Intelligent Hydrogels in Myocardial Regeneration and Engineering
Abstract
:1. Introduction
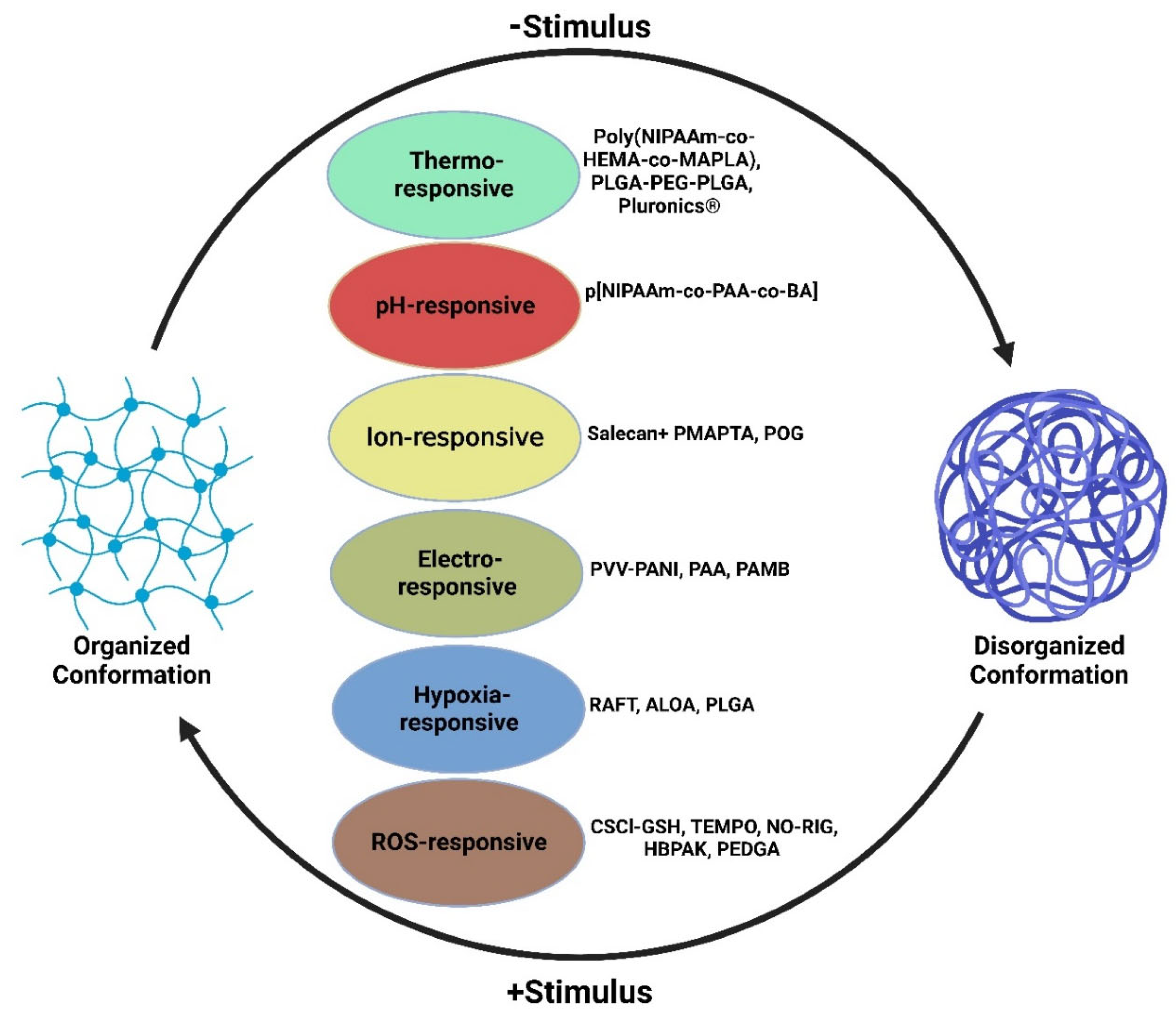
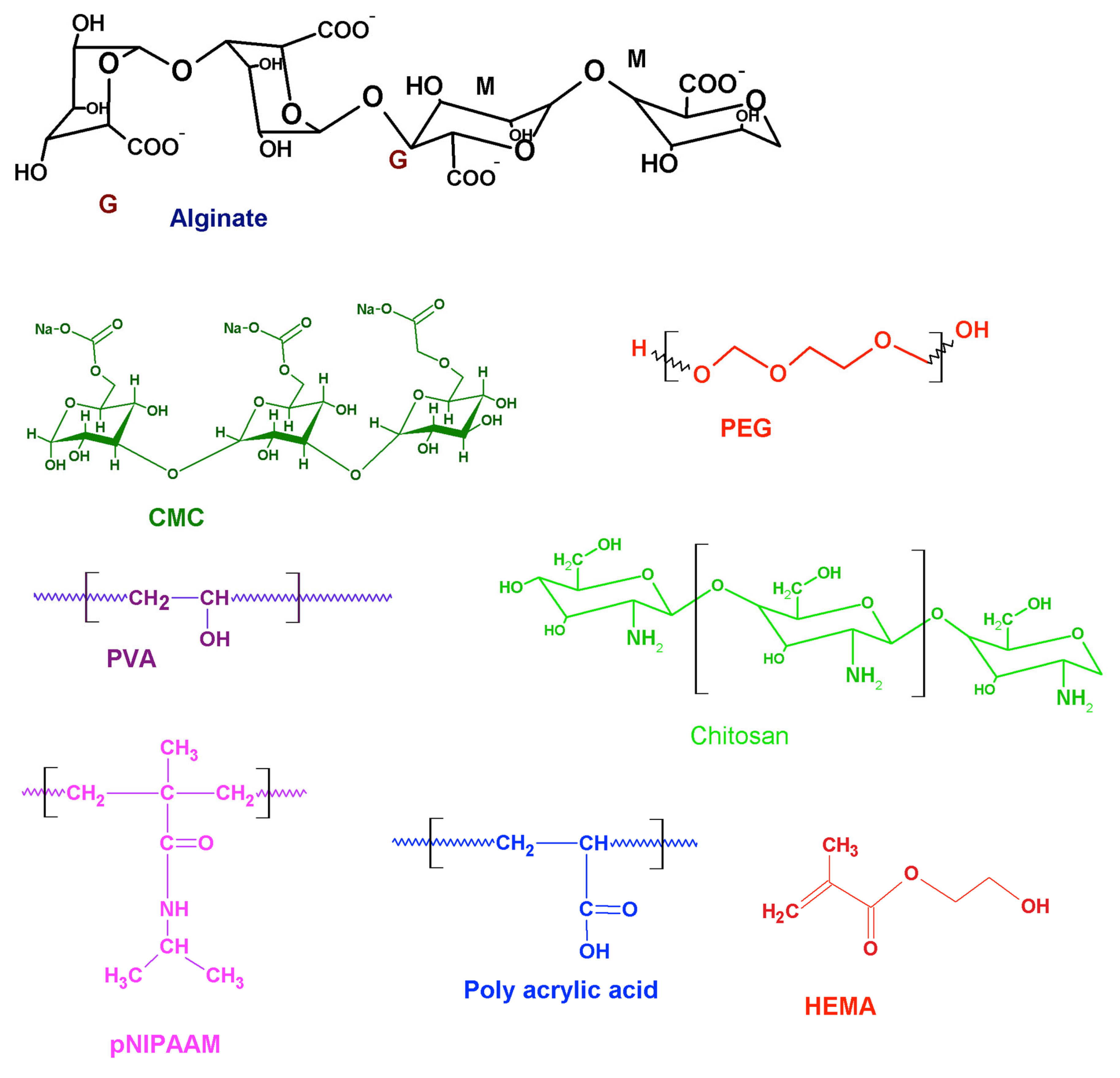
2. Intelligent Hydrogels and Cardiac Tissue Engineering
3. Temperature-Responsive Hydrogels
4. pH-Responsive Hydrogel
5. Ion-Responsive Hydrogels
| Type of Smart Hydrogel | Molecular Compound | Function of Hydrogel | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Temperature -Responsive | Poly(NIPAAm-co-HEMA-co-MAPLA) | Provides mechanical support to left ventricular wall via thickening and decreasing mechanical stress | Biodegradable through modification of copolymers, effective site-specific drug delivery, decrease in systemic side effects, evade toxic solvents, high solvent swelling | Decreased pH via acidic degradation, lacks biocompatibility | [44,49,50] |
| Temperature-Responsive | PLGA-PEG-PLGA | Liquid between the temperatures of 2 °C and 15 °C and transitions into a gel at body temperature | Biocompatible, water-soluble, and non-immunogenic, gradual drug release for both hydrophobic and hydrophilic drugs | Hydrophobic/hydrophilic imbalance could lead to no phase change, narrow gel transition temperature window | [23,24,25,26,27,28,29,30,31,32,33,38,39,40,41,42,43,44,45,46] |
| Temperature -Responsive | Pluronics® | At concentration of 20 wt%, exist in liquid form <25 °C and transitions to a gel at 37 °C | Sustained drug release, good bioadhesiveness, good biocompatibility | Poor gel durability, weak mechanical strength | [27,28,30,31,32,41] |
| Temperature- Responsive and pH-responsive | p [NIPAAm-co-PAA-co-BA] | Exists in liquid form at room temperature with a pH of 7.4 but transitions into a gel at 37 °C with a pH of 6.8. Able to deliver drug motifs such as bFGF | Gel dissolution and elimination once target is back at normal physiology pH | Increased inflammatory response | [51] |
| Electroconductive | PVV-PANI, PAA, PAMB | Enhanced neural and glial differentiation with electrical stimulation | Drug loading capacity, high bioactivity and cytocompatibility, increased tensile strength and compression | Enhanced cell growth leading to cell death, loss of conductivity, inability to control arrhythmia | [52,53,54,55] |
| Ion-responsive | Salecan + PMAPTA, POG | Binding with negatively charged drugs and stable drug release. Display uniform conductivity and elasticity. | Drug loading capacity, biocompatible, injectable liquid form, controlled biodegradation | Drug release impacted by pH changes, differing affinities to drug binding, and release dependent on charge strength | [10,47,48] |
| Hypoxia-responsive | RAFT, ALOA, PLGA | Increase cell retention, greater oxygen partial pressure capabilities | Excellent biocompatibility, no substantial increase in inflammation | Can trigger ROS burst | [56,57,58,59,60,61] |
| ROS-responsive | CSCl-GSH, TEMPO, NO-RIG, HBPAK, PEDGA | Antioxidant properties effective in facilitating tissue recovery, ROS scavenging, and reduce inflammation | Successfully diminished ROS microenvironment and alleviated hypoxia | Limited retention time to optimize ROS-scavenging capability | [56,57,58,61,62,63,64] |
