Emulsion Gels Formed by Electrostatic Interaction of Gelatine and Modified Corn Starch via pH Adjustments: Potential Fat Replacers in Meat Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Compositions
2.2. pH Values
2.3. Color
2.4. Syneresis and Thermal Stability
2.5. Gel Strength and Hardness
2.6. Particle Size and Zeta Potential
2.7. Lipid Oxidation
2.8. Oil Binding Capacity
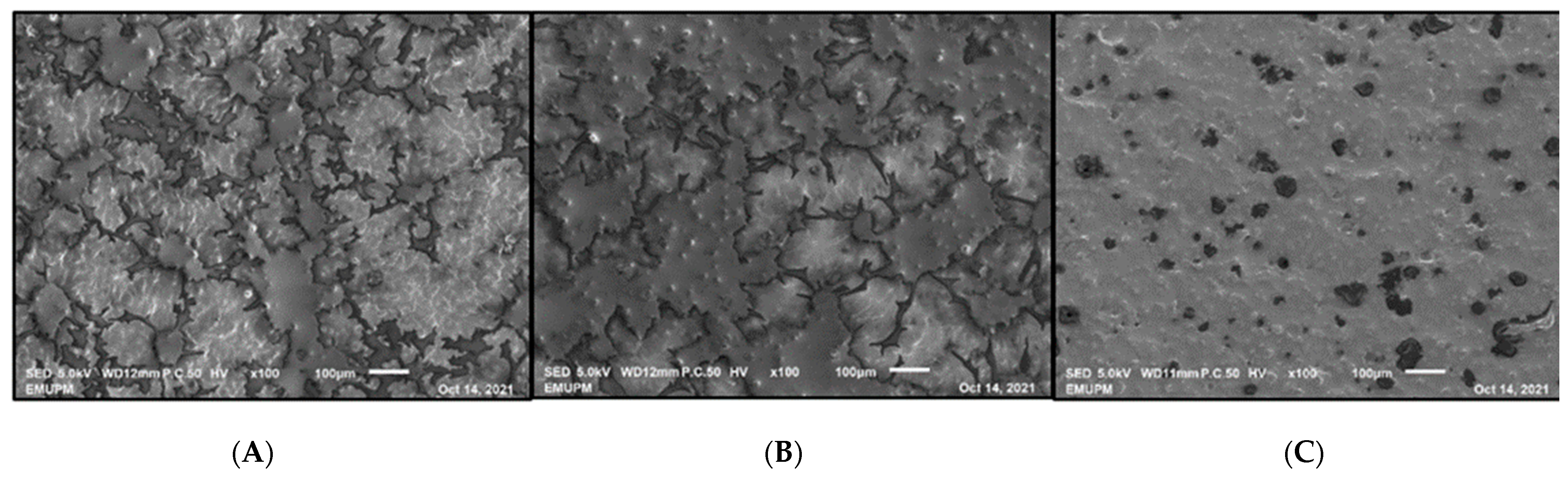
2.9. Microstructure
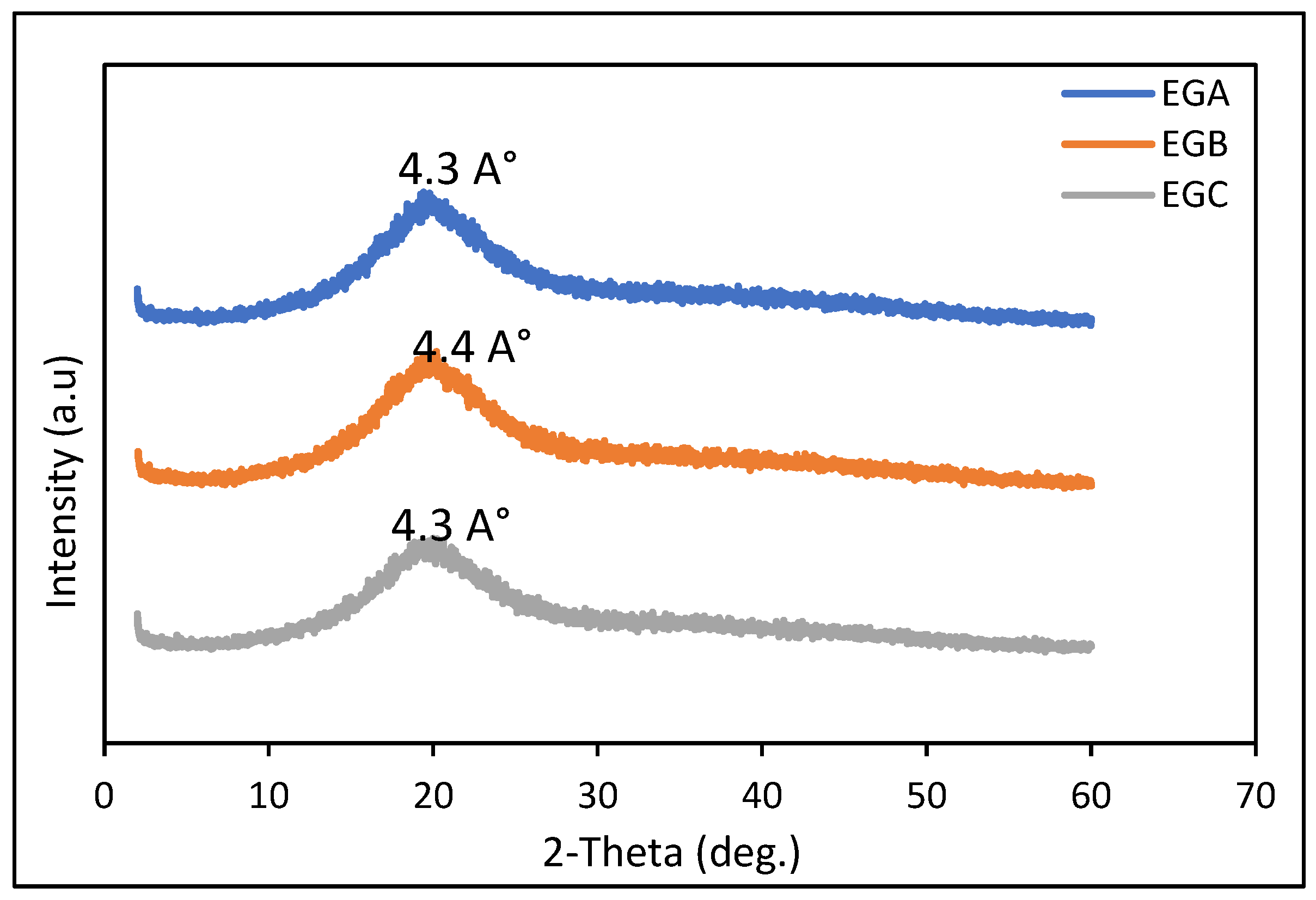
2.10. X-ray Diffraction
2.11. Fatty Acids Profile
2.12. Differential Scanning Calorimetry
3. Conclusions
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Proximate Composition Analysis
4.3. pH Analysis
4.4. Color Measurement
4.5. Syneresis and Thermal Stability
4.6. Gel Strength and Hardness
4.7. Particle Size and Zeta Potential
4.8. Lipid Oxidation
4.9. Oil Binding Capacity (OBC)
4.10. Microstructure
4.11. X-ray Diffraction
4.12. Fatty Acids Analysis
4.13. Differential Scanning Calorimetry
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Ruiz-Capillas, C.; Herrero, A.M. New lipid materials based on chia emulsion gels: Application in meat products. Biomed. J. Sci. Technol. Res. 2019, 18, 13215–13218. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Emulsion gels as potential fat replacers delivering β-glucan and healthy lipid content for food applications. J. Food Sci. Technol. 2016, 53, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Asyrul-Izhar, A.B.; Bakar, J.; Sazili, A.Q.; Meng, G.Y.; Ismail-Fitry, M.R. Incorporation of Different Physical Forms of Fat Replacers in the Production of Low-Fat/Reduced-Fat Meat Products: Which is More Practical? Food Rev. Int. 2022, 38, 1–33. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Okuro, P.K.; Santos, M.; de Souza Paglarini, C.; da Cunha, R.L.; Ruiz-Capillas, C.; Herrero, A.M.; Pollonio, M.A.R. Understanding the role of chia (Salvia Hispanica L.) mucilage on olive oil-based emulsion gels as a new fat substitute in emulsified meat products. Eur. Food Res. Technol. 2020, 246, 909–922. [Google Scholar] [CrossRef]
- de Souza Paglarini, C.; de Figueiredo Furtado, G.; Biachi, J.P.; Vidal, V.A.S.; Martini, S.; Forte, M.B.S.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels with potential application in meat products. J. Food Eng. 2018, 222, 29–37. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Hubinger, M.D. Effect of pH and Pea Protein: Xanthan Gum Ratio on Emulsions with High Oil Content and High Internal Phase Emulsion Formation. Molecules 2021, 26, 5646. [Google Scholar] [CrossRef]
- Freire, M.; Cofrades, S.; Pérez-Jiménez, J.; Gómez-Estaca, J.; Jiménez-Colmenero, F.; Bou, R. Emulsion gels containing n-3 fatty acids and condensed tannins designed as functional fat replacers. Food Res. Int. 2018, 113, 465–473. [Google Scholar] [CrossRef]
- Khalesi, H.; Emadzadeh, B.; Kadkhodaee, R.; Fang, Y. Effect of Persian gum on whey protein concentrate cold-set emulsion gel: Structure and rheology study. Int. J. Biol. Macromol. 2019, 125, 17–26. [Google Scholar] [CrossRef]
- dos Santos, R.S.; Rosseto, H.C.; da Silva, J.B.; Vecchi, C.F.; Caetano, W.; Bruschi, M.L. The effect of carbomer 934P and different vegetable oils on physical stability, mechanical and rheological properties of emulsion-based systems containing propolis. J. Mol. Liq. 2020, 307, 112969. [Google Scholar] [CrossRef]
