Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
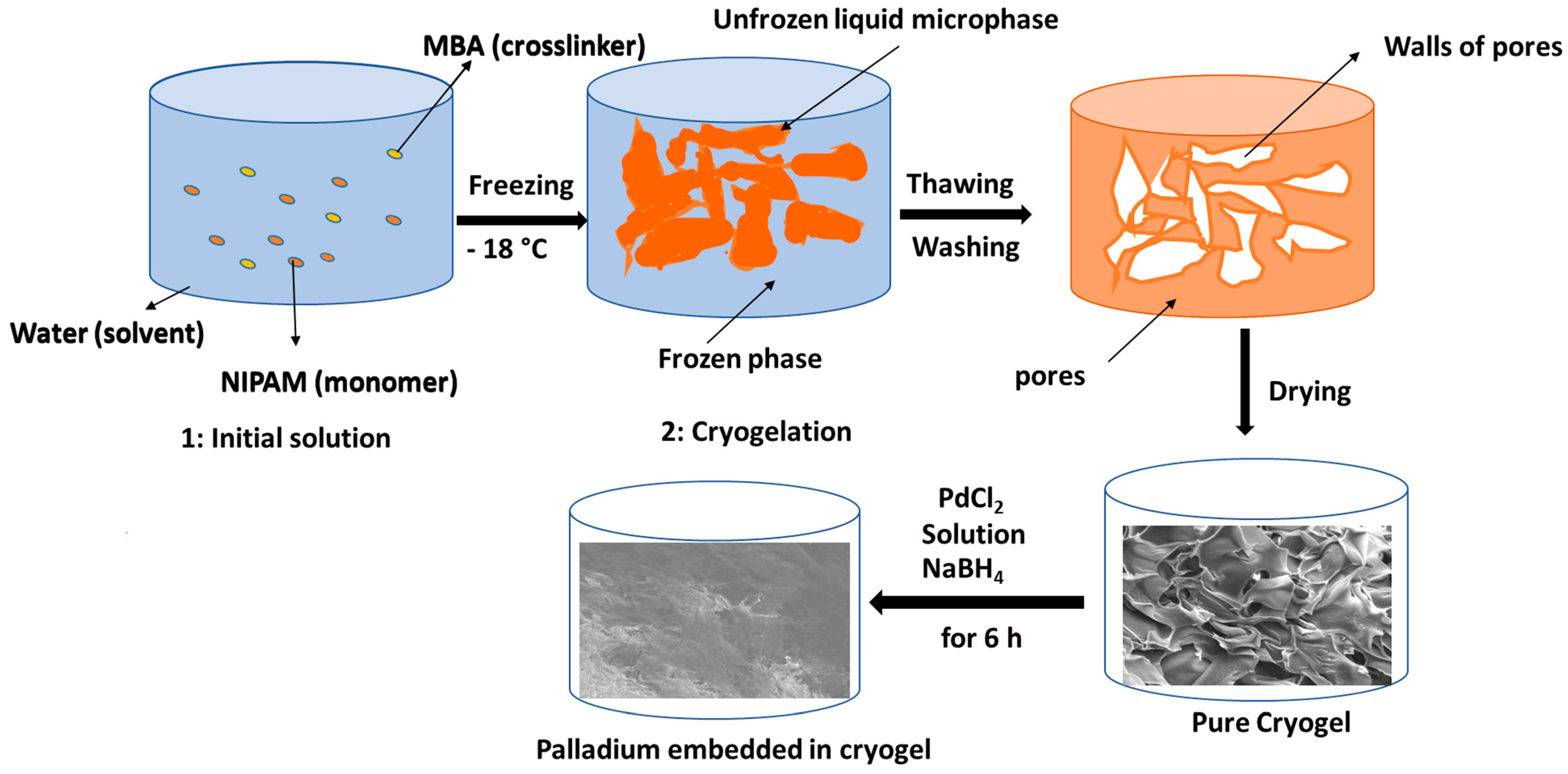
2.2. Synthesis of Pure Cryogel
2.3. Synthesis of Hybrid Cryogels
2.3.1. Synthesis of Silver Based Cryogel
2.3.2. Synthesis of Palladium Based Cryogel
2.4. Synthesis of Hydrogel and Its Hybrid with Silver Nanoparticles
2.5. Catalytic Study
2.6. Antibacterial Study
2.7. Instrumentation
3. Results and Discussion
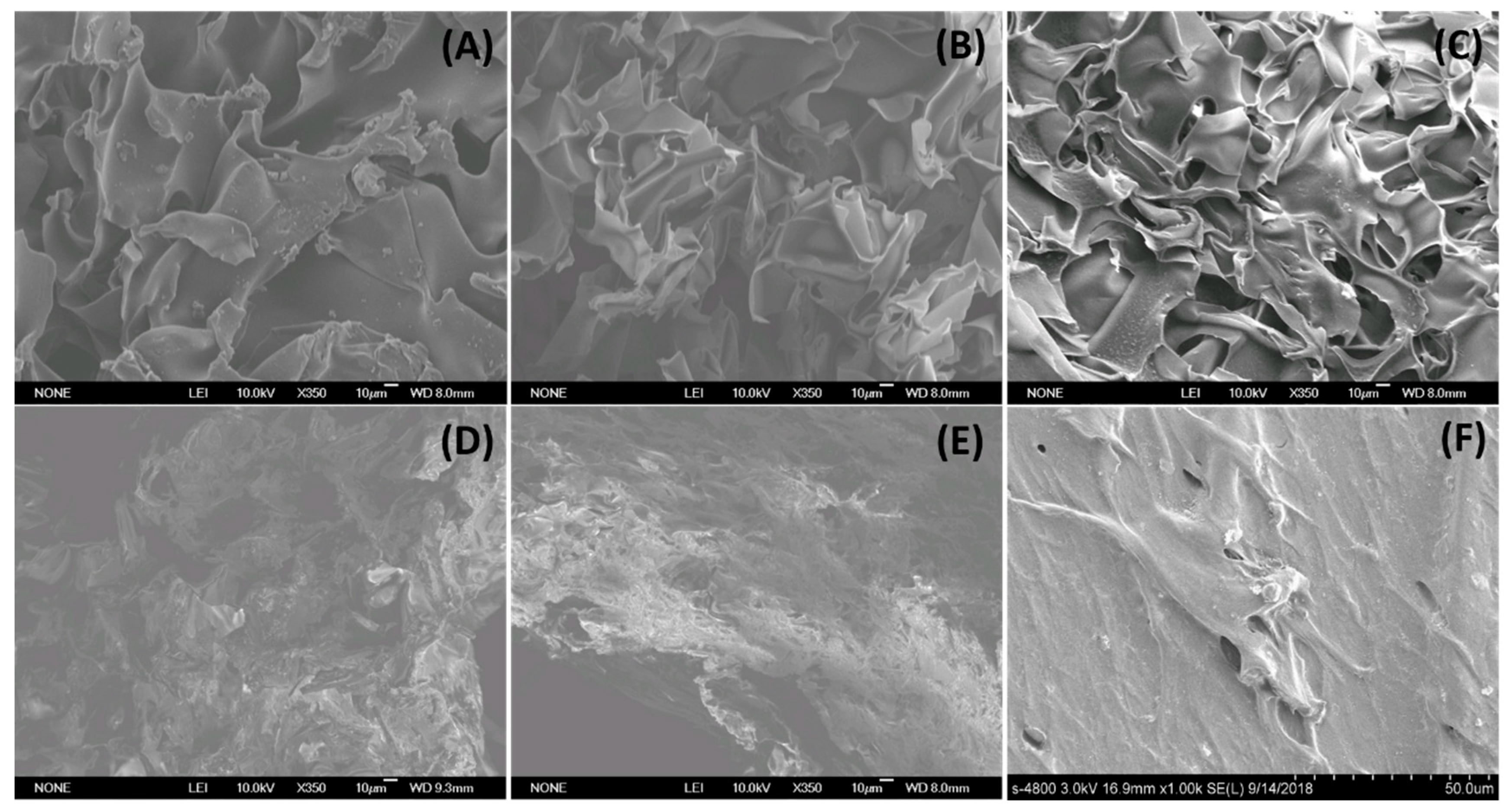
3.1. Scanning Electron Microscopy (SEM) of Cryogels and a Hydrogel
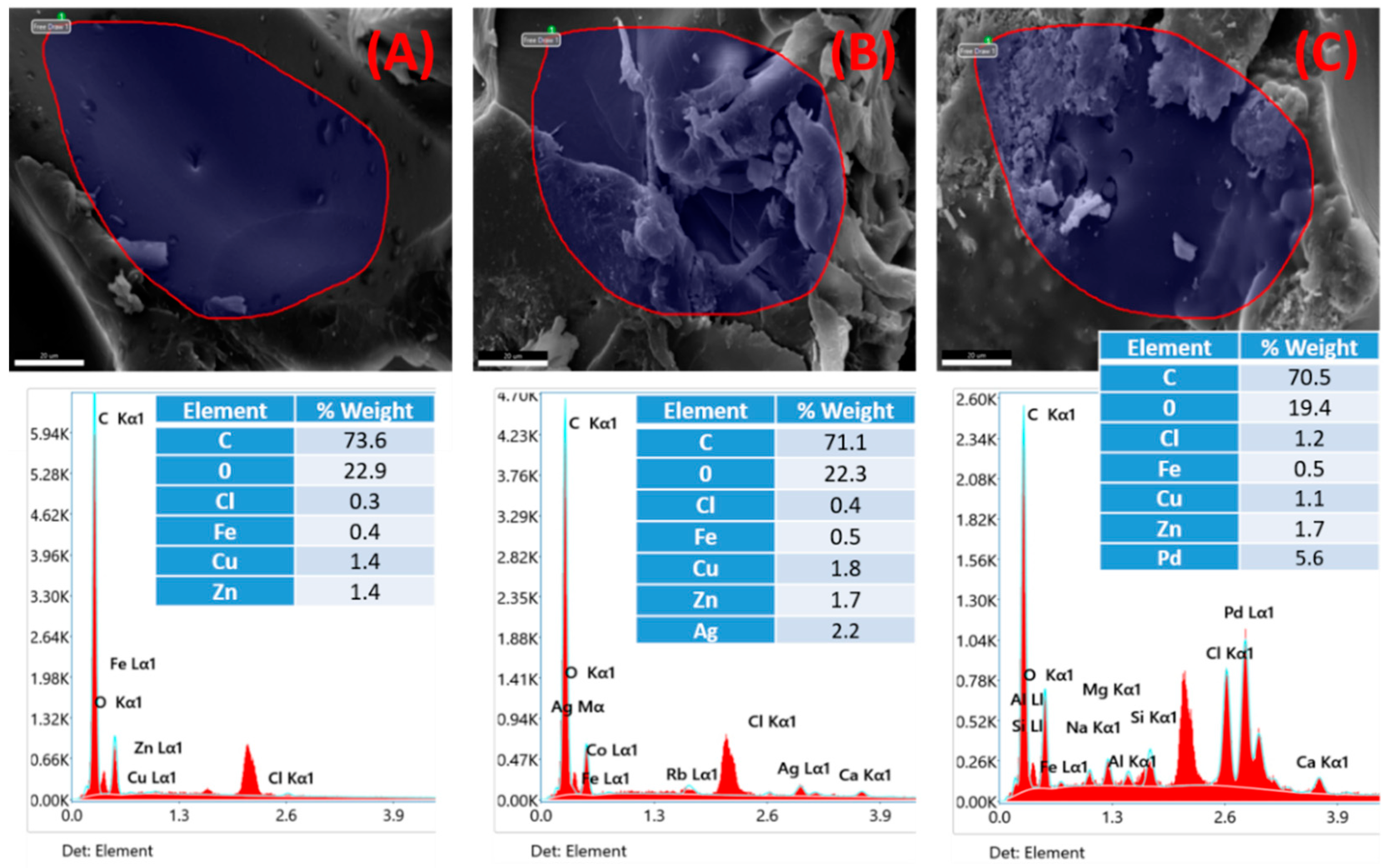
3.2. Chemical Analysis of Pure and Hybrid Cryogels
4. Application of Hybrid Cryogels
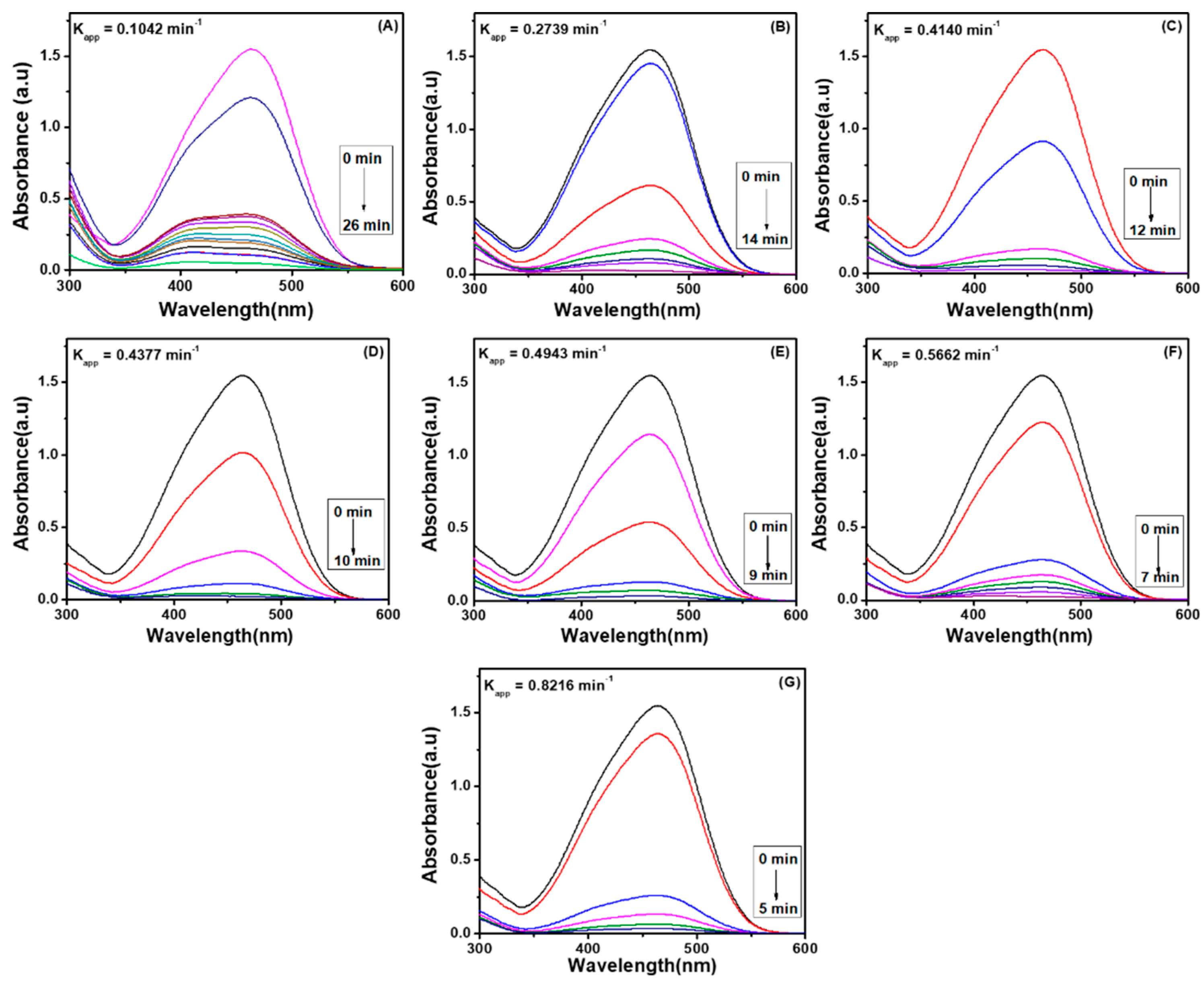
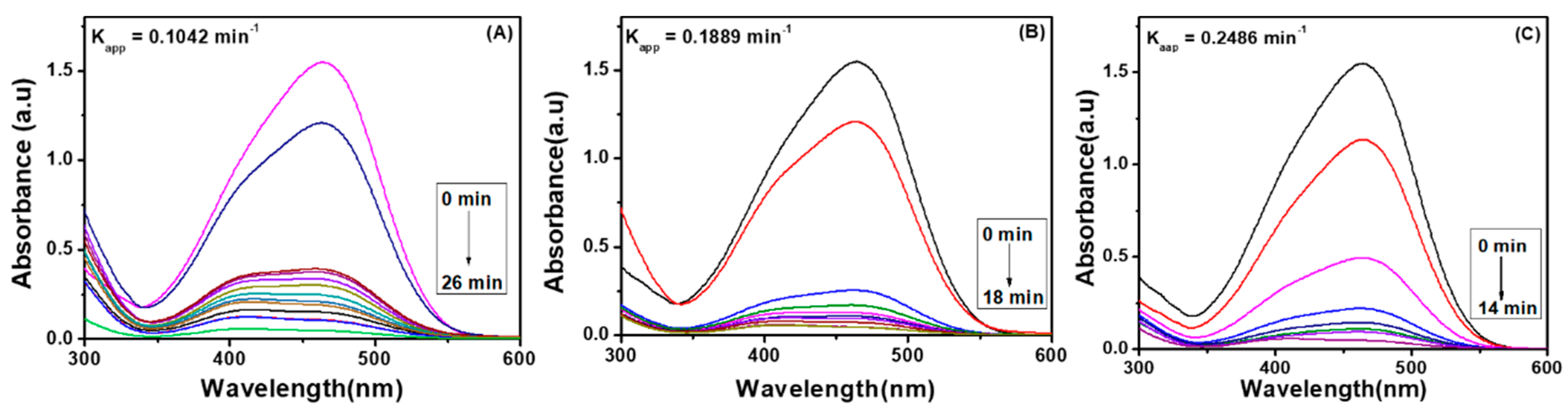
4.1. Degradation of Methyl Orange via Ag Embedded Hybrid Cryogel
4.1.1. Effect of Temperature on Catalytic Activity of Ag Embedded Hybrid Cryogel
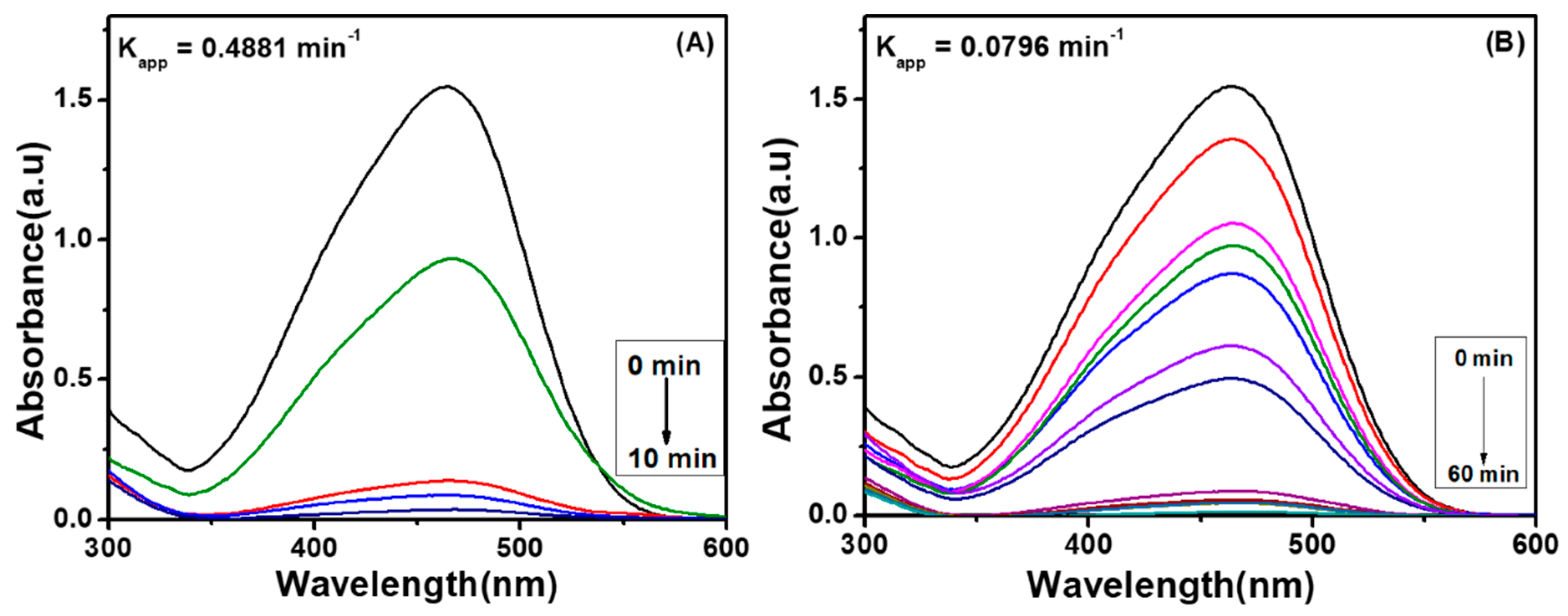
4.1.2. Effect of Dosage of Catalyst on Degradation Performance of Ag Embedded Hybrid Cryogels
4.2. Degradation of Methyl Orange via Pd Embedded Hybrid Cryogel
4.3. Comparative Study of Catalytic Performance of Fabricated Hybrid Cryogels with Ag-Hybrid Hydrogel for Degradation of Methyl Orange
4.4. Antibacterial Activities of Pure NIPAM Cryogel and Silver (Ag) and Palladium (Pd) Embedded Hybrid Cryogels
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradi, O.; Sharma, G. Emerging novel polymeric adsorbents for removing dyes from wastewater: A comprehensive review and comparison with other adsorbents. Environ. Res. 2021, 201, 111534. [Google Scholar] [CrossRef]
- Whelton, A.J.; McMillan, L.; Connell, M.; Kelley, K.M.; Gill, J.P.; White, K.D.; Gupta, R.; Dey, R.; Novy, C. Residential tap water contamination following the freedom industries chemical spill: Perceptions, water quality, and health impacts. Environ. Sci. Technol. 2015, 49, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotubes functional groups: Critical review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar]