6. Hypoxia-Responsive Hydrogels
7. ROS-Responsive Hydrogels
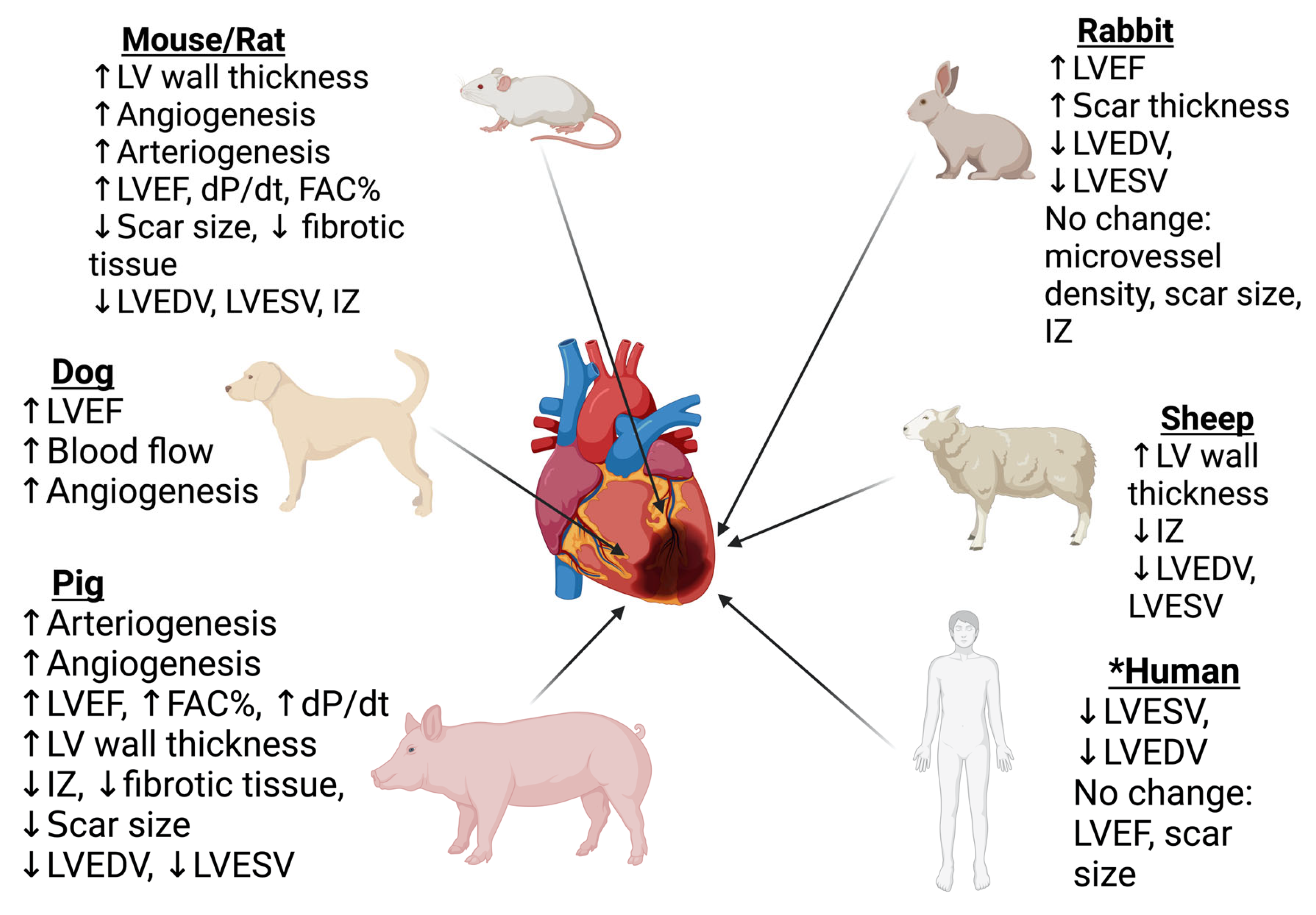
8. Evidence from Translational Models
9. Degradation-Dependent Hydrogels
10. Hypoxia-Responsive Hydrogels
11. Electroconductive Hydrogels
12. Thermo-Sensitive Hydrogels
13. ROS-Responsive Hydrogels
14. Angiogenesis Promoting Hydrogels
15. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [PubMed]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Sadek, H.A.; Bergmann, O. Cardiomyocyte Renewal in the Human Heart: Insights from the Fall-Out. Eur. Heart J. 2017, 38, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Agrawal, D.K. Infarct Zone: A Novel Platform for Exosome Trade in Cardiac Tissue Regeneration. J. Cardiovasc. Transl. Res. 2020, 13, 686–701. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of Natural Hydrogels for Cardiac, Neural, and Bone Tissue Engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Peng, H.; Ning, X.; Wei, G.; Wang, S.; Dai, G.; Ju, A. The Preparations of Novel Cellulose/Phenylboronic Acid Composite Intelligent Bio-Hydrogel and Its Glucose, PH Responsive Behaviors. Carbohydr. Polym. 2018, 195, 349–355. [Google Scholar] [CrossRef]
- Chen, M.H.; Chung, J.J.; Mealy, J.E.; Zaman, S.; Li, E.C.; Arisi, M.F.; Atluri, P.; Burdick, J.A. Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci. 2018, 19, 1800248. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Seliktar, D. Hydrogels for Therapeutic Cardiovascular Angiogenesis. Adv. Drug Deliv. Rev. 2016, 95, 31–39. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A Tunable Self-Healing Ionic Hydrogel with Microscopic Homogeneous Conductivity as a Cardiac Patch for Myocardial Infarction Repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel Drug Delivery System with Predictable and tunable Drug Release and Degradation Rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Tian, Y.; Liu, Z. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Available online: https://pubmed-ncbi-nlm-nih-gov.proxy.westernu.edu/27729419/ (accessed on 10 August 2022).
- Shu, Y.; Hao, T.; Yao, F.; Qian, Y.; Wang, Y.; Yang, B.; Li, J.; Wang, C. RoY Peptide-Modified Chitosan-Based Hydrogel to Improve Angiogenesis and Cardiac Repair under Hypoxia. ACS Appl. Mater. Interfaces 2015, 7, 6505–6517. [Google Scholar] [CrossRef]
- Wang, L.L.; Chung, J.J.; Li, E.C.; Uman, S.; Atluri, P.; Burdick, J.A. Injectable and Protease-Degradable Hydrogel for SiRNA Sequestration and Triggered Delivery to the Heart. J. Control. Release 2018, 285, 152–161. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-Responsive Chitosan Hydrogel for Healing of Full-Thickness Wounds Infected with XDR Bacteria Isolated from Burn Patients: In Vitro and in Vivo Animal Model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef]
- Ter Boo, G.J.; Schmid, T.; Zderic, I.; Nehrbass, D.; Camenisch, K.; Richards, R.G.; Grijpma, D.W.; Moriarty, T.F.; Eglin, D. Local Application of a Gentamicin-Loaded Thermo-Responsive Hydrogel Allows for Fracture Healing upon Clearance of a High Staphylococcus Aureus Load in a Rabbit Model. Eur. Cell Mater. 2018, 35, 151–164. [Google Scholar] [CrossRef]
