Evaluation of Mitigation Role of L-Phenylalanine-Based Low-Molecular-Weight Gelator against Oil Pollution-Induced Nile Tilapia Toxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Synthesis of the Gelator and the Gelation Efficiency
2.2. Morphological Toxicity Symptoms
2.3. Effect of Crude Engine Oil and Organogelator on Water Quality Parameters
2.4. Bacterial Community Affected by Crude Oil in Nile Tilapia Fish
2.5. Heavy Metal Analysis of Water Samples
2.6. Biochemical Activity
2.7. Micronucleus Test
2.8. Expression of Stress-Related Genes
2.9. LMWGs as a Smart Remediation Tool for Oil Spills
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Analysis
4.3. Synthesis of Low-Molecular-Weight Gelator (Z-Phe-C18)
4.4. Experimental Fish and Study Setup
4.5. Water Physicochemical Parameters
4.6. Microbiological Analysis
4.6.1. Detection of Total Coliform and E. coli
4.6.2. Detection of Staphylococcus aureus
4.6.3. Detection of Salmonella spp.
4.6.4. Detection of Pseudomonas aeruginosa
4.7. Total Heavy Metals Determination
4.8. Enzyme Activity Analysis
4.8.1. Glutathione S Transferase (GST) and Superoxide Dismutase (SOD) Activities
4.8.2. Catalase Activity
4.9. Micronucleus Assay
4.10. Expression of Stress-Related Genes
4.10.1. Isolation of Total RNA and Reverse Transcription (RT) Reaction
4.10.2. Real-Time Polymerase Chain Reaction (RT-PCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Magd, I.A.; Zakzouk, M.; Ali, E.M.; Abdulaziz, A.M. An open source approach for near-real time mapping of oil spills along the Mediterranean coast of Egypt. Remote Sens. 2021, 13, 2733. [Google Scholar] [CrossRef]
- Pichler, J.; Eder, R.M.; Besser, C.; Pisarova, L.; Dörr, N.; Marchetti-Deschmann, M.; Frauscher, M. A comprehensive review of sustainable approaches for synthetic lubricant components. Green Chem. Lett. Rev. 2023, 16, 2185547. [Google Scholar] [CrossRef]
- Ek, H.; Dave, G.; Sturve, J.; Camey, B.; Stephensen, E.; Birgerson, G. Tentative Biomarkers for 2,4,6, Trinitrololune (TNT) in fish on Corhnchus mykiss. J. Aquat. Pollut. Toxicol. 2005, 77, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.; Moustafa, M.; El-Husseiny, I.; Saeid, S.; Saleh, O.; Borhan, T. Identification of some skeletal deformities in freshwater teleosts raised in Egyptian aquaculture. Chemosphere 2009, 77, 419–425. [Google Scholar] [CrossRef]
- Soliman, G.; Abdelaziz, M.; Eissa, A.E.; Elias, N.; Moustafa, M. Impacts of natural and experimental phenol pollution on the reproductive performance, vitellogenin synthesis and pathological alterations of male Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2020, 24, 479–495. [Google Scholar] [CrossRef]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar]
- Sujatha, K.; Senthilkumaar, P.; Sangeetha, S.; Gopalakrishnan, M. Isolation of human pathogenic bacteria in two edible fishes, Priacanthus hamrur and Megalaspis cordyla at Royapuram waters of Chennai, India. Indian J. Sci. Technol. 2011, 4, 539–541. [Google Scholar] [CrossRef]
- Jisr, N.; Younes, G.; El Omari, K.; Hamze, M.; Sukhn, C.; El-Dakdouki, M.H. Levels of heavy metals, total petroleum hydrocarbons, and microbial load in commercially valuable fish from the marine area of Tripoli, Lebanon. Environ. Monit. Assess. 2020, 192, 705. [Google Scholar] [CrossRef]
- Lunestad, B.T.; Nesse, L.; Lassen, J.; Svihus, B.; Nesbakken, T.; Fossum, K.; Rosnes, J.T.; Kruse, H.; Yazdankhah, S. Salmonella in fish feed; occurrence and implications for fish and human health in Norway. Aquaculture 2007, 265, 1–8. [Google Scholar] [CrossRef]
- Fingas, M. Physical spill countermeasures. In Oil Spill Science and Technology, 1st ed.; Gulf Professional Publishing: Oxford, UK, 2010; pp. 303–337. [Google Scholar]
