Effects of Potassium Bicarbonate on Gel, Antioxidant and Water Distribution of Reduced-Phosphate Silver Carp Surimi Batter under Cold Storage
Abstract
1. Introduction
2. Results and Discussion
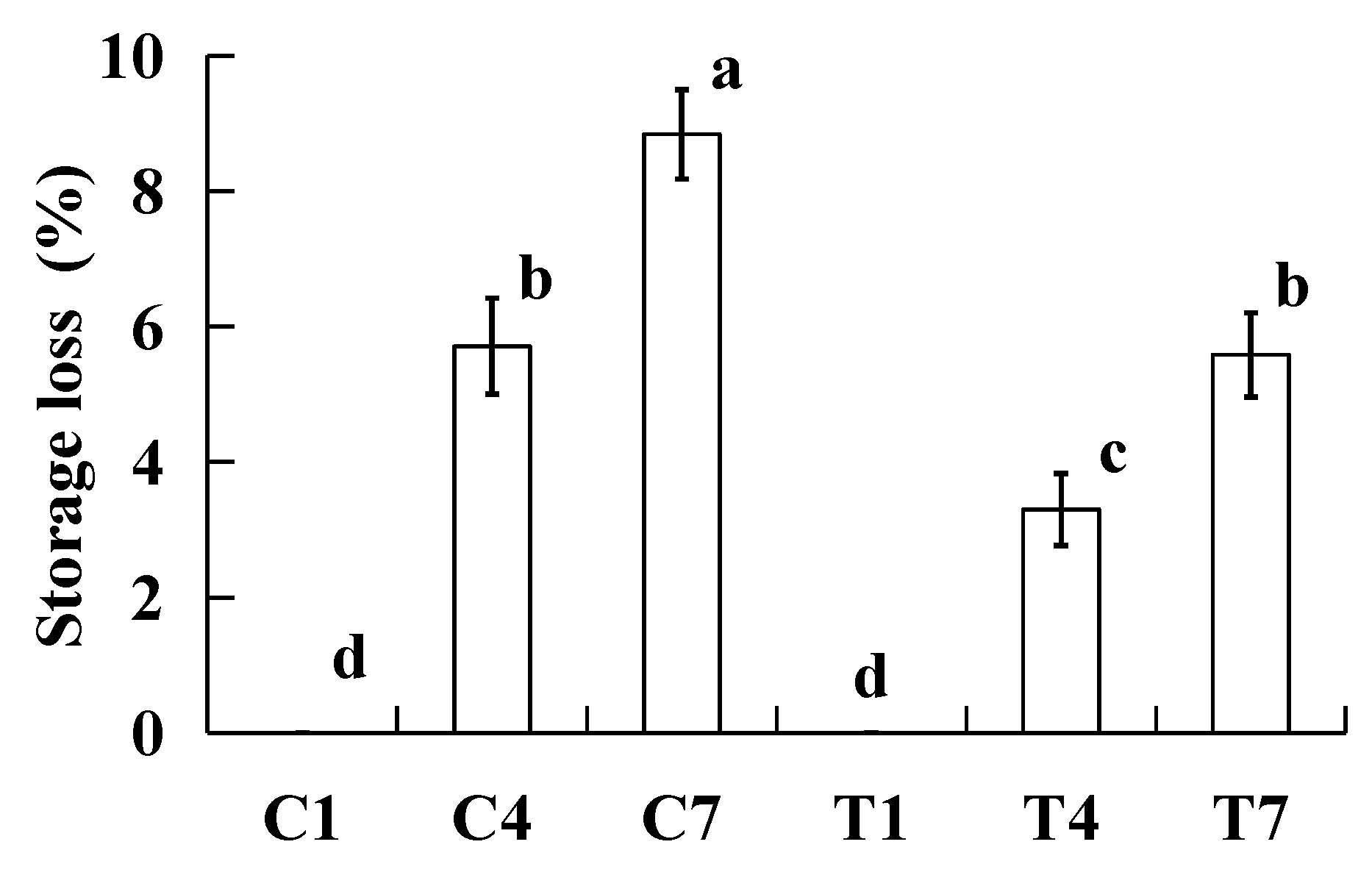
2.1. Storage Loss
2.2. Low-Field NMR
2.3. pH
2.4. Whiteness
2.5. Texture Properties
2.6. TBARS
2.7. Total Volatile Basic Nitrogen
2.8. Total Plate Count
3. Conclusions
4. Materials and Methods
4.1. Materials and Silver Carp Surimi Batters Prepared
4.2. Cold Storage
4.3. Determination of Storage Loss
4.4. Low-Field NMR Measurement
4.5. pH Measurement
4.6. Whiteness Measurement
4.7. Texture Properties Measurement
4.8. TBARS Measurement
4.9. Total Volatile Basic Nitrogen Measurement
4.10. Total Plate Count Measurement
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.Q.; Wang, Y.D.; Chen, M.; Zhong, Q.; Zhuang, Y.; Su, H.C.; Yang, H. Effect of high-content ultrasonically emulsified lard on the physicochemical properties of surimi gels from silver carp enhanced by microbial transglutaminase. Int. J. Food Sci. Technol. 2023, 58, 2974–2984. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, H.; Xue, Y.; Xue, C.H. Effects of soybean phospholipids, ovalbumin, and starch sodium octenyl succinate on the mechanical, microstructural, and flavor properties of emulsified surimi gels. LWT Food Sci. Technol. 2022, 161, 113260. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Lautrou, M.; Narcy, A.; Dourmad, J.Y.; Pomar, C.; Schmidely, P.; Montminy, M.P.L. Dietary Phosphorus and Calcium Utilization in Growing Pigs: Requirements and Improvements. Front. Vet. Sci. 2021, 8, 734365. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.E.; Bohrer, B.M.; Mejia, S.M.V. Phosphate alternatives for meat processing and challenges for the industry: A critical review. Food Res. Int. 2023, 166, 112624. [Google Scholar] [CrossRef]
- Krithika, M.V.; Balakrishnan, U.; Amboiram, P.; Shaik, M.S.J.; Chandrasekaran, A.; Ninan, B. Early calcium and phosphorus supplementation in VLBW infants to reduce metabolic bone disease of prematurity: A quality improvement initiative. BMJ Open Qual. 2022, 11, e001841. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shi, B.; Liu, G.; Li, S.; Kang, Z. Using Potassium Bicarbonate to Improve the Water-HoldingCapacity, Gel and Rheology Characteristics of Reduced-Phosphate Silver Carp Batters. Molecules 2023, 28, 5608. [Google Scholar] [CrossRef]
- Li, M.; Luo, J.; Zhang, Y.; Zhang, K.; Guan, Z.Q.; Ling, C.M. Effects of different phosphorus-free water-retaining agents on the quality of frozen tilapia fillets. Food Sci. Nutr. 2023, 10, 633–644. [Google Scholar] [CrossRef]