- Zhang, X.G.; Wang, Y.; Cai, Y.M.; Wilson, K.; Lee, A.F. Bio/hydrochar sorbents for environmental remediation. Energy Environ. Mater. 2020, 3, 453–468. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng. J. 2016, 284, 687–697. [Google Scholar]
- Liu, X.; Pang, H.W.; Liu, X.P.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.Q.; Hu, B.W.; Yang, H.; Wang, X.K. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2020, 2, 100076. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science, and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Shaha, Z.; Hassana, S.; Shaheen, K.; Khan, S.A.; Gul, T.; Anward, Y.; Al-shaeri, M.A.; Khan, M.; Haleem, M.L. Synthesis of AgNPs coated with secondary metabolites of Acacia nilotica: An efficient antimicrobial and detoxification agent for environmental toxic organic pollutants. Mater. Sci. Eng. C 2020, 111, 110829. [Google Scholar]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of photocatalytic degradation of methyl orange using immobilized scoria Ni/TiO2 nanoparticles. J. Nanostructure Chem. 2020, 10, 143–159. [Google Scholar]
- Zhao, P.X.; Feng, X.W.; Huang, D.S.; Yang, G.Y.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Wu, Y.G.; Wen, M.; Wu, Q.S.; Fang, H. Ni/graphene nanostructure and its electron-enhanced catalytic action for hydrogenation reaction of nitrophenol. J. Phys. Chem. C, 2014; 118, 6307–6313. [Google Scholar]