- Dewling, R.T.; McCarthy, L.T. Chemical treatment of oil spills. Environ. Int. 1980, 3, 155–162. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Sreedhar, A.; Noh, J.S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Bonifazi, E.L.; Edelsztein, V.C.; Menéndez, G.O.; Samaniego, L.C.; Spagnuolo, C.C.; Di Chenna, P.H. Versatile supramolecular organogel with outstanding stability toward aqueous interfaces. ACS Appl. Mater. Interfaces 2014, 6, 8933–8936. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-molecular-weight gels: The state of the art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Heeres, A.; van der Pol, C.; Stuart, M.; Friggeri, A.; Feringa, B.L.; van Esch, J. Orthogonal self-assembly of low molecular weight hydrogelators and surfactants. J. Am. Chem. Soc. 2003, 125, 14252–14253. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Abdellatif, M.; Averlant-Petit, M.C.M.; Loic, S.; Pickaert, G. New C-terminal hydrazide L-Leucine derivatives as multi-solvent low molecular weight organogelators. J. Text. Color. Polym. 2018, 15, 73–83. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Ibrahim, S.; Nomura, K. Efficient and eco-friendly low-molecular-weight gelators based on L-phenylalanine as promising remediation tool for oil pollution. J. King Saud Univ. Sci. 2020, 32, 946–951. [Google Scholar] [CrossRef]
- Feng, G.; Chen, H.; Cai, J.; Wen, J.; Liu, X. L-Phenylalanine based low-molecular-weight efficient organogelators and their selective gelation of oil from oil/water mixtures. Soft. Mater. 2014, 12, 403–410. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Zhang, G.; Xu, B. L-Lysine-based gelators for the formation of oleogels in four vegetable oils. Molecules 2022, 27, 1369. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sato, T.; Kurose, A.; Shirai, H.; Hanabusa, K. New low-molecular weight gelators based on L-valine and L-isoleucine with various terminal groups. Tetrahedron Lett. 2005, 46, 2741–2745. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Zhou, Y.; Zhou, Z.; Luo, C.; Wang, Y.; Yao, B.; Ji, X. Comparative study of the micro-rheological properties and microstructure of edible oil gels prepared by amino acid gelator. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127421. [Google Scholar] [CrossRef]
- Miroslaw, B.; Demchuk, O.M.; Luboradzki, R.; Tyszczuk-Rotko, K. Low-molecular-weight organogelators Based on N-dodecanoyl-L-amino Acids—Energy frameworks and supramolecular synthons. Materials 2023, 16, 702. [Google Scholar] [CrossRef]
- Rodriguez-Cea, A.; Ayllon, F.; Garcia-Vazquez, E. Micronucleus test in freshwater fish species: An evaluation of its sensitivity for application in field surveys. Ecotoxicol. Environ. Saf. 2003, 56, 442–448. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Głowacki, E.D.; Voss, G.; Bauer, S.; Sariciftci, N.S. Green and biodegradable electronics. Mater. Today 2012, 15, 340–346. [Google Scholar] [CrossRef]
- Ewida, A.Y. Oil spills: Impact on water quality and microbial community on the Nile River, Egypt. Int. J. Environ. 2014, 3, 192–198. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. APHA Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Liu, K.; He, P.; Fang, Y. Progress in the studies of low-molecular mass gelators with unusual properties. Sci. China Chem. 2011, 54, 575–586. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, ON, Canada, 2002; 11p. [Google Scholar]
- Evaluation of Certain Food Additives and Contaminants: 57th Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Rome, Italy, 2001; p. 171.
