrochar for catalytic decomposition of phenol. Appl. Catal. B Environ. 2017, 204, 566–576. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y. Fabrication strategies of Ni-based catalysts in reforming of biomass tar/tar model compounds. Appl. Energy Combust. Sci. 2022, 9, 100053. [Google Scholar] [CrossRef]
- Gai, C.; Zhu, N.; Hoekman, S.K.; Liu, Z.; Jiao, W.; Peng, N. Highly dispersed nickel nanoparticles supported on hydrochar for hydrogen-rich syngas production from catalytic reforming of biomass. Energy Convers. Manag. 2019, 183, 474–484. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon N. Y. 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Sieben, J.M.; Duarte, M.M.E.; Mayer, C.E. Electro-Oxidation of Methanol on Pt—Ru Nanostructured Catalysts Electrodeposited onto Electroactivated Carbon Fiber Materials. ChemCatChem 2010, 2, 182–189. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; et al. Multi-Component Fe-Ni Hydroxide Nanocatalyst for Oxygen Evolution and Methanol Oxidation Reactions under Alkaline Conditions. ACS Catal. 2017, 7, 365–379. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; He, F.; Zuo, Y.; Zhang, C.; Liu, J.; Yu, X.; Du, R.; Zhang, T.; Infante-Carrió, M.F.; et al. Colloidal Ni–Co–Sn nanoparticles as efficient electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 22915–22924. [Google Scholar] [CrossRef]