- Ajmal, M.; Aftab, F.; Bibi, I.; Iqbal, M.; Ambreen, J.; Ahmad, H.B.; Akhtar, N.; Haleem, A.; Siddiq, M. Facile synthesis of porous anionic hydrogel embedded with nickel nanoparticles and evaluation of its catalytic performance for the rapid reduction of 4-nitrophenol. J. Porous Mater. 2019, 26, 281–290. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khan, S.R.; Begum, R. Temperature-responsive hybrid microgels for catalytic applications: A review. Mater. Sci. Technol. 2017, 33, 129–137. [Google Scholar]
- Ajmal, M.; Anwar, S.; Naeem, H.; Siddiq, M. Poly(acrylic acid) hydrogel microparticles fabricated with silver nanoparticles: Synthesis, characterization, and catalytic applications. Polym. Eng. Sci. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Haleem, A.; Syaal, S.B.; Ajmal, M.; Ambreen, J.; Rauf, S.; Ali, N.; Muhammad, S.; Shah, A.; Zia, M.A.; Siddiq, M. Silver and palladium nanoparticle embedded poly(n-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hybrid microgel catalyst with pH and temperature dependent catalytic activity. Korean J. Chem. Eng. 2020, 37, 614–622. [Google Scholar] [CrossRef]
- Liu, J.; Shu, T.; Su, L.; Zhang, X.; Serpe, M.J. Synthesis of poly(N-isopropylacrylamide)-co-(acrylic acid) microgel-entrapped CdS quantum dots and their photocatalytic degradation of an organic dye. RSC Adv. 2018, 8, 16850–16857. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Ahmad, E.; Sharif, E.; Wu, W.; Irfan, A. Fundamentals and applications of acrylamide based microgels and their hybrids: A review. RSC Adv. 2019, 9, 13838–13854. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, C.; Guo, J.; Fan, Y.; Zhang, Y. Synthesis of silver nanoparticles loaded onto polymer-inorganic composite materials and their regulated catalytic activity. Polymers 2019, 11, 401. [Google Scholar] [CrossRef]
- Chen, X.L.; Sui, W.W.; Ren, D.Y.; Ding, Y.Y.; Zhu, X.L.; Chen, Z.Y. Synthesis of hydrophobic polymeric cryogels with supermacroporous structure. Macromol. Mater. Eng. 2016, 301, 659–664. [Google Scholar] [CrossRef]