- Regnell, O.; Tesson, S.V.M.; Oskolkov, N.; Nerentorp, M. Mercury–selenium accumulation patterns in muscle tissue of two freshwater fish species, Eurasian perch (Perca fluviatilis) and Vendace (Coregonus albula). Water Air Soil Pollut. 2022, 233, 236. [Google Scholar] [CrossRef]
- Gimenes, T.C.; Penteado, J.O.; dos Santos, M.; da Silva Júnior, F.M.R. Methylmercury in fish from the Amazon region—A Review focused on eating habits. Water Air Soil Pollut. 2021, 232, 199. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Krishnan-Ghosh, Y. First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Commun. 2001, 185–186. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting–self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [PubMed]
- Gad, N. Oxidative stress and antioxidant enzymes in Oreochromis niloticus as biomarkers of exposure to crude oil pollution. IJESE 2011, 1, 49–58. [Google Scholar]
- Zhang, J.F.; Shen, H.; Xu, T.L.; Wang, X.R.; Li, W.M.; Gu, Y.F. Effects of long term exposure of low level diesel oil on the antioxidant defense system of fish. Bull. Environ. Contam. Toxicol. 2003, 71, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Simonata, J.; Guedes, C.; Martinez, C. Biochemical, physiological and histological changes in neotropical fish Prochildus lineatus exposed to diesel oil. Ecotoxicol. Environ. Saf. 2008, 69, 112–120. [Google Scholar] [CrossRef]
- Sturve, J.; Hasselberg, L.; Falth, H.; Celander, M.; Forlin, L. Effect of North sea oil and alkaylphenole on biomarker response in Juvenile Atlantic cod (Gadus morhua). Aquat. Toxicol. 2006, 78, 73–78. [Google Scholar] [CrossRef]
- Wengu, X.; Yuangou, I.; Qingyang, W.; Shuqi, W.; Huaiping, Z.; Wenhua, L. Effect of phenanthrene on hepatic enzymatic activities in tilapia Oreochromis niloticus. J. Environ. Sci. 2009, 21, 854–857. [Google Scholar]
- Jiminez, B.D.; Stegeman, J.J. Detoxification enzymes as indicators of environmental stress on fish. Am. Fish. Soc. Symp. 1990, 8, 67–79. [Google Scholar]
- Londis, W.G.; Yu, M.H. Introduction to Environmental Toxicology: Impacts of Chemical upon Ecological System; Lewis Publishers: Boca Raton, FL, USA, 2000; pp. 231–232. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in Environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Ali, F.K.; El-Shehawi, A.M.; Seehy, M.A. Micronucleus test in fish genome: A sensitive monitor for water pollution. Afr. J. Biotechnol. 2008, 7, 606–612. [Google Scholar]
