Characterizing a Cost-Effective Hydrogel-Based Transparent Soil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transparency
2.2. Mechanical Properties
2.3. Allowed Width and Height of Transparent Soil
2.4. Optimized Conditions for Making Transparent Soil
2.5. Bead Size and Porosity
2.6. Stability of Bead Size
3. Conclusions
4. Materials and Methods
4.1. Experiment Design
4.2. The Process of Making Transparent Soil
- Disinfect all equipment, including the laboratory bench, mesh metal sieve, glass bottles, syringe barrels, support stand, stirring hotplate, stirring bar, syringe, and needles, with 75% ethanol for 5 min, followed by four rinses with reverse osmosis water.
- Prepare the polymer solution with the required concentration (mixing Phytagel and sodium alginate powder in a 4:1 wt. ratio).
- Prepare the MgCl2 solution with the necessary concentration as the crosslinker solution.
- Prepare the Murashige & Skoog (MS) solution at twice the regular concentration.
- Heat the polymer solution (400 mL) in a universal oven to ensure complete dissolution at a temperature of 121 °C for 60 min. Before heating, ensure that the bottle cap is loose. After heating, tighten the bottle cap and allow the solution to cool to room temperature.
- Assemble the automatic dropping system for TS beads with a syringe barrel fixed on an iron support stand. Connect a stopcock to the syringe barrel with the required capacity and a metal flathead needle with the appropriate inner diameter (total length: 25 mm, exposed length: 13 mm). Ensure the stopcock is turned off.