- Hu, C.-C.; Chiu, Y.-C.; Chaw, J.-R.; Chen, C.-F.; Liu, H.-W. Thermo-Responsive Hydrogel as an Anti-VEGF Drug Delivery System to Inhibit Retinal Angiogenesis in Rex Rabbits. Technol. Health Care 2019, 27, 153–163. [Google Scholar] [CrossRef]
- Liao, H.-T.; Chen, C.-T.; Chen, J.-P. Osteogenic Differentiation and Ectopic Bone Formation of Canine Bone Marrow-Derived Mesenchymal Stem Cells in Injectable Thermo-Responsive Polymer Hydrogel. Tissue Eng. C Methods 2011, 17, 1139–1149. [Google Scholar] [CrossRef]
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O’Doherty, M.; Chalanqui, M.; Kelly, D.J.; Haut-Donahue, T.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Macromol. Biosci. 2017, 17, 1700118. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-Based Thermosensitive Hydrogel Containing SWCNTs for Stem Cell Transplantation in Myocardial Repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Sun, T.; Tsou, Y.-H.; Chen, H.; Xu, X. Dual-Functional Dextran-PEG Hydrogel as an Antimicrobial Biomedical Material. Macromol. Biosci. 2018, 18, 1700325. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble Collagen Based Biodegradable Hybrid Hydrogel for Biomedical Scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 2199–2219. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Liu, S.; Li, X.; Zhao, X.; Hu, R.; Zhang, J.; Han, H.; Dong, A. Adjustable Degradation and Drug Release of a Thermosensitive Hydrogel Based on a Pendant Cyclic Ether Modified Poly(ε-Caprolactone) and Poly(Ethylene Glycol)Co-Polymer. Acta Biomater. 2012, 8, 3963–3973. [Google Scholar] [CrossRef]
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA–PEG–PLGA Thermosensitive Hydrogels with Suitable Thermosensitivity and Properties for Use in a Drug Delivery System. J. Mater. Chem. B 2017, 5, 1551–1565. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 Thermosensitive Injectable Smart Hydrogels for Controlled Drug Delivery System Development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Zou, S.; He, Q.; Wang, Q.; Wang, B.; Liu, G.; Zhang, F.; Cheng, X.; Wang, B.; Zhang, L. Injectable Nanosponge-Loaded Pluronic F127 Hydrogel for Pore-Forming Toxin Neutralization. Int. J. Nanomed. 2021, 16, 4239–4250. [Google Scholar] [CrossRef]
- Norouzi, M.; Firouzi, J.; Sodeifi, N.; Ebrahimi, M.; Miller, D.W. Salinomycin-Loaded Injectable Thermosensitive Hydrogels for Glioblastoma Therapy. Int. J. Pharm. 2021, 598, 120316. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive Chitosan–Pluronic Hydrogel as an Injectable Cell Delivery Carrier for Cartilage Regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.; Quintanar, D.; Ganem, A. Applications of Thermo-Reversible Pluronic F-127 Gels in Pharmaceutical Formulations. J. Pharm. Pharm. Sci. A Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2006, 9, 339–358. [Google Scholar]