- Amin, R.S.; Hameed, R.M.A.; El-Khatib, K.M. Microwave heated synthesis of carbon supported Pd, Ni and Pd–Ni nanoparticles for methanol oxidation in KOH solution. Appl. Catal. B Environ. 2014, 148, 557–567. [Google Scholar] [CrossRef]
- An, Y.; Ijaz, H.; Huang, M.; Qu, J.; Hu, S. The one-pot synthesis of CuNi nanoparticles with a Ni-rich surface for the electrocatalytic methanol oxidation reaction. Dalt. Trans. 2020, 49, 1646–1651. [Google Scholar] [CrossRef]
- Guo, M.; Yu, Y.; Hu, J. Nickel Nanoparticles for the Efficient Electrocatalytic Oxidation of Methanol in an Alkaline Medium. Electrocatalysis 2017, 8, 392–398. [Google Scholar] [CrossRef]
- Ferdowsi, G.S.; Seyedsadjadi, S.A.; Ghaffarinejad, A. Ni nanoparticle modified graphite electrode for methanol electrocatalytic oxidation in alkaline media. J. Nanostructure Chem. 2014, 5, 17–23. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Meera, M.A.; El-Newehy, M.H. In Situ Preparation of Novel Porous Nanocomposite Hydrogel as Effective Adsorbent for the Removal of Cationic Dyes from Polluted Water. Polymers 2020, 12, 3002. [Google Scholar] [CrossRef] [PubMed]