- Malla, T.M.; Ganesh, N. Cytogenetic and tissue toxicity by synthetic sindoor in fresh water catfish Heteropneustes fossilis. Biomed. Pharmacol. J. 2009, 2, 85–89. [Google Scholar]
- Abdel-Gawad, F.; Khalil, W.; Bassem, S.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.; Nassar, H.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Fathy, R.; Abdel-Rahim, E.; Shallan, M.; Khalil, W.; Bassem, S.; Abdel-Gawad, F. Assessment of Holothuria Atra Extracts Against Synthetic Textile Dyes Induced Endocrine Disorders, Biochemical and Genetic Alterations in Nile Tilapia (Oreochromis niloticus). Int. J. Pharm. Res. 2021, 13, 3392–3399. [Google Scholar]
- Abdel-Gawad, F.K.; Guerriero, G.; Khalil, W.K.B.; Abbas, H.H. Evaluation of Oxidative Stress, Genotoxicity and Gene Expression Alterations as Oil Pollution Markers in Solea vulgaris, from Suez Canal. Quantum Matter 2016, 5, 291–296. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; El-Kady, A.A.; Waly, A.I.; Abdel-Wahhab, M.A. Carboxymethyl chitosan modulates the genotoxic risk and oxidative stress of perfluorooctanoic acid in Nile tilapia (Oreochromis niloticus). J. Saudi Soc. Agric. Sci. 2016, 15, 57–66. [Google Scholar] [CrossRef]
- Guerriero, G.; Bassem, S.; Khalil, W.; Temraz, T.; Ciarcia, G.; Abdel-Gawad, F. Temperature changes and marine fish species (Epinephelus coioides and Sparus aurata): Role of oxidative stress biomarkers in toxicological food studies. Emir. J. Food Agric. 2018, 30, 205–211. [Google Scholar] [CrossRef]
- Cajaraville, M.P.; Bebianno, M.J.; Blasco, J.; Porte, C.; Sarasquete, C.; Viarengo, A. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci. Total Environ. 2000, 247, 295–311. [Google Scholar] [CrossRef]
- Dondero, F.; Dagnino, A.; Jonsson, H.; Capri, F.; Gastaldi, L.; Viarengo, A. Assessing the occurrence of a stress syndrome in mussels (Mytilus edulis) using a combined biomarker/gene expression approach. Aquat. Toxicol. 2006, 78, S13–S24. [Google Scholar] [CrossRef]
- Santana, M.; Domingues de Melo, G.; Sandrini-Neto, L.; Di Domenico, M.; Prodocimo, M.M. A meta-analytic review of fish antioxidant defense and biotransformation systems following pesticide exposure. Chemosphere 2022, 291, 132730. [Google Scholar] [CrossRef]
- Diaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Girón-Pérez, M.I. Modulation of immune response by organophosphorus pesticides: Fishes as a potential model in immunotoxicology. J. Immunol. Res. 2015, 2015, 213836. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuro Endocrinol. Lett. 2009, 30, 2–12. [Google Scholar] [PubMed]