- Transfer the polymer solution into the syringe barrel of the dropping system, passing it through a fine mesh metal sieve. If smaller beads are desired through the needles, cap the bottle and connect it to an air pump.
- Position a magnetic stirrer beneath the dispensing system. Pour 2.0 L of MgCl2 solution into a plastic container fitted with a stirring bar and place the container on the magnetic stirrer. Stir the solution at a rate of 200 rpm.
- Open the stopcock and initiate the dropping process, adjusting the stopcock to determine an appropriate dropping speed.
- Once the dropping process is complete, allow the beads to remain in the MgCl2 solution for 15 min, then collect the TS beads from the MgCl2 solution using a metal sieve.
- Soak the beads in the 2-fold concentrated MS solution for 12 h (overnight is preferable) at a beads-to-nutrient solution ratio of 1:1 (v:v).
- Filter the beads from the solution using a metal sieve and dry them with paper towels.
- The TS beads are now ready for use.
4.3. Measurements and Methods
4.3.1. Transparency Analysis
4.3.2. Mechanical Properties Analysis
4.3.3. Particle Size Analysis
4.3.4. Porosity Analysis
4.3.5. Stability Analysis of Transparent Soil Beads
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, J.P. Harnessing root architecture to address global challenges. Plant J. 2022, 109, 415–431. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.Y.; Fang, Q.; Yan, Z.Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- McGrail, R.K.; Van Sanford, D.A.; McNear, D.H. Trait-Based Root Phenotyping as a Necessary Tool for Crop Selection and Improvement. Agronomy 2020, 10, 1328. [Google Scholar] [CrossRef]