- Kim, Y.C.; Shin, M.D.; Hackett, S.F.; Hsueh, H.T.; Lima E Silva, R.; Date, A.; Han, H.; Kim, B.-J.; Xiao, A.; Kim, Y.; et al. Gelling Hypotonic Polymer Solution for Extended Topical Drug Delivery to the Eye. Nat. Biomed. Eng. 2020, 4, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Kharlampieva, E. Self-Assemblies of Thermoresponsive Poly(N-Vinylcaprolactam) Polymers for Applications in Biomedical Field. ACS Appl. Polym. Mater. 2020, 2, 26–39. [Google Scholar] [CrossRef]
- Sala, R.L.; Kwon, M.Y.; Kim, M.; Gullbrand, S.E.; Henning, E.A.; Mauck, R.L.; Camargo, E.R.; Burdick, J.A. Thermosensitive Poly(N-Vinylcaprolactam) Injectable Hydrogels for Cartilage Tissue Engineering. Tissue Eng. A 2017, 23, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, T.; Orakdogen, N. Poly(N,N-Dimethylaminoethyl Methacrylate-Co-2-Acrylamido-2-Methyl-Propanosulfonic Acid)/Laponite Nanocomposite Hydrogels and Cryogels with Improved Mechanical Strength and Rapid Dynamic Properties. Appl. Clay Sci. 2016, 121–122, 162–173. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Tan, M.J.; Thoniyot, P.; Loh, X.J. Unusual Thermogelling Behaviour of Poly [2-(Dimethylamino)Ethyl Methacrylate] (PDMAEMA)-Based Polymers Polymerized in Bulk. RSC Adv. 2015, 5, 62314–62318. [Google Scholar] [CrossRef]
- Yu, P.; Xie, J.; Chen, Y.; Liu, J.; Liu, Y.; Bi, B.; Luo, J.; Li, S.; Jiang, X.; Li, J. A Thermo-Sensitive Injectable Hydroxypropyl Chitin Hydrogel for Sustained Salmon Calcitonin Release with Enhanced Osteogenesis and Hypocalcemic Effects. J. Mater. Chem. B 2020, 8, 270–281. [Google Scholar] [CrossRef]
- Li, Z.; Shim, H.; Cho, M.O.; Cho, I.S.; Lee, J.H.; Kang, S.-W.; Kwon, B.; Huh, K.M. Thermo-Sensitive Injectable Glycol Chitosan-Based Hydrogel for Treatment of Degenerative Disc Disease. Carbohydr. Polym. 2018, 184, 342–353. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 ShRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-Sensitive Injectable Hydrogel Based on the Physical Mixing of Hyaluronic Acid and Pluronic F-127 for Sustained NSAID Delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, Z.; Niu, H.; Sui, Y.; Li, H.; Ma, J.; Guan, J. Spatiotemporal Delivery of Basic Fibroblast Growth Factor to Directly and Simultaneously Attenuate Cardiac Fibrosis and Promote Cardiac Tissue Vascularization Following Myocardial Infarction. J. Control. Release 2019, 311–312, 233–244. [Google Scholar] [CrossRef]
- Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Kumar, P.; Pillay, V. Rheological and Swelling Behavior of PH Sensitive Hydrogel Particles. APCBEE Procedia 2014, 9, 192–196. [Google Scholar] [CrossRef]
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of Basic Fibroblast Growth Factor with a pH-Responsive, Injectable Hydrogel to Improve Angiogenesis in Infarcted Myocardium. Biomaterials 2011, 32, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Yasin, T.; Heng, J.Y.Y.; Akhter, Z. Synthesis and Characterization of Novel pH-, Ionic Strength and Temperature- Sensitive Hydrogel for Insulin Delivery. Polymer 2010, 51, 1687–1693. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators Based on Stimulus-Responsive Hydrogels and Their Emerging Biomedical Applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Li, J.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a novel cationic hydrogel base on salecan-g-PMAPTAC. Int. J. Biol. Macromol. 2017, 101, 474–480. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Matsumura, Y.; Zhu, Y.; Jiang, H.; D’Amore, A.; Luketich, S.K.; Charwat, V.; Yoshizumi, T.; Sato, H.; Yang, B.; Uchibori, T.; et al. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289. [Google Scholar] [CrossRef]