- Georgiev, G.L.; Trzebicka, B.; Kostovac, B.; Petrova, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Nikonorov, V.V.; Ivanov, R.V.; Kildeev, N.R.; Bulatnikova, L.N.; Lozinsky, V.I. Synthesis and characteristics of cryogels of chitosan crosslinked by glutaric aldehyde. Polym. Sci. 2010, 52, 828–834. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis and structure-property Raltionship of cryogels, in polymeric cryogels: Macroporous gels with remarkable proeprties. Adv. Polym. Sci. 2009, 263, 104–160. [Google Scholar]
- Plieva, F.; Huiting, X.; Galaev, I.Y.; Bergenstahl, B.; Mattiasson, B. Macroporous elastic polyacrylamide gels prepared at subzero temperatures: Control of porous structure. J. Mater. Chem. 2006, 16, 4065–4073. [Google Scholar] [CrossRef]
- Zhao, S.L.; Zou, Y.L.; Liu, X.Y.; Zhang, H.X. Ecofriendly construction of enzyme reactor based on three-dimensional porous cryogel composites. Chem. Eng. J. 2019, 361, 286–293. [Google Scholar] [CrossRef]
- Petrov, P.D.; Tsvetanov, C.B. Cryogel via UV irradiation, in polymeric cryogels: Macroporous gels with remarkable properties. Adv. Polym. Sci. 2009, 263, 199–223. [Google Scholar]
- Sahiner, N.; Seven, F.; Al-Lohedan, H. Super-fast hydrogen generation via super porous QP (VI)-M cryogel catalyst systems from hydrolysis of NaBH4. Int. J. Hydrog. Energy 2015, 40, 4605–4616. [Google Scholar] [CrossRef]
- Cimen, D.; Denizli, A. Immobilized metal affinity monolithic cryogels for cytochrome c purification. Biointerfaces 2012, 93, 29–35. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Bobnar, V.; Finsgar, M.; Grohens, Y.; Thomas, S.; Kokol, V. Cellulose nanofibrils-reduced graphene oxide xerogels and cryogels for dielectric and electrochemical storage applications. Polymer 2018, 147, 260–270. [Google Scholar]