- Abbas, M.; Abid, M.A.; Meng, Z.; Abbas, M.; Wang, P.; Lu, C.; Askari, M.; Akram, U.; Ye, Y.; Wei, Y.; et al. Integrating advancements in root phenotyping and genome-wide association studies to open the root genetics gateway. Physiol. Plant 2022, 174, e13787. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhu, L.; Xu, W.; Liu, L.; Teng, G. Recent advances in methods for in situ root phenotyping. PeerJ 2022, 10, e13638. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Uga, Y. Improving the efficiency of plant root system phenotyping through digitization and automation. Breed. Sci. 2022, 72, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Gao, W.; der Bom, F.; Weng, Z.; Doolette, C.L.; Maksimenko, A.; Hausermann, D.; Zheng, Y.; Tang, C.; Lombi, E.; et al. Use of X-ray tomography for examining root architecture in soils. Geoderma 2022, 405, 115405. [Google Scholar] [CrossRef]
- Zhou, H.; Whalley, W.R.; Hawkesford, M.J.; Ashton, R.W.; Atkinson, B.; Atkinson, J.A.; Sturrock, C.J.; Bennett, M.J.; Mooney, S.J. The interaction between wheat roots and soil pores in structured field soil. J. Exp. Bot. 2021, 72, 747–756. [Google Scholar] [CrossRef]
- Girdthai, T.; Jogloy, S.; Kesmala, T.; Vorasoot, N.; Akkasaeng, C.; Wongkaew, S.; Holbrook, C.C.; Patanothai, A. Relationship between Root Characteristics of Peanut in Hydroponics and Pot Studies. Crop Sci. 2010, 50, 159–167. [Google Scholar] [CrossRef]