- Zhang, C.; Hsieh, M.-H.; Wu, S.-Y.; Li, S.-H.; Wu, J.; Liu, S.-M.; Wei, H.-J.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials 2020, 231, 119672. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020, 105, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, J.; Wang, F.; Xing, R.; Sun, B.; Zhou, Y. Polyacrylamide-Sodium Alginate Hydrogel Releasing Oxygen and Vitamin C Promotes Bone Regeneration in Rat Skull Defects. Front. Mater. 2021, 8, 469. [Google Scholar] [CrossRef]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Park, K.M.; Park, K.D. Calcium peroxide-mediated in situ formation of multifunctional hydrogels with enhanced mesenchymal stem cell behaviors and antibacterial properties. J. Mater. Chem. B 2020, 8, 11033–11043. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Guan, J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials 2012, 33, 5914–5923. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 18458–18469. [Google Scholar] [CrossRef]
- Komeri, R.; Thankam, F.G.; Muthu, J. Free Radical Scavenging Injectable Hydrogels for Regenerative Therapy. Mater. Sci. Eng. C 2017, 71, 100–110. [Google Scholar] [CrossRef]
- Finosh, G.T.; Jayabalan, M. Reactive Oxygen Species—Control and Management Using Amphiphilic Biosynthetic Hydrogels for Cardiac Applications. Adv. Biosci. Biotechnol. 2013, 4, 1134–1146. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, Z.; Niu, H.; Gao, N.; Guan, Y.; Li, C.; Dang, Y.; Cui, X.; Liu, X.L.; Duan, Y.; et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci. Rep. 2018, 8, 1371. [Google Scholar] [CrossRef]
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-Generating Photo-Cross-Linkable Hydrogels Support Cardiac Progenitor Cell Survival by Reducing Hypoxia-Induced Necrosis. ACS Biomater. Sci. Eng. 2017, 3, 1964–1971. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A chitosan–glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef]
- Xie, J.; Yao, Y.; Wang, S.; Fan, L.; Ding, J.; Gao, Y.; Li, S.; Shen, L.; Zhu, Y.; Gao, C. Alleviating Oxidative Injury of Myocardial Infarction by a Fibrous Polyurethane Patch with Condensed ROS-Scavenging Backbone Units. Adv. Healthc. Mater. 2022, 11, 2101855. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Heusch, G.; Kleinbongard, P.; Rassaf, T. Cardioprotection Beyond Infarct Size Reduction. Circ. Res. 2019, 124, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Wang, Y.; Lin, Q.; Yao, A.; Cao, F.; Li, D.; Zhou, J.; Duan, C.; Du, Z.; et al. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 2012, 33, 3093–3106. [Google Scholar] [CrossRef] [PubMed]