- Hu, C.S.; Li, H.J.; Wang, J.Y.; Haleem, A.; Li, X.C.; Siddiq, M.; He, W.D. Mushroom-Like rGO/PAM Hybrid Cryogels with Efficient Solar-Heating Water Evaporation. ACS Appl. Energy Mater. 2019, 10, 7554–7563. [Google Scholar] [CrossRef]
- Yang, X.; Debeli, D.K.; Shan, G.; Pan, P. Selective adsorption and high recovery of La3+ using graphene oxide/poly(N-isopropyl acrylamide-maleic acid) cryogel. Chem. Eng. J. 2020, 379, 122335. [Google Scholar] [CrossRef]
- Radhouani, H.; Bicho, D.; Goncalves, C.; Maia, F.R.; Reis, R.L.; Oleivera, J.M. Kefiran, cryogels as potential scaffolds for drug delivery and tissue engineering applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E. Physicochemical, complexation and catalytic properties of polyampholyte cryogels. Gels 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Chullasat, K.; Nurerk, P.; Kanatharana, P.; Kueseng, P.; Sukchuay, T.; Bunkoed, O. Hybrid monolith sorbent of polypyrrole-coated graphene oxide incorporated into a polyvinyl alcohol cryogel for extraction and enrichment of sulfonamides from water samples. Anal. Chim. Acta 2017, 961, 59–66. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, J.; Guo, X.X.; Hou, S.C.; Chen, S.Q.; Siddiq, M.; He, W.D. Radiation-induced synthesis of hydrophobic cryogels with rapid and high absorption of organic solvents and oils. Microporous Mesoporous Mater 2022, 330, 111486. [Google Scholar] [CrossRef]
- Wei, G.; Miao, Y.E.; Zhang, C.; Yang, Z.; Liu, Z.; Tjiu, W.W.; Liu, T. Ni-doped graphene/carbon cryogels and their applications as versatile sorbents for water purification. ACS Appl. Mater. Interfaces 2013, 5, 7584–7591. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Tercan, M.; Demirci, S.; Dayan, O.; Sahiner, N. Simultaneous degradation and reduction of multiple organic compounds by poly (vinyl imidazole) cryogel-templated Co, Ni, and Cu metal nanoparticles. New J. Chem. 2020, 44, 4417–4425. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Chen, X.; Chen, M.; He, W.; Chen, Z. Supermacroporous polydivinylbenzene cryogels with high surface area: Synthesis by solvothermal postcrosslinking and their adsorption behaviors for carbon dioxide and aniline. J. Appl. Polym. Sci 2019, 136, 47716. [Google Scholar] [CrossRef]
- Haleem, A.; Li, H.J.; Li, P.Y.; Hu, C.S.; Li, X.C.; Wang, J.Y.; Chen, S.Q.; He, W.D. Rapid UV-radiation synthesis of polyacrylate cryogel oil-sorbents with adaptable structure and performance. Environ. Res. 2020, 187, 109488. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, J.; Guo, X.X.; Wang, J.Y.; Li, H.J.; Li, P.Y.; Chen, S.-Q.; He, W.-D. Hybrid Cryogels Composed of P(NIPAM-co-AMPS) and Metal Nanoparticles for Rapid Reduction of p-Nitrophenol. Polymer 2020, 193, 122352. [Google Scholar] [CrossRef]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.R.; Gomez, D.; Mikhalovsky, S.; Galaev, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter. 2005, 1, 303–309. [Google Scholar]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Coll. Int. Sci. 2013, 187, 1–46. [Google Scholar]
- Zhu, D.Y.; Lu, M.; Guo, J.; Liang, L.; Lan, Y. Effect of adamantyl methacrylate on the thermal and mechanical properties of thermosensitive poly(N-isopropylacrylamide) hydrogels. J. Appl. Polym. Sci. 2012, 124, 1–9. [Google Scholar]
