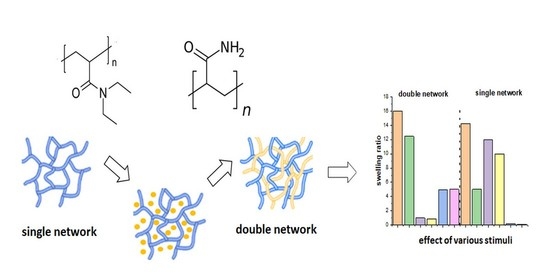
External Stimuli-Responsive Characteristics of Poly(N,N′-diethylacrylamide) Hydrogels: Effect of Double Network Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogels Synthesis
2.2. Effect of the Temperature on the Hydrogels’ Behavior
2.3. Effect of Solvent Composition on the Hydrogels’ Behavior
2.4. Effect of Salt on the Hydrogels’ Behavior
2.5. Effect of All Stimuli on the Hydrogels’ Behavior
2.6. NMR Relaxation of the Solvent Molecules
2.7. Deswelling and Swelling Kinetics
3. Conclusions
4. Materials and Methods
4.1. Swelling Experiments
4.1.1. Thermo-Responsive Properties
4.1.2. Solvent- and Salt-Responsive Properties
4.1.3. Deswelling and Swelling Kinetics
4.2. 1H NMR Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Asoh, T.A.; Matsusaki, M.; Kaneko, T.; Akashi, M. Fabrication of temperature-responsive bending hydrogels with a nanostructured gradient. Adv. Mater. 2008, 20, 2080–2083. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, H.; Xue, J.; Jiang, J.; Liu, Z.; Liu, Z.; Li, G.; Zhao, Y. Programmable humidity-responsive actuation of polymer films enabled by combining shape memory property and surface-tunable hygroscopicity. ACS Appl. Mater. Interfaces 2021, 13, 38773–38782. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B.; Ahmed, S. Peptide-basedlow molecular weight photosensitive supramolecular gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Ohmine, I.; Tanaka, T. Salt effects on the phase transition of ionic gels. J. Chem. Phys. 1982, 77, 5725–5729. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.; Smoukov, S.K. Electroactive polymers for sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase transitions in ionic gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Sheikhi, A.; Afewerki, S.; Oklu, R.; Gaharwar, A.K.; Khademhosseini, A. Effect of ionic strength on shear-thinning nanoclay–polymer composite hydrogels. Biomater. Sci. 2018, 6, 2073–2083. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Ju, X.J.; Xie, R.; Chu, L.Y. Smart thermo-triggered squirting capsules for nanoparticle delivery. Soft Matter 2010, 6, 3759–3763. [Google Scholar] [CrossRef]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Ilavský, M.; Hrouz, J.; Havlíček, I. Phase transition in swollen gels: 7. Effect of charge concentration on the temperature collapse of poly(N,N-diethylacrylamide) networks in water. Polymer 1985, 26, 1514–1518. [Google Scholar] [CrossRef]
- Tirumala, R.; Ilavský, J.; Ilavský, M. Effect of chemical structure on the volume-phase transition in neutral and weakly charged poly(N,N-alkyl(meth)acrylamide) hydrogels studied by ultrasmall-angle X-ray scattering. J. Chem. Phys. 2006, 124, 234911. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Tanaka, T. Phase transitions in gels and a single polymer. Polymer 1979, 20, 1404–1412. [Google Scholar] [CrossRef]
- Ilavský, M. Phase Transition in Swollen Gels. 2. Effect of charge concentration on the collapse and mechanical behavior of polyacrylamide networks. Macromolecules 1982, 15, 782–788. [Google Scholar] [CrossRef]
- Caykara, T.; Dogmu, M. The effect of solvent composition on swelling and shrinking properties of poly(acrylamide-co-itaconic acid) hydrogels. Eur. Polym. J. 2004, 40, 2605–2609. [Google Scholar] [CrossRef]
- Amiya, T.; Hirokawa, Y.; Hirose, Y.; Li, Y.; Tanaka, T. Reentrant phase transition of N-isopropylacrylamide gels in mixed solvents. J. Chem. Phys. 1987, 86, 2375–2379. [Google Scholar] [CrossRef]
- Hirotsu, S. Critical points of the volume phase transition in N-isopropylacrylamide gels. J. Chem. Phys. 1988, 88, 427–431. [Google Scholar] [CrossRef]