- Ronson, R.S.; Nakamura, M.; Vinten-Johansen, J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc. Res. 1999, 44, 47–59. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Wang, Z.; Ding, J.; Wang, S.; Baiqiang, H.; Ke, S.; Gao, C. Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mater. Chem. B 2019, 7, 5019–5037. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Redox nanoparticles: Synthesis, properties and perspectives of use for treatment of neurodegenerative diseases. J. Nanobiotechnol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Ding, J.; Yao, Y.; Li, J.; Duan, Y.; Nakkala, J.R.; Feng, X.; Cao, W.; Wang, Y.; Hong, L.; Shen, L.; et al. A Reactive Oxygen Species Scavenging and O2 Generating Injectable Hydrogel for Myocardial Infarction Treatment In vivo. Small 2020, 16, 2005038. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, F.; Huang, X.P.; Chung, J.C.; Konecny, F.; Weisel, R.D.; Li, R.K. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials 2011, 32, 579–586. [Google Scholar] [CrossRef]
- Zhang, H.; Fazel, S.; Tian, H.; Mickle, D.A.G.; Weisel, R.D.; Fujii, T.; Li, R.-K. Increasing Donor Age Adversely Impacts Beneficial Effects of Bone Marrow but Not Smooth Muscle Myocardial Cell Therapy. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H2089–H2096. [Google Scholar] [CrossRef]
- Anderl, J.N.; Robey, T.E.; Stayton, P.S.; Murry, C.E. Retention and Biodistribution of Microspheres Injected into Ischemic Myocardium. J. Biomed. Mater. Res. A 2009, 88, 704–710. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Li, Y.; Ma, Y.; Zhang, Y.; Liu, Z.; Zhou, J.; Lin, Q.; Wang, Y.; Duan, C.; et al. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive chitosan hydrogel. J. Heart Lung Transpl. 2010, 29, 881–887. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Tous, E.; Minakawa, M.; Morita, M.; Robb, J.D.; Koomalsingh, K.J.; Gorman, J.H., 3rd; Gorman, R.C.; Burdick, J.A. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovitz, L.J.; Savage, E.B.; Ratcliffe, M.B.; Bavaria, J.E.; Kreiner, G.; Iozzo, R.V.; Hargrove, W.C.; Bogen, D.K.; Edmunds, L.H. Large Animal Model of Left Ventricular Aneurysm. Ann. Thorac. Surg. 1989, 48, 838–845. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.; Radwan, M.; Keklikian, A.; Atwal, M.; Rai, T.; Agrawal, D. Fluoroscopy Guided Minimally Invasive Swine Model of Myocardial Infarction by Left Coronary Artery Occlusion for Regenerative Cardiology. Cardiol. Cardiovasc. Med. 2022, 6, 416. [Google Scholar]
- Rane, A.A.; Chuang, J.S.; Shah, A.; Hu, D.P.; Dalton, N.D.; Gu, Y.; Peterson, K.L.; Omens, J.H.; Christman, K.L. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS ONE 2011, 6, e21571. [Google Scholar] [CrossRef]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Influence of Plasma Protein-Hydrogel Interaction Moderated by Absorption of Water on Long-Term Cell Viability in Amphiphilic Biosynthetic Hydrogels. RSC Adv. 2013, 3, 24509–24520. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Influence of Physical and Mechanical Properties of Amphiphilic Biosynthetic Hydrogels on Long-Term Cell Viability. J. Mech. Behav. Biomed. Mater. 2014, 35, 111–122. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J.; Sankar, V.; Gopal, R.K. Growth and Survival of Cells in Biosynthetic Poly Vinyl Alcohol-Alginate IPN Hydro-gels for Cardiac Applications. Colloids Surf. B Biointerfaces 2013, 107, 137–145. [Google Scholar] [CrossRef]