- Jadhav, S.R.; Vemula, P.K.; Kumar, R.; Raghavan, S.R.; John, G. Sugar-derived phase-selective molecular gelators as model solidifiers for oil spills. Angew. Chem. Int. Ed. 2010, 49, 7695–7698. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, K.; Liu, X.; Xia, H.; Liu, J.; Fang, Y. New dicholesteryl-based gelators: Gelling ability and selective gelation of organic solvents from their mixtures with water at room temperature. New J. Chem. 2008, 32, 2218–2224. [Google Scholar] [CrossRef]
- Mukherjee, S.; Shang, C.; Chen, X.; Chang, X.; Liu, K.; Yu, C.; Fang, Y. N-Acetylglucosamine-based efficient, phase-selective organogelators for oil spill remediation. Chem. Commun. 2014, 50, 13940–13943. [Google Scholar] [CrossRef]
- Wang, D.; Niu, J.; Wang, Z.; Jin, J. Monoglyceride-based organogelator for broad-range oil uptake with high capacity. Langmuir 2015, 31, 1670–1674. [Google Scholar] [CrossRef]
- Suzuki, M.; Sato, T.; Shirai, H.; Hanabusa, K. Powerful low-molecular-weight gelators based on L-valine and L-isoleucine with various terminal groups. New J. Chem. 2006, 30, 1184–1191. [Google Scholar] [CrossRef]
- Santos, C.A.; Lenz, D.; Brandão, G.P.; Chippari-Gomes, A.R.; Gomes, L.C. Acute toxicity of the water-soluble fraction of diesel in Prochilodus vimboides Kner (Characiformes: Prochilodontidae). Neotrop. Ichthyol. 2013, 11, 193–198. [Google Scholar] [CrossRef]
- Mosquera Narvaez, L.E.; Ferreira, L.M.d.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; Hartigan, P.; Fanning, S.; Fitzpatrick, E. Veterinary Microbiology and Microbial Disease, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; p. 928. [Google Scholar]
- Qasim, D.A. Molecular detection of pseudomonas aeruginosa isolated from minced meat and studies the pyocyanin effectiveness on pathogenic bacteria. Iraqi J. Agric. Sci. 2019, 50, 1199–1207. [Google Scholar] [CrossRef]
- APHA; AWWA (American Water Works Association); WEF (Water Environment Federation). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; p. 40. [Google Scholar]
- Haque, R.; Bin-Hafeez, B.; Parvez, S.; Pandey, S.; Sayeed, I.; Ali, M.; Raisuddin, S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. Hum. Exp. Toxicol. 2003, 22, 473–480. [Google Scholar] [CrossRef]
- Faheem, M.; Lone, K.P. Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Braz. J. Pharm. Sci. 2018, 53, 1–9. [Google Scholar] [CrossRef]