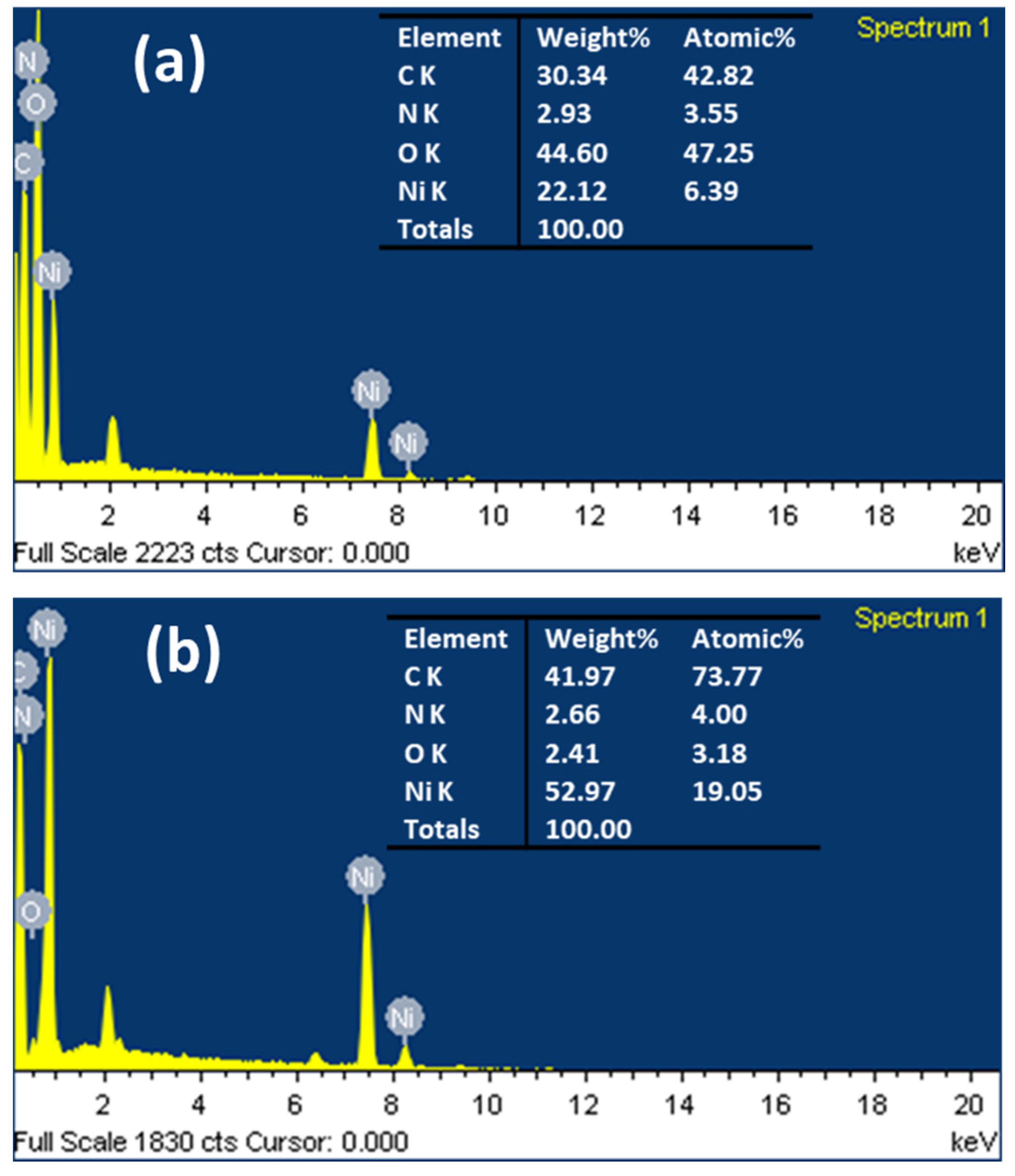
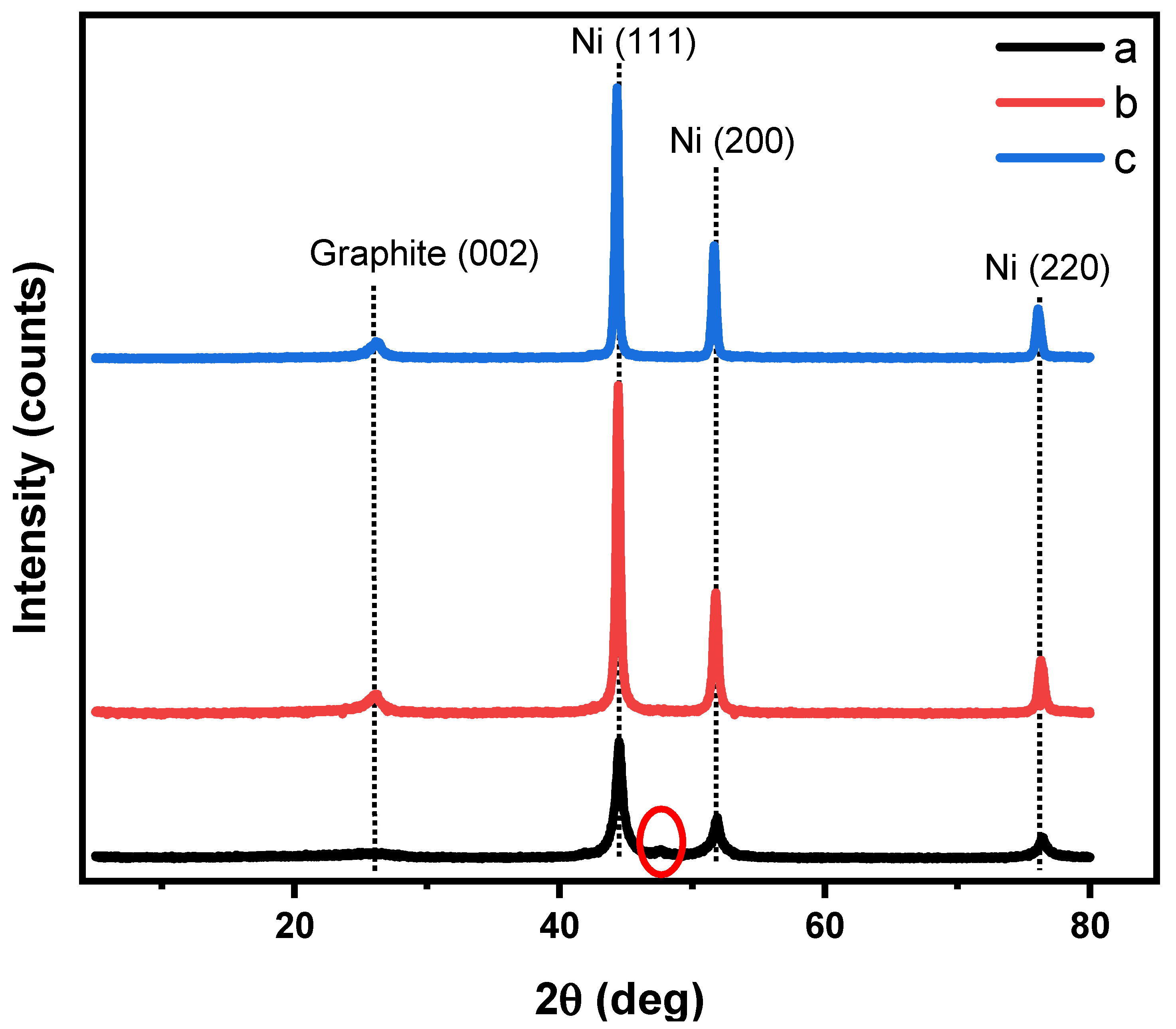
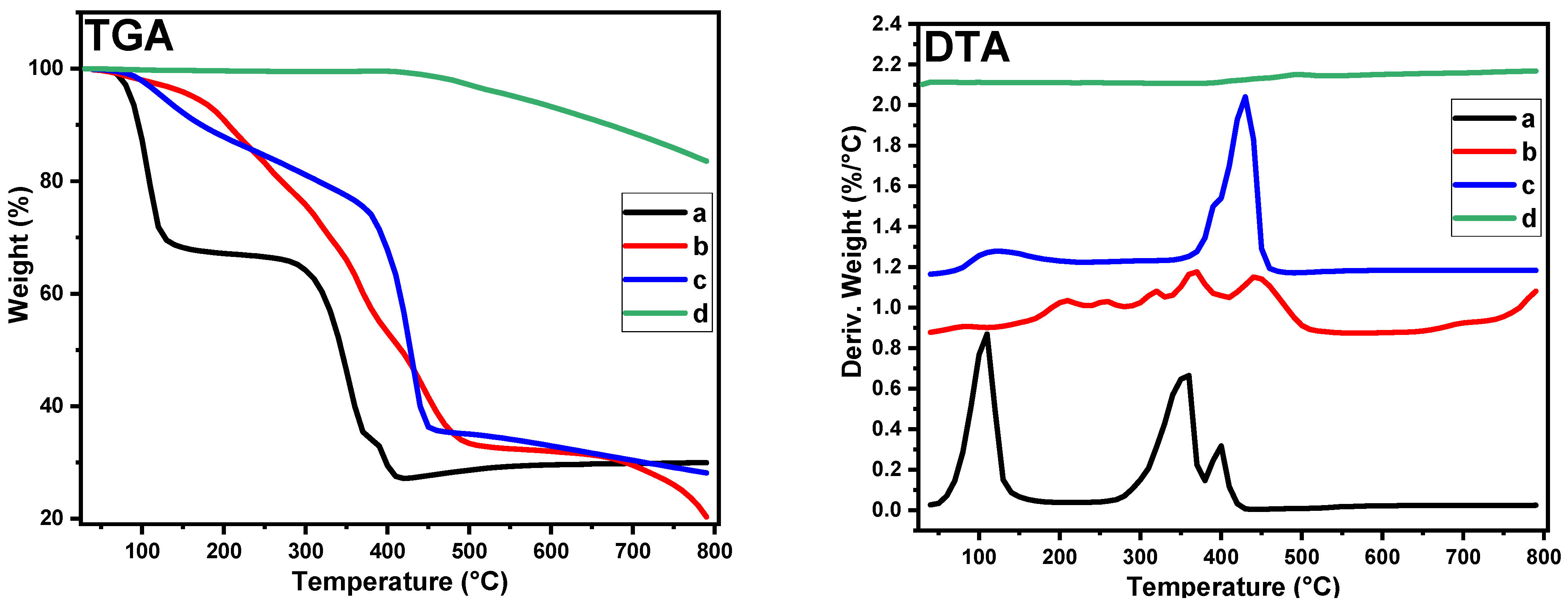

| Nickel | Peak Position (2 Theta) | FWHM | Crystallite Size (D) (nm) | Aver. of D (nm) | d-Space (nm) |
| Ni@PCFs-6 | 44.50 | 0.89392 | 9.60 | 9.50 | 0.20343 |
| 51.83 | 1.08946 | 8.11 | 0.17623 | ||
| 76.42 | 0.93777 | 10.78 | 0.12453 | ||
| Ni@PCFs-7 | 44.42 | 0.41449 | 20.70 | 15.21 | 0.20380 |
| 51.77 | 0.49561 | 14.42 | 0.17646 | ||
| 76.32 | 0.59526 | 10.49 | 0.12467 | ||
| Ni@PCFs-8 | 44.32 | 0.34699 | 24.72 | 22.84 | 0.20421 |
| 51.65 | 0.39374 | 22.42 | 0.17682 | ||
| 76.11 | 0.47196 | 21.38 | 0.12497 | ||
| Carbon | Peak Position (2 Theta) | FWHM | % Graphitization | d-Space (nm) | |
| Ni@PCFs-6 | 24.78 | 6.37 | 0.00 | 0.35898 | |
| Ni@PCFs-7 | 25.95 | 1.76 | 10.23 | 0.34312 | |
| Ni@PCFs-8 | 26.12 | 1.36 | 36.86 | 0.34083 | |
| Catalyst | Conditions | Current Density (mA/cm2) | Ref. |
|---|---|---|---|
| Ni2.5Co0.5Sn2 | CH3OH (2M)/KOH (1M), E = 0.6 V vs. Hg/HgO, ν = 0.1 V/s | 65.5 | [39] |
| Ni-NPs/RCQDs/GCE | CH3OH (2M)/KOH (1M), E = 0.56 V vs. Ag/AgCl, ν = 0.05 V/s | 32 | [20] |
| Ni NPs@r-GO | CH3OH (0.08M)/NaOH (0.11M), E = 0.536 V vs. Ag/AgCl ν = 0.1 V/s | 20 | [19] |
| Ni/C | CH3OH (0.6M)/KOH (0.5M), E = 0.735 V vs. Hg/HgO, ν = 0.01 V/s | 22.13 | [40] |