- Redjala, T.; Zelko, I.; Sterckeman, T.; Legué, V.; Lux, A. Relationship between root structure and root cadmium uptake in maize. Environ. Exp. Bot. 2011, 71, 241–248. [Google Scholar] [CrossRef]
- Shrestha, R.; Al-Shugeairy, Z.; Al-Ogaidi, F.; Munasinghe, M.; Radermacher, M.; Vandenhirtz, J.; Price, A.H. Comparing simple root phenotyping methods on a core set of rice genotypes. Plant Biol. J. 2014, 16, 632–642. [Google Scholar] [CrossRef]
- Su, R.; Zhou, R.; Mmadi, M.A.; Li, D.; Qin, L.; Liu, A.; Wang, J.; Gao, Y.; Wei, M.; Shi, L.; et al. Root diversity in sesame (Sesamum indicum L.): Insights into the morphological, anatomical and gene expression profiles. Planta 2019, 250, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.T.; MacCurdy, R.B.; Jung, J.K.; Shaff, J.E.; McCouch, S.R.; Aneshansley, D.J.; Kochian, L.V. Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiol. 2011, 156, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Floro, E.; Bray, A.L.; Laws, B.; Duncan, K.E.; Topp, C.N. Three-Dimensional Time-Lapse Analysis Reveals Multiscale Relationships in Maize Root Systems with Contrasting Architectures. Plant Cell 2019, 31, 1708–1722. [Google Scholar] [CrossRef]
- Topp, C.N.; Iyer-Pascuzzi, A.S.; Anderson, J.T.; Lee, C.R.; Zurek, P.R.; Symonova, O.; Zheng, Y.; Bucksch, A.; Mileyko, Y.; Galkovskyi, T.; et al. 3D phenotyping and quantitative trait locus mapping identify core regions of the rice genome controlling root architecture. Proc. Natl. Acad. Sci. USA 2013, 110, 1695–1704. [Google Scholar] [CrossRef]
- Han, T.H.; Kuo, Y.F. Developing a system for three-dimensional quantification of root traits of rice seedlings. Comput. Electron. Agric. 2018, 152, 90–100. [Google Scholar] [CrossRef]