- Santos, R.M.; Petry, A.C.; Sousa, V.L.; Souza, H.O.; Azevedo, A.; Soares, A.R.; Weber, L.I. Acute and subchronic effects of petroleum on the freshwater fish Hoplias aff. malabaricus. Braz. J. Biol. 2022, 84, e253731. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS User’s Guide: Statistics SAS; SAS Institute Inc.: Cary, NC, USA, 1982. [Google Scholar]
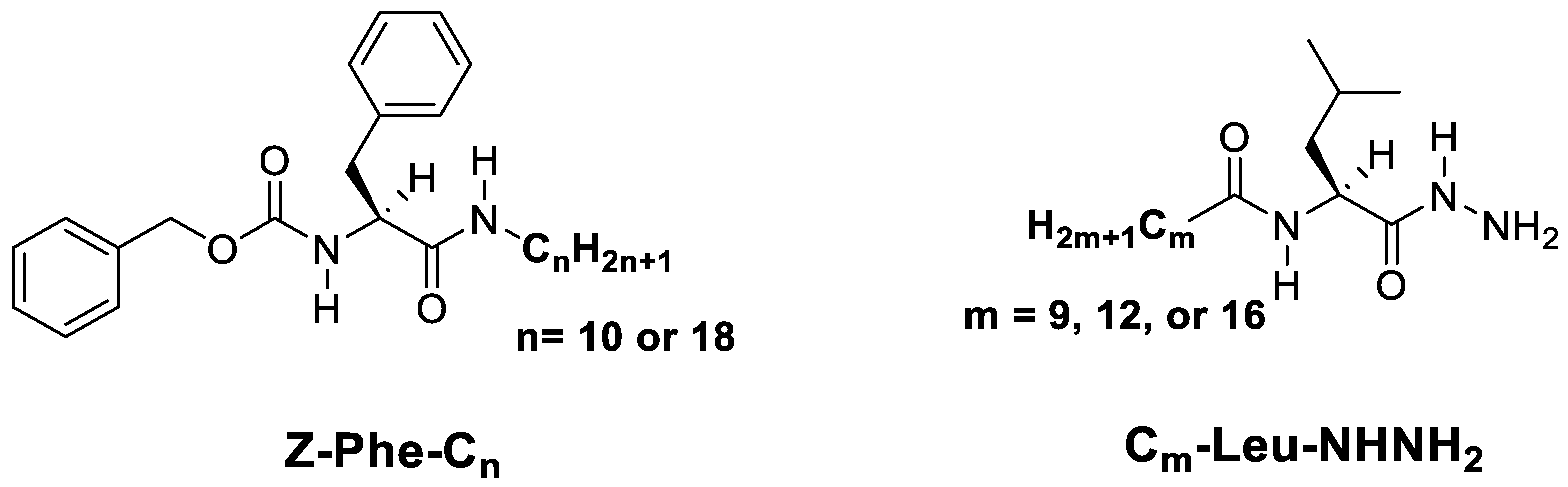








| Control | Pollution Group | Treatment Group (with Gelator) | Gelator | Guideline CCME, 2007 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Oil (L) | Oil (M) | Oil (H) | Oil (L) | Oil (M) | Oil (H) | ||||
| pH | 7.5 ± 0.2 ab | 7.2 ± 0.2 ab | 7.0 ± 0.2 ab | 6.0 ± 0.2 b | 7.9 ± 0.2 a | 7.6 ± 0.2 ab | 6.9 ± 0.2 ab | 8.0 ± 0.2 a | 6.5–9 |
| EC (µs/cm) | 236 ± 4.4 c | 272 ± 5.3 ab | 286 ± 6.7 ab | 292 ± 3.6 a | 248 ± 1.22 b | 260 ± 8.9 b | 272 ± 4.5 ab | 243 ± 7.8 c | 0.0 |
| TDS (mg/L) | 141 ± 5.3 d | 177 ± 6.1 c | 219 ± 7.3 b | 289 ± 1.3 a | 152 ± 6.2 cd | 163 ± 1.9 c | 172 ± 4.8 c | 146 ± 3.9 cd | 500 |
| DO (mgO2/L) | 8.0 ± 1.29 a | 4.8 ± 0.6 b | 3.8 ± 7.49 c | 2.9 ± 5.29 c | 5.9 ± 2.29 ab | 5.3 ± 3.8 b | 4.5 ± 2.6 b | 7.5 ± 1.9 a | 5.5 |
| BOD (mgO2/L) | 3.0 ± 0.22 e | 44.8 ± 0.9 b | 67.2 ± 0.4 a | 80.6 ± 0.7 a | 9.31 ± 1.1 d | 14.8 ± 1.4 c | 17.2 ± 0.4 c | 4.9 ± 0.2 e | 0.0 |
| COD (mgO2/L) | 7.0 ± 0.3 e | 108 ± 4.3 b | 117 ± 0.3 b | 152 ± 0.3 a | 31 ± 0.3 d | 41 ± 0.3 d | 83 ± 0.3 c | 8 ± 0.3 e | 7 |
| Control | Pollution Group | Treatment Group (with Gelator) | Gelator | Guideline FAO/WHO 2002 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Oil (L) | Oil (M) | Oil (H) | Oil (L) | Oil (M) | Oil (H) | ||||
| Total Coliform | ND | 2.5 ± 1.06 a | 2.9 ± 1.3 a | 3.7 ± 0.98 a | 0.52 ± 1.06 b | 0.7 ± 1.06 b | 0.9 ± 1.06 b | ND | <1 |
| E. coli | ND | 2.0 ± 0.9 | 2.3 ± 0.2 | 3.3 ± 0.11 | ND | ND | ND | ND | <1 |
| S. aureus | ND | ND | 2.4 ± 0.6 a,b | 3.5 ± 1.06 a | ND | 1.7 ± 1.06 b | 1.2 ± 1.06 b | ND | <2 |
| Salmonella spp. | ND | ND | ND | 2.6 ± 0.3 | ND | ND | ND | ND | 0.0 |
| P. aeruginosa | ND | ND | ND | 2.08 ± 0.4 | ND | ND | ND | ND | 0.0 |
| Metal Ions | Pollution Group | Treatment Group (with Gelator) | Gelator | Reference Oil Only | ||||
|---|---|---|---|---|---|---|---|---|
| Oil (L) | Oil (M) | Oil (H) | Oil (L) | Oil (M) | Oil (H) | |||
| As | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.1 |
| Cd | 0.02 | 0.02 | 0.03 | <0.001 | <0.001 | <0.001 | <0.001 | 0.07 |
| Cr | 0.02 | 0.02 | 0.04 | <0.01 | <0.01 | <0.01 | <0.001 | 0.25 |
| Cu | 0.02 | 0.06 | 0.1 | 0.01 | 0.02 | 0.03 | <0.001 | 1.4 |
| Fe | 0.12 | 0.37 | 0.6 | <0.01 | <0.01 | 0.02 | <0.001 | 28 |