- Wijaya, W.; Sun, Q.Q.; Vermeir, L.; Dewettinck, K.; Patel, A.R.; Van der Meeren, P. pH and protein to polysaccharide ratio control the structural properties and viscoelastic network of HIPE-templated biopolymeric oleogels. Food Struct. 2019, 21, 100112. [Google Scholar] [CrossRef]
- Nicoletti Telis, V.R. O/W emulsions stabilized by interactions between proteins and polysaccharides. In Encyclopedia of Food Chemistry; Varelis, P., Melton, L., Shahidi, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 494–498. [Google Scholar]
- Yang, X.; Li, A.; Yu, W.; Li, X.; Sun, L.; Xue, J.; Guo, Y. Structuring oil-in-water emulsion by forming egg yolk/alginate complexes: Their potential application in fabricating low-fat mayonnaise-like emulsion gels and redispersible solid emulsions. Int. J. Biol. Macromol. 2020, 147, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; She, Y.; Zhang, R.; Wang, J.; Zhang, X.; Gou, X. Use of starch-based fat replacers in foods as a strategy to reduce dietary intake of fat and risk of metabolic diseases. Food Sci. Nutr. 2020, 8, 16–22. [Google Scholar] [CrossRef]
- Chavan, R.S.; Khedkar, C.D.; Bhatt, S. Fat replacer. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: London, UK, 2016; Volume 2, pp. 589–595. [Google Scholar]
- Peng, X.; Yao, Y. Carbohydrates as fat replacers. Annu. Rev. Food Sci. Technol. 2017, 8, 331–351. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Liang, B.; Wu, T.; Sui, W.; Zhang, M. Microparticulated whey protein-pectin complex: A texture-controllable gel for low-fat mayonnaise. Food Res. Int. 2018, 108, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lobato-Calleros, C.; Ramírez-Santiago, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Impact of native and chemically modified starches addition as fat replacers in the viscoelasticity of reduced-fat stirred yogurt. J. Food Eng. 2014, 131, 110–115. [Google Scholar] [CrossRef]
- Wu, B.C.; McClements, D.J. Microgels formed by electrostatic complexation of gelatin and OSA starch: Potential fat or starch mimetics. Food Hydrocoll. 2015, 47, 87–93. [Google Scholar] [CrossRef]
- Morimura, S.; Nagata, H.; Uemura, Y.; Fahmi, A.; Shigematsu, T.; Kida, K. Development of an effective process for utilization of collagen from livestock and fish waste. Process Biochem. 2002, 37, 1403–1412. [Google Scholar] [CrossRef]
- Simpson, B.K.; Nollet, L.M.; Toldrá, F.; Benjakul, S.; Paliyath, G.; Hui, Y.H. Food Biochemistry and Food Processing, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Segtnan, V.H.; Kvaal, K.; Rukke, E.O.; Schüller, R.B.; Isaksson, T. Rapid assessment of physico-chemical properties of gelatine using near infrared spectroscopy. Food Hydrocoll. 2003, 17, 585–592. [Google Scholar] [CrossRef]
- Ch’ng, S.E.; Ng, M.D.; Pindi, W.; Kang, O.L.; Abdullah, A.; Babji, A.S. Chicken sausages formulated with gelatin from different sources: A comparison of sensory acceptability and storage stability. World Appl. Sci. J. 2014, 31, 2062–2067. [Google Scholar]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Cavalheiro, C.P.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. The role of mixing sequence in structuring O/W emulsions and emulsion gels produced by electrostatic protein-polysaccharide interactions between soy protein isolate-coated droplets and alginate molecules. Food Hydrocoll. 