- Ou, L.; Li, W.; Zhang, Y.; Wang, W.; Liu, J.; Sorg, H.; Furlani, D.; Gäbel, R.; Mark, P.; Klopsch, C.; et al. Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J. Cell. Mol. Med. 2011, 15, 1310–1318. [Google Scholar] [CrossRef]
- Yoon, S.J.; Fang, Y.H.; Lim, C.H.; Kim, B.S.; Son, H.S.; Park, Y.; Sun, K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 163–171. [Google Scholar] [CrossRef]
- Singelyn, J.M.; Sundaramurthy, P.; Johnson, T.D.; Schup-Magoffin, P.J.; Hu, D.P.; Faulk, D.M.; Wang, J.; Mayle, K.M.; Bartels, K.; Salva-tore, M.; et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [Google Scholar] [CrossRef] [Green Version]
- Tous, E.; Ifkovits, J.L.; Koomalsingh, K.J.; Shuto, T.; Soeda, T.; Kondo, N.; Gorman, J.H., 3rd; Gorman, R.C.; Burdick, J.A. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules 2011, 12, 4127–4135. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Huan, Y.; Zhao, H.; Deng, J. Application of bFGF and BDNF to improve angiogenesis and cardiac function. J. Surg. Res. 2006, 136, 85–91. [Google Scholar] [CrossRef]
- Dorsey, S.M.; McGarvey, J.R.; Wang, H.; Nikou, A.; Arama, L.; Koomalsingh, K.J.; Kondo, N.; Gorman, J.H.; Pilla, J.J.; Gorman, R.C.; et al. MRI evaluation of injectable hyaluronic acid-based hydrogel therapy to limit ventricular remodeling after myo-cardial infarction. Biomaterials 2015, 69, 65–75. [Google Scholar] [CrossRef]
- Leor, J.; Tuvia, S.; Guetta, V.; Manczur, F.; Castel, D.; Willenz, U.; Petneházy, O.; Landa, N.; Feinberg, M.S.; Konen, E.; et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023. [Google Scholar] [CrossRef]
- Mukherjee, R.; Zavadzkas, J.A.; Saunders, S.M.; McLean, J.E.; Jeffords, L.B.; Beck, C.; Stroud, R.E.; Leone, A.M.; Koval, C.N.; Rivers, W.T.; et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann. Thorac. Surg. 2008, 86, 1268–1276. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Ye, G.; He, Y.; Li, B.; Guan, Y.; Gong, B.; Mequanint, K.; Xing, M.M.Q.; Qiu, X. Injectable and Conductive Cardiac Patches Repair Infarcted Myocardium in Rats and Minipigs. Nat. Biomed. Eng. 2021, 5, 1157–1173. [Google Scholar] [CrossRef]
- Fujimoto, K.L.; Ma, Z.; Nelson, D.M.; Hashizume, R.; Guan, J.; Tobita, K.; Wagner, W.R. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials 2009, 30, 4357–4368. [Google Scholar] [CrossRef]
- Lu, W.N.; Lü, S.H.; Wang, H.B.; Li, D.X.; Duan, C.M.; Liu, Z.Q.; Hao, T.; He, W.J.; Xu, B.; Fu, Q.; et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng. A 2009, 15, 1437–1447. [Google Scholar] [CrossRef]