- Haleem, A.; Chen, S.-Q.; Mohib, U.; Siddiq, M.; He, W.-D. Highly porous cryogels loaded with bimetallic nanoparticles as an efficient antimicrobial agent and catalyst for rapid reduction of water-soluble organic contaminants. J. Environ. Chem. Eng. 2021, 9, 106510. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, X.-Y.; Yang, J.-M.; Lin, D.-L.; Chen, X.; Zha, L.-S. Investigation of Ag nanoparticles loading temperature responsive hybrid microgels and their temperature controlled catalytic activity. Colloids Surf. A 2012, 393, 105–110. [Google Scholar]
- Verma, N. A green synthetic approach for size tunable nanoporous gold nanoparticles and its glucose sensing application. Appl. Surf. Sci. 2018, 462, 753–759. [Google Scholar] [CrossRef]
- Shah, L.A.; Sayed, M.; Siddiq, M. Synthesis of sensitive hybrid polymer microgels for catalytic reduction of organic pollutants. J. Environ. Chem. Eng. 2016, 4, 3492–3497. [Google Scholar] [CrossRef]
- Tallal, B.; Aftab, A.H.; Li, D. Application of a novel bimetallic hydrogel based on iron and cobalt for the synergistic catalytic degradation of Congo Red dye. J. Chin. Chem. Soc. 2019, 66, 919–927. [Google Scholar]
- Ganapuram, B.R.; Alle, M.; Dadigala, R.; Dasari, A.; Maragoni, V.; Guttena, V. Catalytic reduction of methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by salmalia malabarica gum. Int. Nano Lett. 2015, 5, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Subair, R.; Tripathi, B.P.; Formanek, P.; Simon, F.; Uhlmann, P.; Stamm, M. Polydopamine modified membranes with in situ synthesized gold nanoparticles for catalytic and environmental applications. J. Chem. Eng. 2016, 295, 358–369. [Google Scholar] [CrossRef]
- Nadaf, N.Y.; Kanase, S.S. Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab. J. Chem. 2019, 12, 4806–4814. [Google Scholar] [CrossRef] [Green Version]
- Safari, G.H.; Nasseri, S.; Mahvi1, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Mohammadi, M.A. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 1–15. [Google Scholar]