2021, 113, 106537. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Gong, Y.; Li, Z.; Guo, Y.; Yu, D.; Pan, M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason Sonochem. 2021, 79, 105756. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Gray, D.A.; Decker, E.A. Stabilization of soybean oil bodies by enzyme (laccase) cross-linking of adsorbed beet pectin coatings. J. Agric. Food Chem. 2010, 58, 9259–9265. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Gibis, M.; Fischer, L.; Weiss, J. Crosslinking of interfacial layers in multilayered oil-in-water emulsions using laccase: Characterization and pH-stability. Food Hydrocoll. 2012, 27, 126–136. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Bispo, M.; Moraes, I.C.; Campanella, O.H.; Pinho, S.C. Stability of curcumin encapsulated in solid lipid microparticles incorporated in cold-set emulsion filled gels of soy protein isolate and xanthan gum. Food Res. Int. 2017, 102, 759–767. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Lin, D.; Lu, W.; Kelly, A.L.; Zhang, L.; Zheng, B.; Miao, S. Interactions of vegetable proteins with other polymers: Structure-function relationships and applications in the food industry. Trends Food Sci. Technol. 2017, 68, 130–144. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein-and polysaccharide-based particles used for Pickering emulsion stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar]
- Turgeon, S.L.; Laneuville, S.I. Protein+ polysaccharide coacervates and complexes: From scientific background to their application as functional ingredients in food products. In Modern Biopolymer Science, 1st ed.; Kasapis, S., Norton, I., Ubbink, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 327–363. [Google Scholar]
- Li, H.; Wang, T.; Hu, Y.; Wu, J.; Van der Meeren, P. Designing delivery systems for functional ingredients by protein/polysaccharide interactions. Trends Food Sci. Technol. 2021, 119, 272–287. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene. Food Chem. 2022, 372, 131262. [Google Scholar] [CrossRef]
- Wu, B.C.; Degner, B.; McClements, D.J. Soft matter strategies for controlling food texture: Formation of hydrogel particles by biopolymer complex coacervation. J. Condens. Matter Phys. 2014, 26, 464104. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Wijaya, C.H.; Patel, A.R. High internal phase emulsions stabilized solely by whey protein isolate-low methoxyl pectin complexes: Effect of pH and polymer concentration. Food Funct. 2017, 8, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.P.; Zhang, H.H.; Huang, G.Q.; Xiao, J.X. Whey protein isolate—Low methoxyl pectin coacervates as a high internal phase Pickering emulsion stabilizer. J. Dispers. Sci. Technol. 2021, 42, 1009–1020. [Google Scholar] [CrossRef]
- Yan, C.; McClements, D.J.; Zhu, Y.; Zou, L.; Zhou, W.; Liu, W. Fabrication of OSA starch/chitosan polysaccharide-based high internal phase emulsion via altering interfacial behaviors. J. Agric. Food Chem. 2019, 67, 10937–10946. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Merino-Álvarez, E.; Salvador, M.; Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Herrero, A.M. Chia (Salvia hispanica L.) a promising alternative for conventional and gelled emulsions: Technological and lipid structural characteristics. Gels 2019, 5, 19. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Olive oil-in-water emulsions stabilized with caseinate: Elucidation of protein–lipid interactions by infrared spectroscopy. Food Hydrocoll. 2011, 25, 12–18. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C. Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil. Gels 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.I.; Gadallah, M.G.E.; Sorour, A.M. Physico-chemical and rheological properties of modified corn starches and its effect on noodle quality. Ann. Agric. Sci. 2012, 57, 19–27. [Google Scholar] [CrossRef]
- Azaripour, A.; Abbasi, H. Effect of type and amount of modified corn starches on qualitative properties of low-protein biscuits for phenylketonuria. Food Sci. Nutr. 2019, 8, 281–290. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Choi, Y.S.; Han, D.J.; Choi, J.H.; Hwang, K.E.; Song, D.H.; Kim, H.W.; Kim, Y.B.; Kim, C.J. Effect of chicken skin on the quality characteristics of semi-dried restructured jerky. Poult. Sci. 2016, 95, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Öztürk-Kerimoğlu, B.; Kara, A.; Urgu-Öztürk, M.; Serdaroğlu, M. A new inverse olive oil emulsion plus carrot powder to replace animal fat in model meat batters. LWT-Food Sci. Technol. 2021, 135, 110044. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Puolanne, E.J.; Ruusunen, M.H.; Vainionpää, J.I. Combined effects of NaCl and raw meat pH on water-holding in cooked sausage with and without added phosphate. Meat Sci. 2001, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.T.; Jeong, H.; Kim, J.; Lee, S.K. Formula optimization of a perilla-canola oil (O/W) emulsion and its potential application as an animal fat replacer in meat emulsion. Korean J. Food Sci. Anim. Resour. 2018, 38, 580. [Google Scholar]
- Chantrapornchai, W.; Clydesdale, F.; McClements, D.J. Influence of droplet characteristics on the optical properties of colored oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1999, 155, 373–382. [Google Scholar] [CrossRef]
- Sato, A.C.K.; Moraes, K.E.F.P.; Cunha, R.L. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll. 2014, 34, 184–192. [Google Scholar] [CrossRef]
- Perrechil, F.A.; Cunha, R.L. Stabilization of multilayered emulsions by sodium caseinate and κ-carrageenan. Food Hydrocoll. 2013, 30, 606–613. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-protein interactions and their relevance in food colloids. In The Complex World of Polysaccharide; Karunaratne, D.N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 395–406. [Google Scholar]
- Tokle, T.; McClements, D.J. Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: Effects of pH, salt and heating. Food Hydrocoll. 2011, 25, 976–982. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Azarikia, F.; Abbasi, S.; Scanlon, M.G.; McClements, D.J. Emulsion stability enhancement against environmental stresses using whey protein–tragacanthin complex: Comparison of layer-by-layer and mixing methods. Int. J. Food Prop. 2017, 20, 2084–2095. [Google Scholar] [CrossRef]