- Jaico, T.; Prabhakar, H.; Adhikari, K.; Singh, R.K.; Mohan, A. Influence of Bicarbonates and Salt on the Physicochemical and Sensory Properties of Meatloaf. J. Food Qual. 2022, 4788425. [Google Scholar] [CrossRef]
- Mohan, A.; Jaico, T.; Kerr, W.; Singh, R. Functional properties of bicarbonates on physicochemical attributes of ground beef. LWT Food Sci. Technol. 2016, 70, 333–341. [Google Scholar] [CrossRef]
- Lee, N.; Sharma, V.; Brown, N.; Mohan, A. Functional properties of bicarbonates and lactic acid on chicken breast retail display properties and cooked meat quality. Poult. Sci. 2015, 94, 302–310. [Google Scholar] [CrossRef]
- Lin, X.X.; Liu, C.S.; Cai, L.; Yang, J.R.; Zhou, J.C.; Jiang, H.Z.; Shi, Y.H.; Gu, Z.F. Effect of High Hydrostatic Pressure Processing on Biochemical Characteristics, Bacterial Counts, and Color of the Red Claw Crayfish Cherax quadricarinatus. J. Shellfish Res. 2021, 40, 177–184. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S.; Yu, D.; Wang, H. Potential nano bacteriostatic agents to be used in meat-based foods processing and storage: A critical review. Trends Food Sci. Technol. 2023, 131, 77–90. [Google Scholar] [CrossRef]
- Varo, M.A.; Martin-Gomez, J.; Serratosa, M.P.; Merida, J. Effect of potassium metabisulphite and potassium bicarbonate on color, phenolic compounds, vitamin C and antioxidant activity of blueberry wine. LWT—Food Sci. Technol. 2022, 163, 113585. [Google Scholar] [CrossRef]
- Micklander, E.; Peshlov, B.; Purslow, P.P.; Engelsen, S.B. Nmr-cooking: Monitoring the changes in meat during cooking by low-field 1H-NMR. Trends Food Sci. Technol. 2002, 13, 341–346. [Google Scholar] [CrossRef]
- Sun, S.; Lin, Z.Y.; Cheng, S.S.; Abd El-Aty, A.M.; Tan, M.Q. Effect of water-retention agents on Scomberomorus niphonius surimi after repeated freeze-thaw cycles: Low-field NMR and MRI studies. Int. J. Food Eng. 2023, 19, 15–25. [Google Scholar] [CrossRef]
- Peng, Z.; Zhu, M.; Zhang, J.; Zhao, S.; He, H.; Kang, Z.; Xu, B. Physicochemical and structural changes in myofibrillar proteins from porcine longissimus dorsi subjected to microwave combined with air convection thawing treatment. Food Chem. 2021, 343, 128412. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, R.; Cheng, S.; Tan, M. Water dynamics changes and protein denaturation in surf clam evaluated by two-dimensional LF-NMR T1-T2 relaxation technique during heating process. Food Chem. 2020, 320, 126622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, Z.; Sukmanov, V.; Ma, H. Effects of soy protein isolate on gel properties and water holding capacity of low-salt pork myofibrillar protein under high pressure processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef]