- Ilyas, H.; Haleem, A.; Muzaffar, I.; Siddiq, M. Influence of GO-Ag nano-filler on the antibacterial, antifouling and hydrophilic characteristics of polyvinyl chloride membrane. J. Water Process. Eng. 2021, 4, 102336. [Google Scholar] [CrossRef]



| Samples | ATCC Bacterial Strains | Zone of Inhibition |
|---|---|---|
| Pure NIPAM | Staphylococcus aureus | No zone of inhibition |
| NIPAM with Ag hybrid | Staphylococcus aureus | 11 mm |
| NIPAM with Pd hybrid | Staphylococcus aureus | 9 mm |
| Positive control | Staphylococcus aureus | 15 mm |
| Pure NIPAM | Escherichia coli | No zone of inhibition |
| NIPAM with Ag hybrid | Escherichia coli | 9 mm |
| NIPAM with Pd hybrid | Escherichia coli | 7 mm |
| Positive control | Escherichia coli | 30 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bhatti, M.A.; Shah Bukhari, S.N.U.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels 2023, 9, 64. https://doi.org/10.3390/gels9010064
Ambreen J, Haleem A, Shah AA, Mushtaq F, Siddiq M, Bhatti MA, Shah Bukhari SNU, Chandio AD, Mahdi WA, Alshehri S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels. 2023; 9(1):64. https://doi.org/10.3390/gels9010064
Chicago/Turabian StyleAmbreen, Jaweria, Abdul Haleem, Aqeel Ahmed Shah, Fozia Mushtaq, Muhammad Siddiq, Muhammad Ali Bhatti, Syed Nizam Uddin Shah Bukhari, Ali Dad Chandio, Wael A. Mahdi, and Sultan Alshehri. 2023. "Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation" Gels 9, no. 1: 64. https://doi.org/10.3390/gels9010064
APA StyleAmbreen, J., Haleem, A., Shah, A. A., Mushtaq, F., Siddiq, M., Bhatti, M. A., Shah Bukhari, S. N. U., Chandio, A. D., Mahdi, W. A., & Alshehri, S. (2023). Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels, 9(1), 64. https://doi.org/10.3390/gels9010064