- Albano, K.M.; Nicoletti, V.R. Ultrasound impact on whey protein concentrate-pectin complexes and in the O/W emulsions with low oil soybean content stabilization. Ultrason Sonochem. 2018, 41, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Wang, J.; Stoddard, F.; Salovaara, H.; Sontag-Strohm, T. Preparation and Characterization of Emulsion Gels from Whole Faba Bean Flour. Foods. 2020, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, R.A.; Cavallieri, Â.L.F.; Cunha, R.L. Gelation of oil-in-water emulsions stabilized by whey protein. J. Food Eng. 2016, 175, 108–116. [Google Scholar] [CrossRef]
- Patel, A.; Longmore, N.; Mohanan, A.; Ghosh, S. Salt and pH-induced attractive interactions on the rheology of food protein-stabilized nanoemulsions. ACS Omega 2019, 4, 11791–11800. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and water-holding properties and microstructures of soy protein isolate emulsion gels induced by CaCl2, glucono-δ-lactone (GDL), and transglutaminase: Influence of thermal treatments before and/or after emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, G.; Shi, X.; Ma, C.; Liu, F. Comparative Study of Heat-and Enzyme-Induced Emulsion Gels Formed by Gelatin and Whey Protein Isolate: Physical Properties and Formation Mechanism. Gels 2022, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 59–94. [Google Scholar]
- Lee, M.C.; Jiang, X.; Brenna, J.T.; Abbaspourrad, A. Oleogel-structured composite for the stabilization of ω3 fatty acids in fish oil. Food Funct. 2018, 9, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Szumała, P.; Wysocka, I. Effect of gelation and storage conditions on the oxidative stability of microemulsion and nanoemulsion delivery systems. Eur. J. Pharm. Sci. 2018, 124, 17–25. [Google Scholar] [CrossRef]
- Charoen, R.; Jangchud, A.; Jangchud, K.; Harnsilawat, T.; Decker, E.A.; McClements, D.J. Influence of interfacial composition on oxidative stability of oil-in-water emulsions stabilized by biopolymer emulsifiers. Food Chem. 2012, 131, 1340–1346. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Lu, W.; Gul, K.; Mata, A.; Fang, Y. Emulsion structure design for improving the oxidative stability of polyunsaturated fatty acids. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2955–2971. [Google Scholar] [CrossRef]
- Huang, Y.; Li, A.; Qiu, C.; Teng, Y.; Wang, Y. Self-assembled colloidal complexes of polyphenol–gelatin and their stabilizing effects on emulsions. Food Funct. 2017, 8, 3145–3154. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Jin, O.J.; Muhamad, N.J.; Ishak, W.R. Physicochemical properties and stability of Moringa oleifera seed oil-in-water emulsions as affected by different types of polysaccharide and emulsifier. Mal. J. Fund. Appl. Sci. 2019, 324–329. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Choi, K.O.; Kim, D.E.; Kang, W.S.; Ko, S. Improvement of oxidative stability of rice bran oil emulsion by controlling droplet size. J. Food Process. Preserv. 2013, 37, 139–151. [Google Scholar] [CrossRef]
- Espert, M.; Hernández, M.J.; Sanz, T.; Salvador, A. Rheological properties of emulsion templated oleogels based on xanthan gum and different structuring agents. Curr. Res. Food Sci. 2022, 5, 564–570. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Sato, A.C.K.; Cunha, R.L. Emulsifying properties of collagen fibers: Effect of pH, protein concentration and homogenization pressure. Food Hydrocoll. 2011, 25, 604–612. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Bai, X. Effects of Polysaccharide Concentrations on the Formation and Physical Properties of Emulsion-Templated Oleogels. Molecules 2022, 27, 5391. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Yanty, N.A.M.; Marikkar, J.M.N.; Miskandar, M.S.; Van Bockstaele, F.; Dewettinck, K.; Nusantoro, B. Compatibility of selected plant-based shortening as lard substitute: Microstructure, polymorphic forms and textural properties. Grasas y Aceites. 2017, 68, e181. [Google Scholar] [CrossRef]