- Kang, Z.; Xie, J.; Li, Y.; Song, W.; Ma, H. Effects of pre-emulsified safflower oil with magnetic field modified soy 11S globulin on the gel, rheological, and sensory properties of reduced-animal fat pork batter. Meat Sci. 2023, 198, 109087. [Google Scholar]
- Ghimire, A.; Paudel, N.; Poudel, R. Effect of pomegranate peel extract on the storage stability of ground buffalo (Bubalus bubalis) meat. LWT Food Sci. Technol. 2022, 154, 112690. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of pigments and color in sardine (sardinella gibbosa) and mackerel (rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005, 93, 607–617. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Residual effect of CO2 on hake (merluccius merluccius L.) stored in modified and controlled atmospheres. Eur. Food Res. Technol. 2001, 212, 413–420. [Google Scholar] [CrossRef]
- Li, Y.; Sukmanov, V.; Kang, Z.; Ma, H. Effect of soy protein isolate on the techno-functional properties and protein conformation of low-sodium pork meat batters treated by high pressure. J. Food Process Eng. 2020, 43, e13343. [Google Scholar] [CrossRef]
- Thomas, R.; Jebin, N.; Saha, R.; Sarma, D.K. Antioxidant and antimicrobial effects of kordoi (Averrhoa carambola) fruit juice and bamboo (Bambusa polymorpha) shoot extract in pork nuggets. Food Chem. 2016, 190, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT Food Sci. Technol. 2020, 117, 108633. [Google Scholar] [CrossRef]
- Kang, Z.L.; Zou, Y.F.; Xu, X.L.; Zhu, C.Z.; Wang, P.; Zhou, G.H. Effect of various amounts of pork and chicken meat on the sensory and physicochemical properties of chinese-style meatball (kung-wan). Food Sci. Technol. Res. 2013, 19, 963–970. [Google Scholar] [CrossRef][Green Version]
- Lu, W.W.; Qin, Y.Y.; Ruan, Z. Effects of high hydrostatic pressure on color, texture, microstructure, and proteins of the tilapia (Orechromis niloticus) surimi gels. J. Texture Stud. 2021, 52, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.L.; Mársico, E.T.; Rosenthal, A.; Conte-Junior, C.A. Synergistic effect of ultraviolet radiation and high hydrostatic pressure on texture, color, and oxidative stability of refrigerated tilapia fillets. J. Sci. Food Agric. 2019, 99, 4474–4481. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, H.; Xiong, Q.; Wu, R.; Hu, Y.; Liu, R. A novel strategy for inhibiting AGEs in fried fish cakes: Grape seed extract surimi slurry coating. Food Control 2023, 154, 109948. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Thangrattana, M.; Kitikangsadan, A. Impact of oyster mushroom (Pleurotus ostreatus) on chemical, physical, microbiological and sensory characteristics of fish burger prepared from salmon and striped catfish filleting by-product. Int. J. Gastron. Food Sci. 2022, 30, 100598. [Google Scholar] [CrossRef]
- Rivas-Caedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuez, M.; Picon, A. Effect of high-pressure processing and chemical composition on lipid oxidation, aminopeptidase activity and free amino acids of serrano dry-cured ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef]
- Ozogul, F.; Polat, A.; Ozogul, Y. The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina pilchardus). Food Chem. 2004, 85, 49–57. [Google Scholar] [CrossRef]
- Fan, W.; Chi, Y.; Zhang, S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT Food Sci. Technol. 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Ye, T.; Chen, X.; Chen, Z.; Liu, R.; Wang, Y.; Lin, L.; Lu, J.F. Quality characteristics of shucked crab meat (eriocheir sinensis) processed by high pressure during superchilled storage. J. Food Biochem. 2021, 45, 13708. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Buamard, N.; Vate, N.K.; Benjakul, S. Effect of squid melanin-free ink and pre-emulsification on properties and stability of surimi gel fortified with seabass oil during refrigerated storage. J. Aquat. Food Prod. Technol. 2018, 27, 919–933. [Google Scholar] [CrossRef]
- Solo-de-Zaldívar, B.; Tovar, C.A.; Borderías, A.J.; Herranz, B. Pasteurization and chilled storage of restructured fish muscle products based on glucomannan gelation. Food Hydrocoll. 2015, 43, 418–426. [Google Scholar] [CrossRef]
- An, Y.; You, J.; Xiong, S.; Yin, T. Short-term frozen storage enhances crosslinking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 257, 216–222. [Google Scholar] [CrossRef]
- Ulu, H. Evaluating of three 2-thiobarbituric acid methods for the measurement of lipid oxidation in various meats and meat products. Meat Sci. 2004, 67, 683–687. [Google Scholar] [CrossRef]



| Sample | Initial Relaxation Time (ms) | Peak Ratio (%) | ||||
|---|---|---|---|---|---|---|
| T2b | T21 | T22 | P2b | P21 | P22 | |
| C1 | 2.32 ± 0.10 a | 46.56 ± 1.26 c | 465.42 ± 12.56 c | 1.38 ± 0.17 a | 90.26 ± 0.87 b | 8.58 ± 0.46 d |