| NiCu@C | CH3OH (1M)/KOH (1M), E = 0.586 V vs. Hg/HgO, ν = 0.05 V/s | 41.12 | [41] |
| NiNPs@CFs | CH3OH (0.5M)/KOH (1M), E = 0.8 V vs. Hg/HgO, ν = 0.05 V/s | 2.0 | [21] |
| NiNPs/ITO | CH3OH (0.5M)/NaOH (0.1M), E = 0.71 V vs. Ag/AgCl, ν = 0.05 V/s | 5.47 | [42] |
| NiNP-GE | CH3OH (0.5M)/NaOH (0.5M), E = 0.8 V vs. SCE, ν = 0.1 V/s | 7.0 | [43] |
| Ni@PCFs-6 | CH3OH (0.5M)/KOH (1M), E = 0.8 V vs. Ag/AgCl, ν = 0.05 V/s | 34.58 | This work |
| Ni@PCFs-7 | 74.16 | ||
| Ni@PCFs-8 | 158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaleb, H.A.; Salah, A.; Thamer, B.M. Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation. Gels 2022, 8, 542. https://doi.org/10.3390/gels8090542
Altaleb HA, Salah A, Thamer BM. Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation. Gels. 2022; 8(9):542. https://doi.org/10.3390/gels8090542
Chicago/Turabian StyleAltaleb, Hamud A., Abdulwahab Salah, and Badr M. Thamer. 2022. "Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation" Gels 8, no. 9: 542. https://doi.org/10.3390/gels8090542
APA StyleAltaleb, H. A., Salah, A., & Thamer, B. M. (2022). Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation. Gels, 8(9), 542. https://doi.org/10.3390/gels8090542