- Tiensa, B.E.; Barbut, S.; Marangoni, A.G. Influence of fat structure on the mechanical properties of commercial pate products. Food Res. Int. 2017, 100, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Svenstrup, G.; Brüggemann, D.; Kristensen, L.; Risbo, J.; Skibsted, L.H. The influence of pretreatment on pork fat crystallization. Eur. J. Lipid Sci. Technol. 2005, 107, 607–615. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yılmaz, E. Storage stability of cod liver oil organogels formed with beeswax and carnauba wax. Int. J. Food Sci. Technol. 2015, 50, 404–412. [Google Scholar] [CrossRef]
- Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar]
- Alejandre, M.; Astiasarán, I.; Ansorena, D.; Barbut, S. Using canola oil hydrogels and organogels to reduce saturated animal fat in meat batters. Food Res. Int. 2019, 122, 129–136. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Yilmaz, E.E.; Vural, H.; Yadigari, R.J. Thermal, microscopic, and quality properties of low-fat frankfurters and emulsions produced by addition of different hydrocolloids. Int. J. Food Prop. 2017, 20, 1987–2002. [Google Scholar] [CrossRef]
- Wen, Y.; Che, Q.T.; Kim, H.W.; Park, H.J. Potato starch altered the rheological, printing, and melting properties of 3D-printable fat analogs based on inulin emulsion-filled gels. Carbohydr. Polym. 2021, 269, 118285. [Google Scholar] [CrossRef]
- Bi, W.; Zhao, W.; Li, D.; Li, X.; Yao, C.; Zhu, Y.; Zhang, Y. Effect of resistant starch and inulin on the properties of imitation mozzarella cheese. Int. J. Food Prop. 2016, 19, 159–171. [Google Scholar] [CrossRef]
- Mounsey, J.S.; O’Riordan, E.D. Alteration of imitation cheese structure and melting behaviour with wheat starch. Eur. Food Res. Technol. 2008, 226, 1013–1019. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Su, C.; Gao, Y.; Li, Q.; Du, S.; Yu, X. Effect of water content on the physical properties and structure of walnut oleogels. RSC Adv. 2022, 12, 8987–8995. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ma, C.; Yan, X.; Zeng, H.; McClements, D.J.; Liu, X.; Liu, F. Structure, rheology and functionality of whey protein emulsion gels: Effects of double cross-linking with transglutaminase and calcium ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernández-Martín, F.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Matsuo, K.; Ueno, S. Formation and Physical Analysis of Oleogels Composed of Edible Oils and High-Melting Fat Crystals. J. Oleo Sci. 2021, 70, 1381–1390. [Google Scholar] [CrossRef]
- Douaire, M.; Di Bari, V.; Norton, J.E.; Sullo, A.; Lillford, P.; Norton, I.T. Fat crystallisation at oil–water interfaces. Adv. Colloid Interface Sci. 2014, 203, 1–10. [Google Scholar] [CrossRef]
- Bi, C.H.; Chi, S.Y.; Zhou, T.; Wang, X.Y.; Zhang, J.Y.; Huang, Z.G.; Gao, F. Characterization of a Novel High Internal Phase Pickering Emulsions Stabilized by Soy Protein Self-Assembled Gel Particles. Front. Nutr. 2021, 8, 795396. [Google Scholar] [CrossRef]