- Rao, Z.; Shen, D.; Chen, J.; Jin, L.; Wu, X.; Chen, M.; Li, L.; Chu, M.; Lin, J. Basic Fibroblast Growth Factor Attenuates Injury in Myocardial Infarction by Enhancing Hypoxia-Inducible Factor-1 Alpha Accumulation. Front. Pharm. 2020, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, T.; Jiang, X.J.; Lin, T.; Wu, D.Q.; Zhang, X.Z.; Okello, E.; Xu, H.X.; Yuan, M.J. Injectable hydrogel helps bone marrow-derived mononuclear cells restore infarcted myocardium. Cardiology 2010, 115, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, D.Q.; Jiang, X.J.; Zhang, X.Z.; Li, X.Y.; Zhang, J.F.; Zheng, Z.B.; Zhuo, R.; Jiang, H.; Huang, C. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur. J. Heart Fail. 2009, 11, 14–19. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, T.; Li, X.Y.; Wu, D.Q.; Zheng, Z.B.; Zhang, J.F.; Chen, J.L.; Peng, B.; Jiang, H.; Huang, C.; et al. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. J. Biomed. Mater. Res. A 2009, 90, 472–477. [Google Scholar] [CrossRef]
- Ma, Z.; Nelson, D.M.; Hong, Y.; Wagner, W.R. Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydro-lytic lability. Biomacromolecules 2010, 11, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, K.A.; Zhu, Y.; Takaba, K.; Ramasubramanian, A.; Badathala, A.; Haraldsson, H.; Collins, A.; Aguayo, E.; Shah, C.; Wallace, A.W.; et al. Myocardial injection of a thermoresponsive hydrogel with reactive oxygen species scavenger properties improves border zone contractility. J. Biomed. Mater. Res. A 2020, 108, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Luo, C.Y.; Hu, Y.N.; Yeh, M.L.; Hsueh, Y.C.; Chang, M.Y.; Tsai, D.C.; Wang, J.N.; Tang, M.J.; Wei, E.I.; et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci. Transl. Med. 2012, 4, 146ra109. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Huang, T.T.; Chen, C.H.; Cheng, B.; Hwang, S.M.; Hsieh, P.C. Injection of human cord blood cells with hyaluronan improves postinfarction cardiac repair in pigs. Stem Cells Transl. Med. 2016, 5, 56–66. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Jin, R.; Chen, L.; Dang, M.; Cao, H.; Dong, Y.; Cai, B.; Bai, G.; Gooding, J.J.; et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, 6740. [Google Scholar] [CrossRef]
- Frey, N.; Linke, A.; Süselbeck, T.; Müller-Ehmsen, J.; Vermeersch, P.; Schoors, D.; Rosenberg, M.; Bea, F.; Tuvia, S.; Leor, J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: A first-in-man study. Circ. Cardiovasc. Interv. 2014, 7, 806–812. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doescher, C.; Thai, A.; Cha, E.; Cheng, P.V.; Agrawal, D.K.; Thankam, F.G. Intelligent Hydrogels in Myocardial Regeneration and Engineering. Gels 2022, 8, 576. https://doi.org/10.3390/gels8090576
Doescher C, Thai A, Cha E, Cheng PV, Agrawal DK, Thankam FG. Intelligent Hydrogels in Myocardial Regeneration and Engineering. Gels. 2022; 8(9):576. https://doi.org/10.3390/gels8090576
Chicago/Turabian StyleDoescher, Christian, An Thai, Ed Cha, Pauline V. Cheng, Devendra K. Agrawal, and Finosh G. Thankam. 2022. "Intelligent Hydrogels in Myocardial Regeneration and Engineering" Gels 8, no. 9: 576. https://doi.org/10.3390/gels8090576
APA StyleDoescher, C., Thai, A., Cha, E., Cheng, P. V., Agrawal, D. K., & Thankam, F. G. (2022). Intelligent Hydrogels in Myocardial Regeneration and Engineering. Gels, 8(9), 576. https://doi.org/10.3390/gels8090576