- Uga, Y.; Assaranurak, I.; Kitomi, Y.; Larson, B.G.; Craft, E.J.; Shaff, J.E.; McCouch, S.R.; Kochian, L.V. Genomic regions responsible for seminal and crown root lengths identified by 2D & 3D root system image analysis. BMC Genom. 2018, 19, 273. [Google Scholar]
- Ma, L.; Chai, C.; Wu, W.; Qi, P.; Liu, X.; Hao, J. Hydrogels as the plant culture substrates: A review. Carbohydr. Polym. 2023, 305, 120544. [Google Scholar] [CrossRef]
- Skaar, J. Fresnel equations and the refractive index of active media. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2006, 73, 026605. [Google Scholar] [CrossRef]
- Downie, H.; Holden, N.; Otten, W.; Spiers, A.J.; Valentine, T.A.; Dupuy, L.X. Transparent soil for imaging the rhizosphere. PLoS ONE 2012, 7, e44276. [Google Scholar] [CrossRef]
- Downie, H.F.; Valentine, T.A.; Otten, W.; Spiers, A.J.; Dupuy, L.X. Transparent soil microcosms allow 3D spatial quantification of soil microbiological processes in vivo. Plant Signal Behav. 2014, 9, e970421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patko, D.; Engelhardt, I.; George, T.S.; Stanley-Wall, N.R.; Ladmiral, V.; Ameduri, B.; Daniell, T.J.; Holden, N.; MacDonald, M.P.; et al. Plant-environment microscopy tracks interactions of Bacillus subtilis with plant roots across the entire rhizosphere. Proc. Natl. Acad. Sci. USA 2021, 118, e2109176118. [Google Scholar] [CrossRef] [PubMed]
- Iskander, M.; Bathurst, R.J.; Omidvar, M. Past, Present, and Future of Transparent Soils. Geotech. Test. J. 2015, 38, 557–573. [Google Scholar] [CrossRef]