- Rousseau, D. Trends in structuring edible emulsions with Pickering fat crystals. Curr. Opin. Colloid Interface Sci. 2013, 18, 283–291. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995; pp. 32–42. [Google Scholar]
- Asyrul-Izhar, A.B.; Sarbon, N.M.; Ismail-Fitry, M.R. Effects of Mixing Duration and Raw Materials on the Physicochemical, Microstructural and Sensorial Properties of Sausages Prepared From Red Tilapia (Oreochromis sp.). Asian Fish. Sci. 2021, 34, 355–364. [Google Scholar] [CrossRef]
- Ismail, M.A.; Chong, G.H.; Ismail-Fitry, M.R. Comparison of the microstructural, physicochemical and sensorial properties of buffalo meat patties produced using bowl cutter, universal mixer and meat mixer. J. Food Sci. Technol. 2021, 58, 4703–4710. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, D.; Bael, K.; Thas, O.; Dewettinck, K. Interactions between κ-carrageenan, milk proteins and modified starch in sterilized dairy desserts. Int. Dairy J. 2006, 16, 482–488. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Carballo, J.; Solas, M.T. The effect of use of freeze thawed pork on properties of bologna sausage with two fat levels. Int. J. Food Sci. Technol. 1995, 30, 335–345. [Google Scholar] [CrossRef]
- Gaudino, N. Development of Lecithin-Based Oleogels and Oleogel Emulsions with Stearic Acid Capable of Enhancing Probiotic Viability and Delaying Oxidation. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2018. [Google Scholar]
- Wang, X.Y.; Wang, J.; Rousseau, D.; Tang, C.H. Chitosan-stabilized emulsion gels via pH-induced droplet flocculation. Food Hydrocoll. 2020, 105, 105811. [Google Scholar] [CrossRef]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Palla, C.; Giacomozzi, A.; Genovese, D.B.; Carrín, M.E. Multi–objective optimization of high oleic sunflower oil and monoglycerides oleogels: Searching for rheological and textural properties similar to margarine. Food Struct. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS, 6th ed.; American Oil Chemists’ Society: Urbana, OH, USA, 2009. [Google Scholar]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]


| Samples | EGA | EGB | EGC |
|---|---|---|---|
| Moisture | 57.14 ± 1.20 a | 58.09 ± 1.02 a | 58.95 ± 1.81 a |
| Ash | 2.35 ± 0.29 a | 2.53 ± 0.26 a | 2.36 ± 0.13 a |
| Protein | 3.28 ± 0.51 a | 2.88 ± 0.35 a | 2.48 ± 0.17 a |
| Fat | 7.03 ± 0.25 a | 6.87 ± 0.99 a | 8.33 ± 0.60 a |
| pH | 5.03 ± 0.06 a | 4.50 ± 0.00 b | 3.63 ± 0.06 c |
| Parameters | EGA | EGB | EGC |
|---|---|---|---|
| L* | 91.61 ± 0.24 a | 91.54 ± 0.23 a | 90.79 ± 0.02 b |
| a* | −0.84 ± 0.03 a | −1.08 ± 0.08 b | −1.34 ± 0.01 c |
| b* | 8.83 ± 0.04 c | 9.63 ± 0.49 b | 10.94 ± 0.01 a |
| Thermal Stability (Total loss) | 0.59 ± 0.03 a | 0.64 ± 0.19 a | 0.87 ± 0.07 a |
| Thermal Stability (Water loss) | 0.04 ± 0.00 a | 0.04 ± 0.04 a | 0.08 ± 0.04 a |
| Gel strength | 40.16 ± 5.71 a | 23.89 ± 2.44 b | 18.13 ± 1.09 b |
| Hardness | 1427.11 ± 37.62 a | 458.88 ± 75.54 b | 136.34 ± 6.56 c |
| Parameters | EGA | EGB | EGC |
|---|---|---|---|
| Particle Size Zeta potential | 44.53 ± 4.05 b −6.5 ± 5.19 | 54.01 ± 1.70 b +3.5 ± 0.04 | 68.34 ± 6.04 a +1.9 ± 1.58 |
| Lipid oxidation (Day 0) | 0.28 ± 0.05 b | 0.29 ± 0.02 b | 0.41 ± 0.06 a |
| Lipid oxidation (Day 13) | 0.34 ± 0.03 b | 0.37 ± 0.02 b | 0.41 ± 0.06 b |