- Fomenko, A.; Sedláková, Z.; Ilavský, M. Phase transition in swollen gels 30. Temperature-induced phase transition in positively charged poly(N-isopropylacrylamide) hydrogels in water and aqueous NaCl solutions. Polym. Bull. 2001, 47, 367–374. [Google Scholar] [CrossRef]
- Patra, L.; Vidyasagar, A.; Toomey, R. The effect of the Hofmeister series on the deswelling isotherms of poly(N-isopropylacrylamide) and poly(N,N-diethylacrylamide). Soft Matter 2011, 7, 6061–6067. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Fei, R.; George, J.T.; Park, J.; Grunlan, M.A. Thermoresponsive nanocomposite double network hydrogels. Soft Matter 2012, 8, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fei, R.; George, J.T.; Park, J.; Means, A.K.; Grunlan, M.A. Ultra-strong thermoresponsive double network hydrogels. Soft Matter 2013, 9, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, N.; Ye, M. Preparation and characterization of dual-sensitive double network hydrogels with clay as a physical crosslinker. Appl. Clay Sci. 2015, 103, 40–45. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Bag, D.S. Highly stretchable and tough thermo-responsive double network (DN) hydrogels: Composed of PVA-borax and poly (AM-co-NIPAM) polymer networks. Eur. Polym. J. 2022, 175, 111347. [Google Scholar] [CrossRef]
- Krakovský, I.; Kouřilová, H.; Hrubovský, M.; Labuta, J.; Hanyková, L. Thermoresponsive double network hydrogels composed of poly(N-isopropylacrylamide) and polyacrylamide. Eur. Polym. J. 2019, 116, 415–424. [Google Scholar] [CrossRef]
- Hanyková, L.; Krakovský, I.; Šestáková, E.; Šťastná, J.; Labuta, J. Poly(N,N’-diethylacrylamide)-based thermoresponsive hydrogels with double network structure. Polymers 2020, 12, 2502. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Radecki, M.; Zhigunov, A.; Št’astná, J.; Valentová, H.; Sedláková, Z. Structures and interactions in collapsed hydrogels of thermoresponsive interpenetrating polymer networks. Colloid Polym. Sci. 2015, 293, 709–720. [Google Scholar] [CrossRef]
- Kouřilová, H.; Spěváček, J.; Hanyková, L. 1H NMR study of temperature-induced phase transitions in aqueous solutions of poly(N-isopropylmethacrylamide)/poly(N-vinylcaprolactam) mixtures. Polym. Bull. 2013, 70, 221–235. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Radecki, M.; Zhigunov, A.; Kouřilová, H.; Sedláková, Z. Phase transition in hydrogels of thermoresponsive semi-interpenetrating and interpenetrating networks of poly(N,N-diethylacrylamide) and polyacrylamide. Eur. Polym. J. 2016, 85, 1–13. [Google Scholar] [CrossRef]
- Costa, R.O.R.; Freitas, R.F.S. Phase behavior of poly(N-isopropylacrylamide) in binary aqueous solutions. Polymer 2002, 43, 5879–5885. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Bian, F.; Wang, B.; Chen, S.; Jin, S. The effect of NaCl on the conformational behavior of acenaphthylene labeled poly(N,N-diethylacrylamide) in dilute aqueous solution. Macromol. Chem. Phys. 2006, 207, 104–110. [Google Scholar] [CrossRef]
- Spěváček, J.; Hanyková, L. 1H NMR study on the hydration during temperature-induced phase separation in concentrated poly(vinyl methyl ether)/D2O solutions. Macromolecules 2005, 38, 9187–9191. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Ilavský, M. 1H NMR study of thermotropic phase transition of linear and crosslinked poly(vinyl methyl ether) in D2O. Polymer 2001, 42, 8607–8612. [Google Scholar] [CrossRef]
- Díez-Peña, E.; Quijada-Garrido, I.; Barrales-Rienda, J.M.; Wilhelm, M.; Spiess, H.W. NMR studies of the structure and dynamics of polymer gels based on N-isopropylacrylamide (N-iPAAm) and methacrylic acid (MAA). Macromol. Chem. Phys. 2002, 203, 491–502. [Google Scholar] [CrossRef]