- Zhang, W.A.; Gu, X.; Zhong, W.H.; Ma, Z.T.; Ding, X.M. Review of transparent soil model testing technique for underground construction: Ground visualization and result digitalization. Undergr. Space 2022, 7, 702–723. [Google Scholar] [CrossRef]
- Ma, L.; Shi, Y.; Siemianowski, O.; Yuan, B.; Egner, T.K.; Mirnezami, S.V.; Lind, K.R.; Ganapathysubramanian, B.; Venditti, V.; Cademartiri, L. Hydrogel-based transparent soils for root phenotyping in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 11063–11068. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohyd Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Jaeger, P.A.; McElfresh, C.; Wong, L.R.; Ideker, T. Beyond Agar: Gel Substrates with Improved Optical Clarity and Drug Efficiency and Reduced Autofluorescence for Microbial Growth Experiments. Appl. Environ. Microbiol. 2015, 81, 5639–5649. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Zhou, Y. A root penetration model of Arabidopsis thaliana in phytagel medium with different strength. J. Plant Res. 2017, 130, 941–950. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocolloid 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Dev, M.J.; Warke, R.G.; Warke, G.M.; Mahajan, G.B.; Patil, T.A.; Singhal, R.S. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour. Technol. 2022, 359, 127498. [Google Scholar] [CrossRef]
- Evageliou, V.; Gerolemou, A.; Zikas, A.; Basios, A.; Komaitis, M. Effect of salts and sugars on the clarity of gellan gels. Int. J. Food Sci. Technol. 2011, 46, 1001–1006. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef]