| Oil binding capacity | 98.04 ± 0.04 a | 97.37 ± 1.12 a | 91.80 ± 1.23 b |
| Fatty Acids (%) | EGA | EGB | EGC | |
|---|---|---|---|---|
| Saturated fatty acids | Butryic | 0.0000 | 0.0000 | 0.0000 |
| Caproic | 0.0069 | 0.0000 | 0.0000 | |
| Caprylic | 0.1144 | 0.0135 | 0.0119 | |
| Capric | 0.0221 | 0.0177 | 0.0169 | |
| Undecanoic | 0.0143 | 0.0000 | 0.0000 | |
| Lauric | 0.0831 | 0.0717 | 0.0681 | |
| Tridecanoic | 0.0135 | 0.0000 | 0.0000 | |
| Myristic | 0.4058 | 0.7792 | 0.3920 | |
| Pentadecanoic | 0.0330 | 0.0868 | 0.0443 | |
| Palmitic | 6.0921 | 6.2293 | 5.5987 | |
| Heptadecanoic | 0.1546 | 0.2059 | 0.1511 | |
| Stearic | 2.5202 | 2.3468 | 2.2594 | |
| Arachidic | 0.5358 | 0.4592 | 0.4648 | |
| Henicosanoic | 0.0000 | 0.0000 | 0.0000 | |
| Behenic | 0.3964 | 0.1748 | 0.1809 | |
| Tricosanoic | 0.2472 | 0.0000 | 0.0000 | |
| Lignoceric | 0.0000 | 0.0000 | 0.0000 | |
| Monounsaturated fatty acids | Myristoleic | 0.1820 | 0.0244 | 0.0113 |
| Cis-10-Pentadecenoic | 0.0000 | 0.0000 | 0.0000 | |
| Palmitoleic | 0.5499 | 1.0102 | 0.6032 | |
| Cis-10-Heptadecanoic | 0.1153 | 0.1413 | 0.1097 | |
| Elaidic (Trans) | 0.0000 | 0.0000 | 0.0000 | |
| Oleic | 63.8491 | 57.6595 | 58.9921 | |
| Cis-11-Eicosenoic | 0.9928 | 0.9516 | 0.9385 | |
| Erucic | 0.2099 | 0.0000 | 0.0000 | |
| Nervonic | 0.4363 | 0.3388 | 0.2966 | |
| Polyunsaturated fatty acids | Linolelaidic (Trans) | 0.0000 | 0.0000 | 0.0000 |
| Linoleic (Cis) | 17.0443 | 19.7278 | 20.2468 | |
| -Linolenic | 0.2248 | 0.3315 | 0.3336 | |
| a-Linolenic | 5.7563 | 8.6076 | 8.8154 | |
| Cis-11,14-Eicosadienoic | 0.0000 | 0.0000 | 0.0000 | |
| Cis-8,11,14-Eicosatrienoic | 0.0000 | 0.0000 | 0.0000 | |
| Cis-11,14,17-Eicosatrienoic | 0.0000 | 0.0000 | 0.0000 | |
| Arachidonic | 0.0000 | 0.0000 | 0.0000 | |
| Cis-5,8,11,14,17- eicosapentaenoic | 0.0000 | 0.4844 | 0.2484 | |
| Cis-13, 16-Docosadienoic | 0.0000 | 0.0000 | 0.0000 | |
| Cis-4,7,10,13,16,19-Docosahexaenoic | 0.0000 | 0.3380 | 0.2162 | |
| Parameters | EGA | EGB | EGC |
|---|---|---|---|
| Starch | 4.4 | 7.5 | 2.6 |
| Gelatin | 5.4 | 4 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asyrul-Izhar, A.B.; Bakar, J.; Sazili, A.Q.; Goh, Y.M.; Ismail-Fitry, M.R. Emulsion Gels Formed by Electrostatic Interaction of Gelatine and Modified Corn Starch via pH Adjustments: Potential Fat Replacers in Meat Products. Gels 2023, 9, 50. https://doi.org/10.3390/gels9010050
Asyrul-Izhar AB, Bakar J, Sazili AQ, Goh YM, Ismail-Fitry MR. Emulsion Gels Formed by Electrostatic Interaction of Gelatine and Modified Corn Starch via pH Adjustments: Potential Fat Replacers in Meat Products. Gels. 2023; 9(1):50. https://doi.org/10.3390/gels9010050
Chicago/Turabian StyleAsyrul-Izhar, Abu Bakar, Jamilah Bakar, Awis Qurni Sazili, Yong Meng Goh, and Mohammad Rashedi Ismail-Fitry. 2023. "Emulsion Gels Formed by Electrostatic Interaction of Gelatine and Modified Corn Starch via pH Adjustments: Potential Fat Replacers in Meat Products" Gels 9, no. 1: 50. https://doi.org/10.3390/gels9010050
APA StyleAsyrul-Izhar, A. B., Bakar, J., Sazili, A. Q., Goh, Y. M., & Ismail-Fitry, M. R. (2023). Emulsion Gels Formed by Electrostatic Interaction of Gelatine and Modified Corn Starch via pH Adjustments: Potential Fat Replacers in Meat Products. Gels, 9(1), 50. https://doi.org/10.3390/gels9010050