- Wang, N.; Ru, G.; Wang, L.; Feng, J. 1H MAS NMR studies of the phase separation of poly(N-isopropylacrylamide) gel in binary solvents. Langmuir 2009, 25, 5898–5902. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Childress, K.K.; Pastoor, K.; Rice, C.V. Characterization of free, restricted, and entrapped water environments in poly(N-isopropyl acrylamide) hydrogels via 1H HRMAS PFG NMR spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1521–1527. [Google Scholar] [CrossRef]
- Hanyková, L.; Labuta, J.; Spěváček, J. NMR study of temperature-induced phase separation and polymer–solvent interactions in poly(vinyl methyl ether)/D2O/ethanol solutions. Polymer 2006, 47, 6107–6116. [Google Scholar] [CrossRef]
- Serizawa, T.; Wakita, K.; Akashi, M. Rapid deswelling of porous poly(N-isopropylacrylamide) hydrogels prepared by incorporation of silica particles. Macromolecules 2002, 35, 10–12. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Li, Z.; Xia, Q.B.; Bajalis, E.; Xi, H.X.; Lin, Y.S. Swelling/deswelling kinetics of PNIPAAm hydrogels synthesized by microwave irradiation. Chem. Eng. J. 2008, 142, 263–270. [Google Scholar] [CrossRef]
- Pastoriza, A.; Pacios, I.E.; Pierola, I.F. Kinetics of solvent responsiveness in poly(N,N-dimethylacrylamide) hydrogels of different morphology. Polym. Int. 2005, 54, 1205–1211. [Google Scholar] [CrossRef]
- Franke, D.; Gerlach, G. Swelling studies of porous and nonporous semi-IPN hydrogels for sensor and actuator applications. Micromachines 2020, 11, 425. [Google Scholar] [CrossRef]
- Ceylan, D.; Ozmen, M.M.; Okay, O. Swelling–deswelling kinetics of ionic poly(acrylamide) hydrogels and cryogels. J. Appl. Polym. Sci. 2006, 99, 319–325. [Google Scholar] [CrossRef]
- Sivanantham, M.; Tata, B.V.R. Swelling/deswelling of polyacrylamide gels in aqueous NaCl solution: Light scattering and macroscopic swelling study. Pramana 2012, 79, 457–469. [Google Scholar] [CrossRef]
- Labuta, J.; Hill, J.P.; Hanyková, L.; Ishihara, S.; Ariga, K. Probing the micro-phase separation of thermo-responsive amphiphilic polymer in water/ethanol solution. J. Nanosci. Nanotechnol. 2010, 10, 8408–8416. [Google Scholar] [CrossRef] [PubMed]
- Farrar, T.C.; Becker, E.D. Pulse and Fourier Transform NMR; Academic Press: New York, NY, USA, 1971. [Google Scholar]











| Solution | NMR Signal | T2 (s) |
|---|---|---|
| Water, T = 25 °C | HDO | 4.1 |
| Water, T = 45 °C | HDO | 5.0 *, 1.0 * |
| 20 vol.% acetone, T = 25 °C | HDO | 4.5 |
| 20 vol.% acetone, T = 25 °C | Acetone | 6.7 |
| 80 vol.% acetone, T = 25 °C | HDO | 6.9 *, 0.007 * |
| 80 vol.% acetone, T = 25 °C | Acetone | 10.3 *, 0.011 * |
| Solution | τ1D (min) | τ2D (min) | τ1S (min) | τ2S (min) |
|---|---|---|---|---|
| 30 vol.% acetone | 11.0 | 121 | 8.2 | 131 |
| 40 vol.% acetone | 7.8 | 119 | 9.0 | 121 |
| 50 vol.% acetone | 9.6 | 82 | 10.0 | 104 |
| 60 vol.% acetone | 5.6 | 71 | 8.4 | 101 |
| 80 vol.% acetone | - | 31 | 10.8 | 108 |
| 100 vol.% acetone | - | 17 | 11.2 | 94 |
| 6 M NaCl | 8.4 | 123 | 10.8 | 127 |
| Water, T = 45 °C | 3.0 | 101 | 4.2 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šťastná, J.; Ivaniuzhenkov, V.; Hanyková, L. External Stimuli-Responsive Characteristics of Poly(N,N′-diethylacrylamide) Hydrogels: Effect of Double Network Structure. Gels 2022, 8, 586. https://doi.org/10.3390/gels8090586
Šťastná J, Ivaniuzhenkov V, Hanyková L. External Stimuli-Responsive Characteristics of Poly(N,N′-diethylacrylamide) Hydrogels: Effect of Double Network Structure. Gels. 2022; 8(9):586. https://doi.org/10.3390/gels8090586
Chicago/Turabian StyleŠťastná, Julie, Vladislav Ivaniuzhenkov, and Lenka Hanyková. 2022. "External Stimuli-Responsive Characteristics of Poly(N,N′-diethylacrylamide) Hydrogels: Effect of Double Network Structure" Gels 8, no. 9: 586. https://doi.org/10.3390/gels8090586