- Miranda-Martinez, A.; Yan, H.; Silveira, V.; Serrano-Olmedo, J.J.; Crouzier, T. Portable Quartz Crystal Resonator Sensor for Characterising the Gelation Kinetics and Viscoelastic Properties of Hydrogels. Gels 2022, 8, 718. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.A.P.; Bonsanto, F.P.; Cunha, R.L. Mechanical properties of gellan gum beads prepared with potassium or calcium ions. J. Texture Stud. 2022, 53, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostănaru-Iliescu, A.-C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726. [Google Scholar] [CrossRef]
- Fiorica, C.; Biscari, G.; Palumbo, F.S.; Pitarresi, G.; Martorana, A.; Giammona, G. Physicochemical and rheological characterization of different low molecular weight gellan gum products and derived ionotropic crosslinked hydrogels. Gels 2021, 7, 62. [Google Scholar] [CrossRef]
- Takahashi, R.; Tokunou, H.; Kubota, K.; Ogawa, E.; Oida, T.; Kawase, T.; Nishinari, K. Solution properties of gellan gum: Change in chain stiffness between single- and double-stranded chains. Biomacromolecules 2004, 5, 516–523. [Google Scholar] [CrossRef]
- Feeney, D.S.; Crawford, J.W.; Daniell, T.; Hallett, P.D.; Nunan, N.; Ritz, K.; Rivers, M.; Young, I.M. Three-dimensional microorganization of the soil-root-microbe system. Microb. Ecol. 2006, 52, 151–158. [Google Scholar] [CrossRef]
- Wendell, D.M.; Luginbuhl, K.; Guerrero, J.; Hosoi, A.E. Experimental Investigation of Plant Root Growth Through Granular Substrates. Exp. Mech. 2012, 52, 945–949. [Google Scholar] [CrossRef]