| C4 | 2.41 ± 0.09 a | 57.48 ± 1.18 b | 498.23 ± 11.27 b | 1.42 ± 0.22 a | 86.75 ± 0.76 c | 11.15 ± 0.39 c |
| C7 | 2.47 ± 0.12 a | 68.15 ± 1.30 a | 536.70 ± 12.04 a | 1.50 ± 0.26 a | 81.58 ± 0.93 d | 17.49 ± 0.33 a |
| T1 | 1.92 ± 0.12 b | 35.51 ± 1.27 d | 336.27 ± 12.33 e | 0.96 ± 0.15 b | 93.32 ± 0.91 a | 6.37 ± 0.35 e |
| T4 | 2.07 ± 0.11 b | 47.22 ± 1.31 c | 387.81 ± 11.48 d | 1.04 ± 0.20 b | 90.47 ± 0.83 b | 8.50 ± 0.39 d |
| T7 | 2.11 ± 0.13 b | 55.36 ± 1.23 b | 448.87 ± 12.19 c | 0.99 ± 0.19 b | 85.44 ± 0.95 c | 13.67 ± 0.44 b |
| Sample | Hardness (N) | Springiness | Cohesiveness | Chewiness (N·mm) |
|---|---|---|---|---|
| C1 | 47.37 ± 1.27 d | 0.853 ± 0.011 c | 0.613 ± 0.011 c | 24.71 ± 1.27 b |
| C4 | 51.33 ± 1.35 c | 0.827 ± 0.009 c | 0.595 ± 0.007 d | 25.24 ± 1.04 b |
| C7 | 57.25 ± 1.16 b | 0.771 ± 0.012 d | 0.557 ± 0.009 e | 24.58 ± 1.16 b |
| T1 | 53.91 ± 1.13 c | 0.911 ± 0.012 a | 0.670 ± 0.009 a | 31.10 ± 1.21 a |
| T4 | 56.02 ± 1.27 b | 0.880 ± 0.015 a | 0.642 ± 0.010 b | 31.63 ± 1.37 a |
| T7 | 59.87 ± 1.21 a | 0.844 ± 0.010 b | 0.615 ± 0.012 c | 30.92 ± 1.16 a |
| Sample | Total Volatile Basic Nitrogen (mg/100 g) | Total Plate Count (CFU/g) |
|---|---|---|
| C1 | 3.34 ± 0.32 c | 3.23 × 10 |
| C4 | 8.50 ± 0.39 b | 6.26 × 102 |
| C7 | 17.31 ± 0.52 a | 5.31 × 104 |
| T1 | 3.63 ± 0.44 c | 4.60 × 10 |
| T4 | 9.30 ± 0.38 b | 5.52 × 102 |
| T7 | 16.68 ± 0.71 a | 4.63 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.-C.; Liu, G.-H.; Wang, K.; Xie, C.; Kang, Z.-L. Effects of Potassium Bicarbonate on Gel, Antioxidant and Water Distribution of Reduced-Phosphate Silver Carp Surimi Batter under Cold Storage. Gels 2023, 9, 836. https://doi.org/10.3390/gels9100836
Fan J-C, Liu G-H, Wang K, Xie C, Kang Z-L. Effects of Potassium Bicarbonate on Gel, Antioxidant and Water Distribution of Reduced-Phosphate Silver Carp Surimi Batter under Cold Storage. Gels. 2023; 9(10):836. https://doi.org/10.3390/gels9100836
Chicago/Turabian StyleFan, Jing-Chao, Guang-Hui Liu, Kai Wang, Chun Xie, and Zhuang-Li Kang. 2023. "Effects of Potassium Bicarbonate on Gel, Antioxidant and Water Distribution of Reduced-Phosphate Silver Carp Surimi Batter under Cold Storage" Gels 9, no. 10: 836. https://doi.org/10.3390/gels9100836
APA StyleFan, J.-C., Liu, G.-H., Wang, K., Xie, C., & Kang, Z.-L. (2023). Effects of Potassium Bicarbonate on Gel, Antioxidant and Water Distribution of Reduced-Phosphate Silver Carp Surimi Batter under Cold Storage. Gels, 9(10), 836. https://doi.org/10.3390/gels9100836