| Mn | 0.01 | 0.01 | 0.03 | <0.01 | <0.01 | <0.01 | <0.001 | 0.35 |
| Ni | 0.01 | 0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.001 | 0.2 |
| Pb | 0.02 | 0.03 | 0.05 | 0.01 | 0.01 | 0.02 | <0.001 | 0.35 |
| Zn | 1.06 | 2.24 | 3.5 | 0.02 | 0.03 | 0.06 | <0.001 | 266 |
| Starting Material | Pollutant | Reference |
|---|---|---|
| N-Lauroyl-L-alanine a | kerosene, petrol, and paraffin | [35] |
| Sugar [D-(+)-Mannitol and D-(+)-Sorbitol] b | diesel, mineral oil, silicone oil, and crude oil fractions | [57] |
| Cholesterol c | xylene and/or kerosene | [58] |
| N-Acetylglucosamines d | petrol, kerosene, diesel, silicone oil, and pump oil | [59] |
| Monoglyceride b | diesel and kerosene | [60] |
| L-Valine and L-Isoleucine a | salad oil | [61] |
| L-Phenyl alanine b | crude oil and heavy metal ions in fish aquariums | This work |
| Gene | Primer Sequence (5′–3′) a | NCBI Reference |
|---|---|---|
| MAPK b | F: ATTCAGAGGGTGGGAAGTGG R: GTGCTTGCATTCCTTCACCA | XM_003449968.5 |
| LDH c | F: CGAAAGCCGTCTCAATCTGG R: CAGAGTCGAGGTTAGTGCCA | EU313200.1 |
| CYP1A1 d | F: TAAACTGCAGAGCGAGAGCA R: CTTTCGACCCCAGATAACCA | XM_019365994.2 |
| β-Actin e | F: CCAGCCTTCCTTCCTTGGTA R: AGGTGGGGCAATGATCTTGA | KJ126772.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatif, M.M.; Sabry, N.M.; Ibrahim, S.; Bassem, S.M.; Khalil, W.K.B.; Abdel-Gawad, F.K. Evaluation of Mitigation Role of L-Phenylalanine-Based Low-Molecular-Weight Gelator against Oil Pollution-Induced Nile Tilapia Toxicity. Gels 2023, 9, 848. https://doi.org/10.3390/gels9110848
Abdellatif MM, Sabry NM, Ibrahim S, Bassem SM, Khalil WKB, Abdel-Gawad FK. Evaluation of Mitigation Role of L-Phenylalanine-Based Low-Molecular-Weight Gelator against Oil Pollution-Induced Nile Tilapia Toxicity. Gels. 2023; 9(11):848. https://doi.org/10.3390/gels9110848
Chicago/Turabian StyleAbdellatif, Mohamed Mehawed, Noha M. Sabry, Saber Ibrahim, Samah M. Bassem, Wagdy K. B. Khalil, and Fagr Kh. Abdel-Gawad. 2023. "Evaluation of Mitigation Role of L-Phenylalanine-Based Low-Molecular-Weight Gelator against Oil Pollution-Induced Nile Tilapia Toxicity" Gels 9, no. 11: 848. https://doi.org/10.3390/gels9110848
APA StyleAbdellatif, M. M., Sabry, N. M., Ibrahim, S., Bassem, S. M., Khalil, W. K. B., & Abdel-Gawad, F. K. (2023). Evaluation of Mitigation Role of L-Phenylalanine-Based Low-Molecular-Weight Gelator against Oil Pollution-Induced Nile Tilapia Toxicity. Gels, 9(11), 848. https://doi.org/10.3390/gels9110848