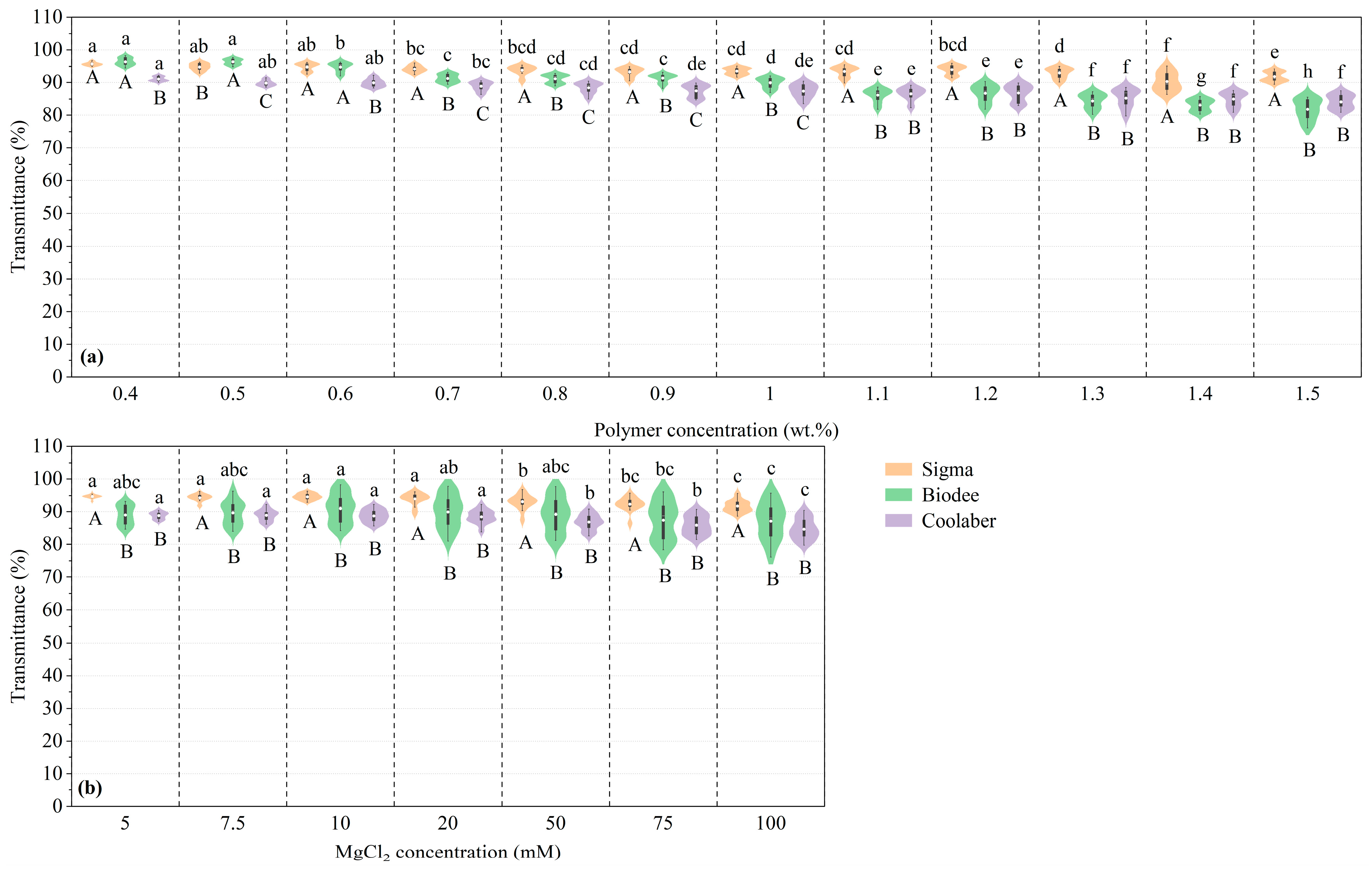
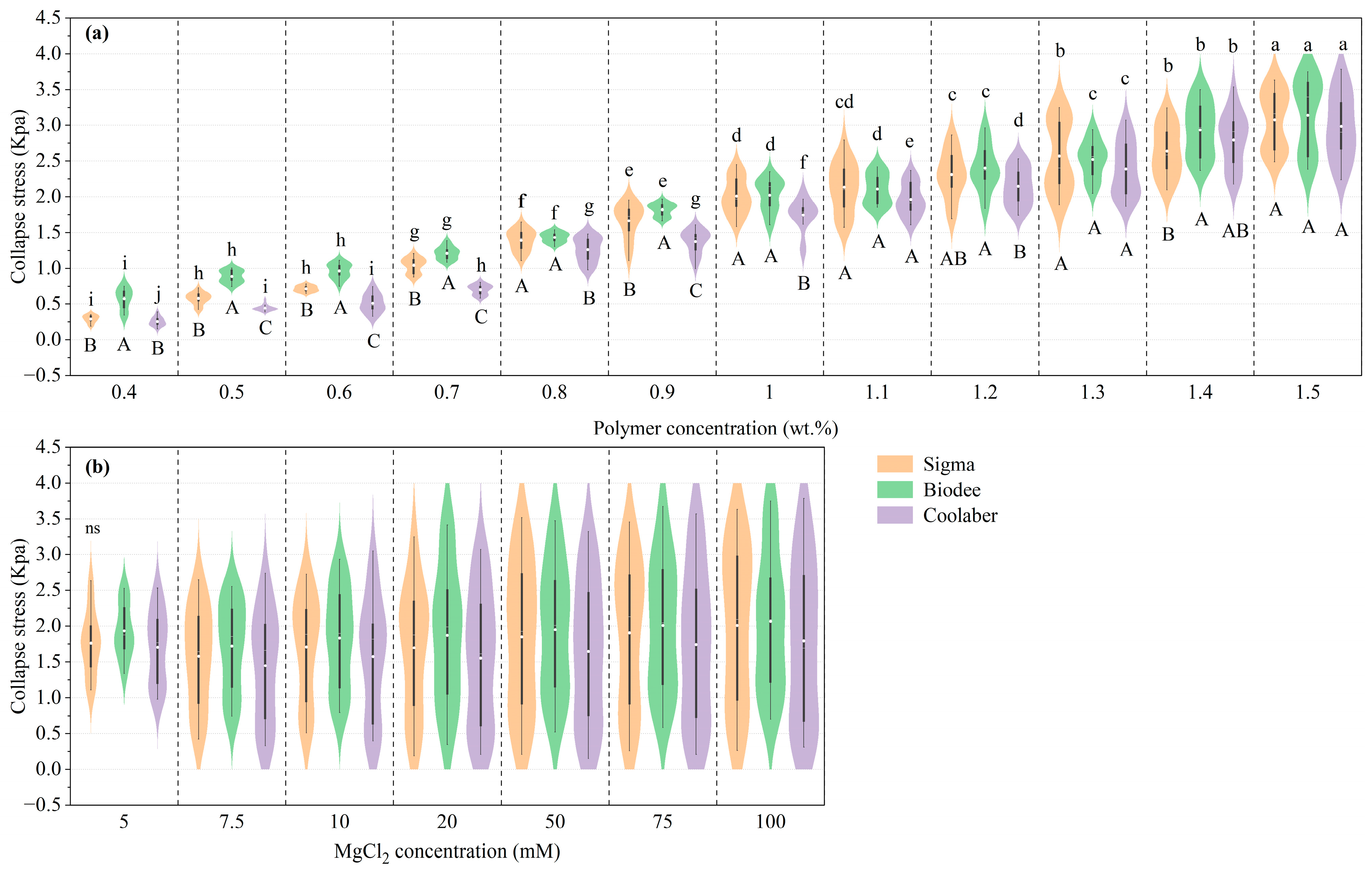
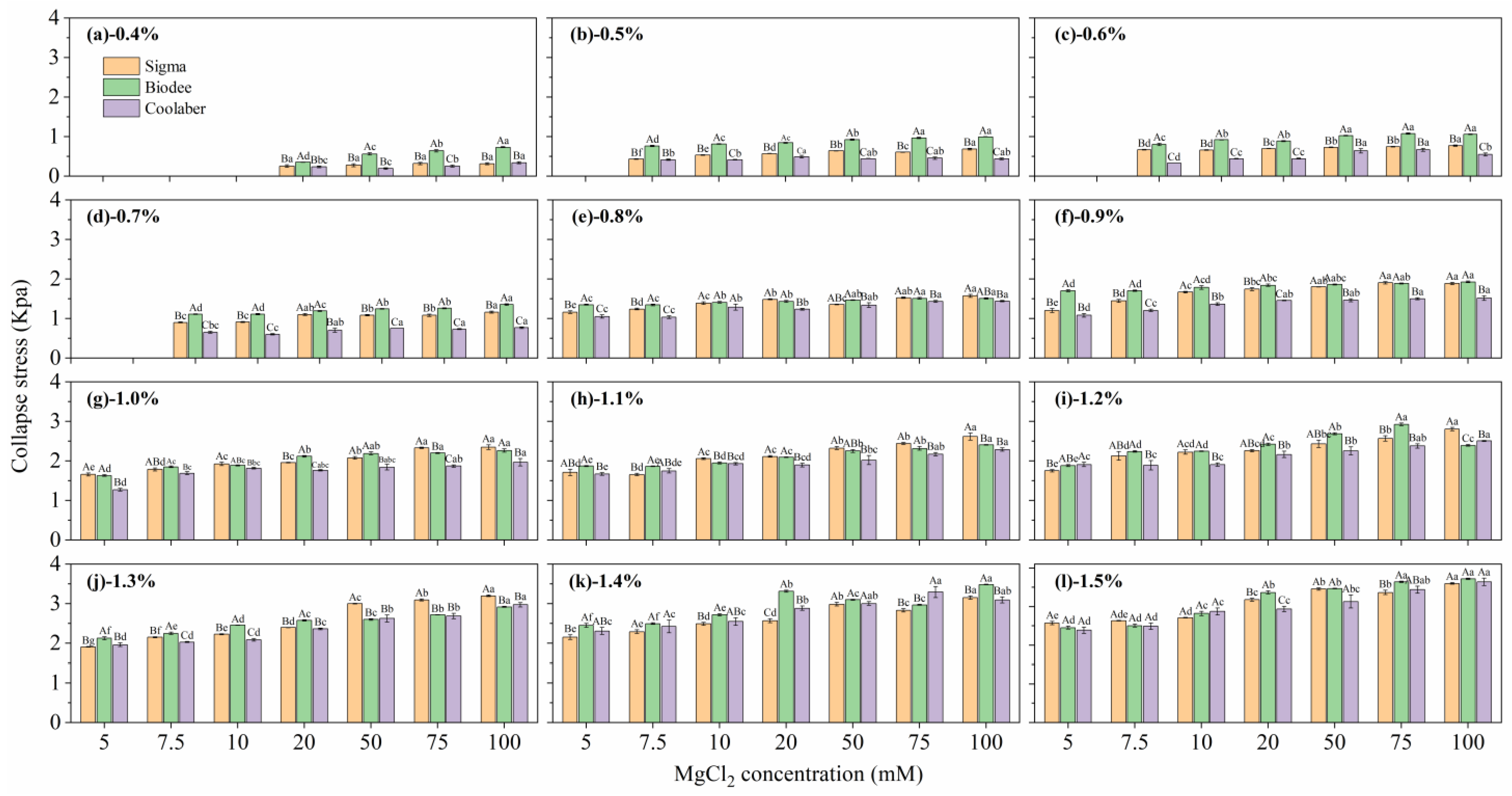
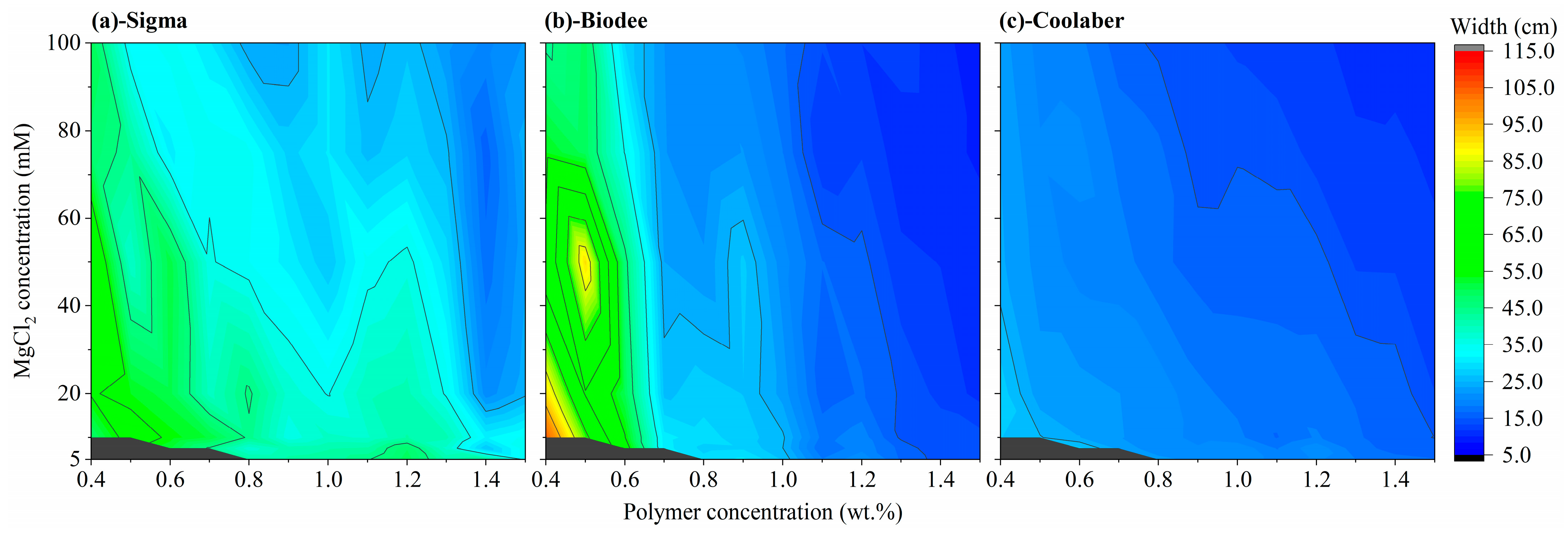
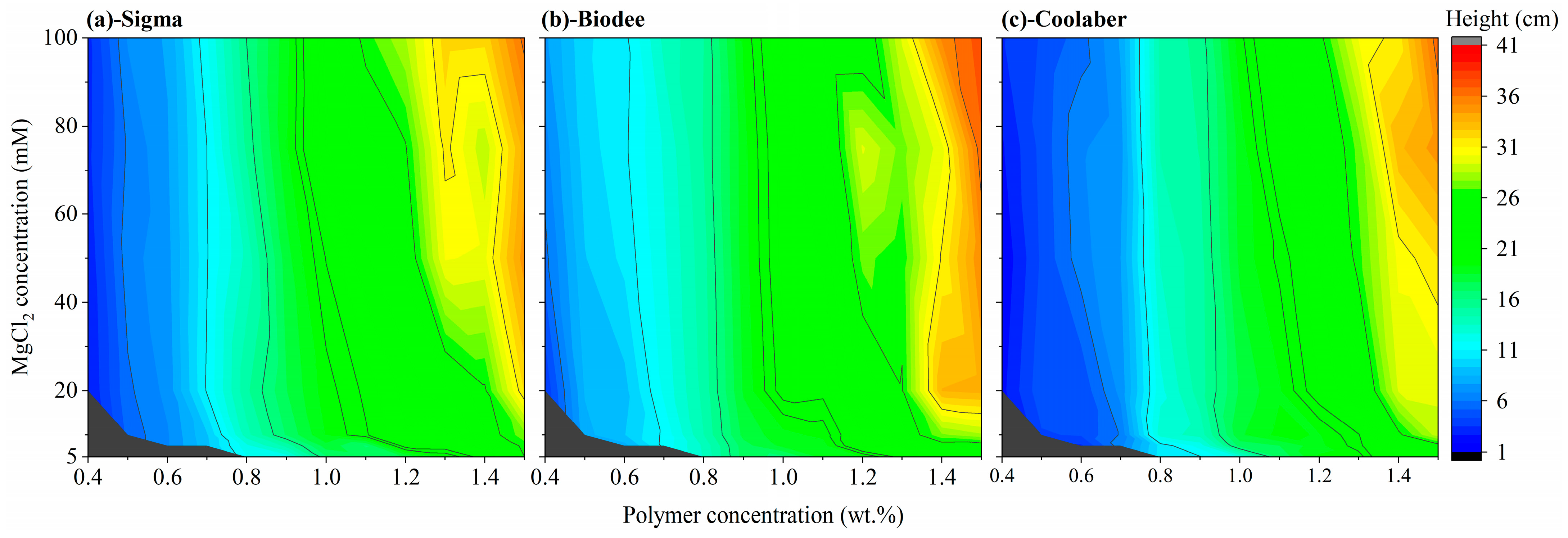
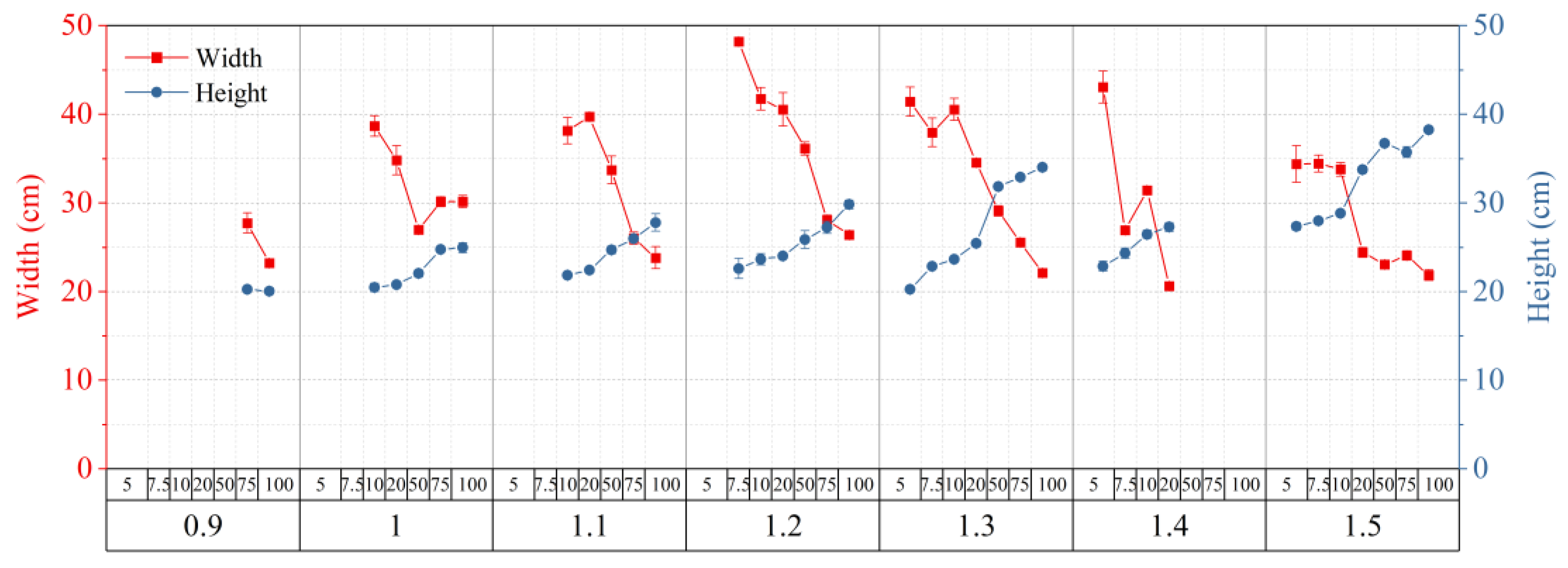


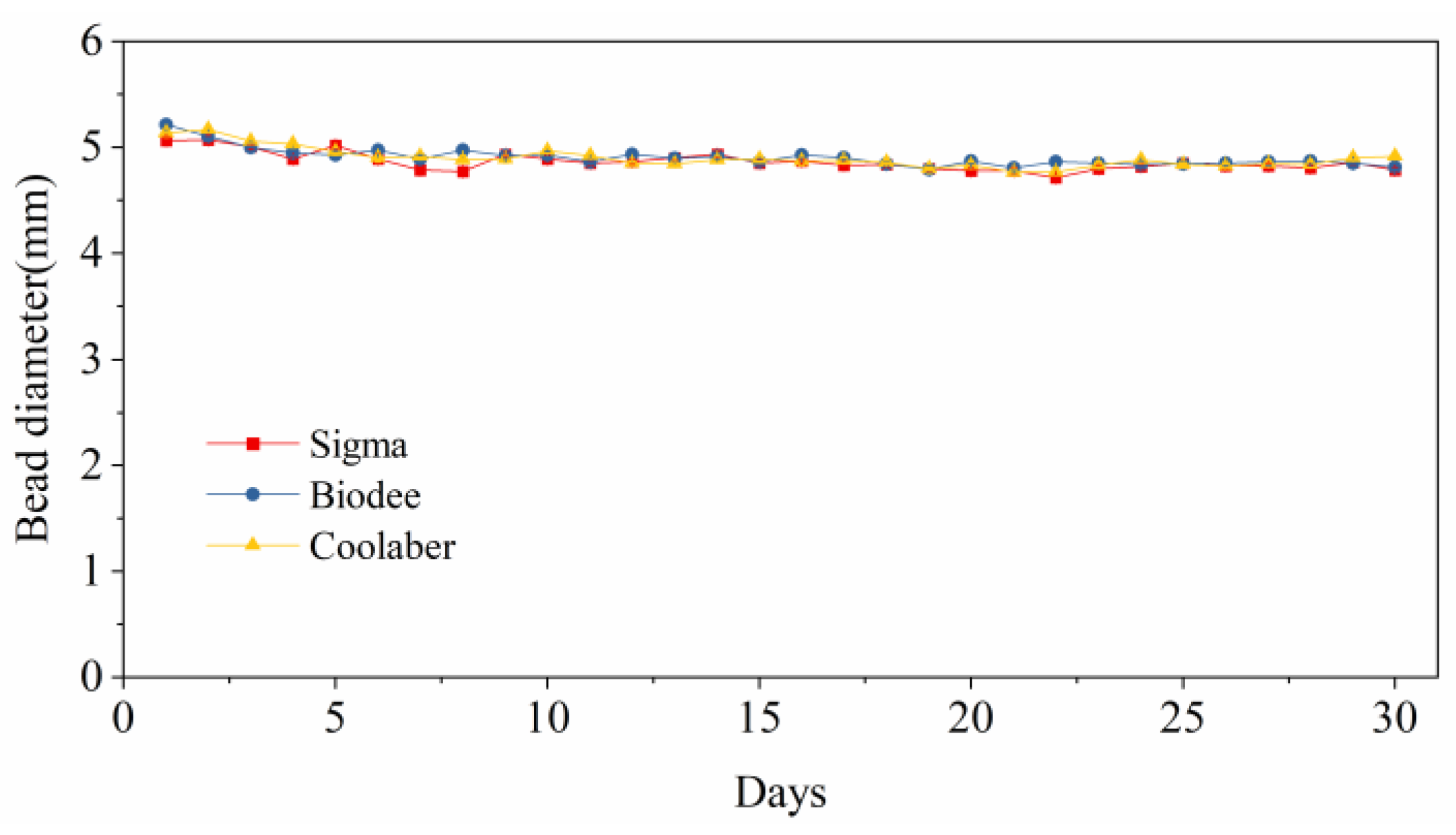
| Source | DF | Transparency | Collapse Stress |
|---|---|---|---|
| PB | 2 | 17,199.6 *** | 818.0 *** |
| PC | 11 | 3987.9 *** | 8014.1 *** |
| CC | 6 | 2273.3 *** | 931.5 *** |
| PB * PC | 22 | 622.0 *** | 31.5 *** |
| PB * CC | 12 | 37.3 *** | 8.2 *** |
| PC * CC | 66 | 36.9 *** | 27.9 *** |
| PB * PC * CC | 132 | 15.7 *** | 6.2 *** |
| Phytagel Brands | Transparency (%) | Stress (Kpa) | Width (cm) | Height (cm) |
|---|---|---|---|---|
| Sigma | 0.934 ± 0.0013 a | 1.790 ± 0.057 ab | 36.80 ± 0.69 a | 18.10 ± 0.58 ab |
| Biodee | 0.890 ± 0.0033 b | 1.912 ± 0.053 a | 27.28 ± 1.33 b | 19.33 ± 0.53 a |
| Coolaber | 0.874 ± 0.0018 c | 1.634 ± 0.059 b | 17.91 ± 0.26 c | 16.54 ± 0.60 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Ma, L.; Gao, Y.; Zhang, J.; Li, S. Characterizing a Cost-Effective Hydrogel-Based Transparent Soil. Gels 2023, 9, 835. https://doi.org/10.3390/gels9100835
Li K, Ma L, Gao Y, Zhang J, Li S. Characterizing a Cost-Effective Hydrogel-Based Transparent Soil. Gels. 2023; 9(10):835. https://doi.org/10.3390/gels9100835
Chicago/Turabian StyleLi, Kanghu, Lin Ma, Yang Gao, Jiyang Zhang, and Sen Li. 2023. "Characterizing a Cost-Effective Hydrogel-Based Transparent Soil" Gels 9, no. 10: 835. https://doi.org/10.3390/gels9100835






