Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing
Abstract
:1. Introduction
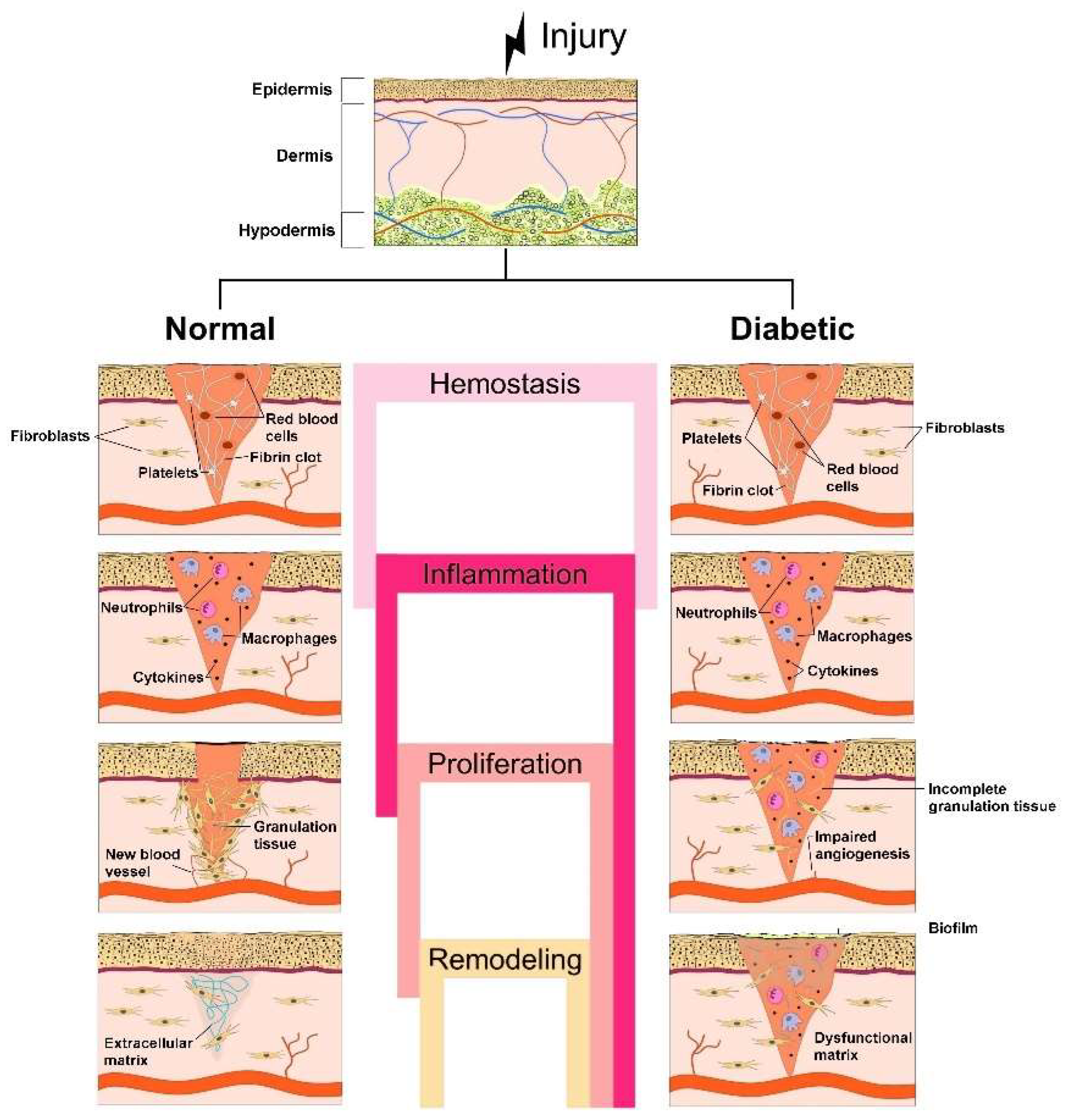
2. Biological and Biochemical Processes during Normal vs. Diabetic Wound Healing
2.1. Stages of Normal Wound Healing
- -
- Vascular constriction that decreases blood circulation at the wound site;
- -
- Platelet aggregation at the wound site owing to the interaction with proteins (collagen (COL) and fibronectin);
- -
- Degranulation;
- -
- Conversion of soluble fibrinogen into insoluble fibrin to arrest bleeding;
- -
- Mediation of hemostasis through key agents, such as fibrin, fibronectin and vitronectin;
- -
- Production of growth factors, such as transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and chemokines, by the clot’s surrounding area to efficiently aid the wound healing [15,16]. PDGF acts also in vascularization by attracting fibroblasts, which stimulate tissue repair through COL deposition [17]. In the first phase of the homeostasis stage, prostaglandin H2 is converted into thromboxane A2 (TXA2) by the action of thromboxane synthase. Then, TXA2 acts as a powerful platelet activator and vasoconstrictor, in addition to participating in the release of macrophages, neutrophils and endothelial cells, playing an important role in the following stages of the wound healing process [18,19].
- -
- Leucocytes’ (especially neutrophils) migration to the injured site to eliminate debris and bacteria;
- -
- Proinflammatory cytokines secretion by neutrophils to promote the expression of adhesion molecules;
- -
- Monocytes’ migration into the wound site and differentiation into macrophages.
- -
- Slow transformation of ECM into a mature scar;
- -
- COL production reorganization in the ECM by replacing COL type III with COL type I and closure of the wound;
- -
- Decrease of blood supply and formation of new blood vessels;
- -
- Formation of a cellular environment and mature avascular tissue [19].
2.2. Pathology of Diabetic Wound Healing
3. Effect of Small Molecules and Natural Polymers in Diabetic Wound Healing
3.1. Small Molecules
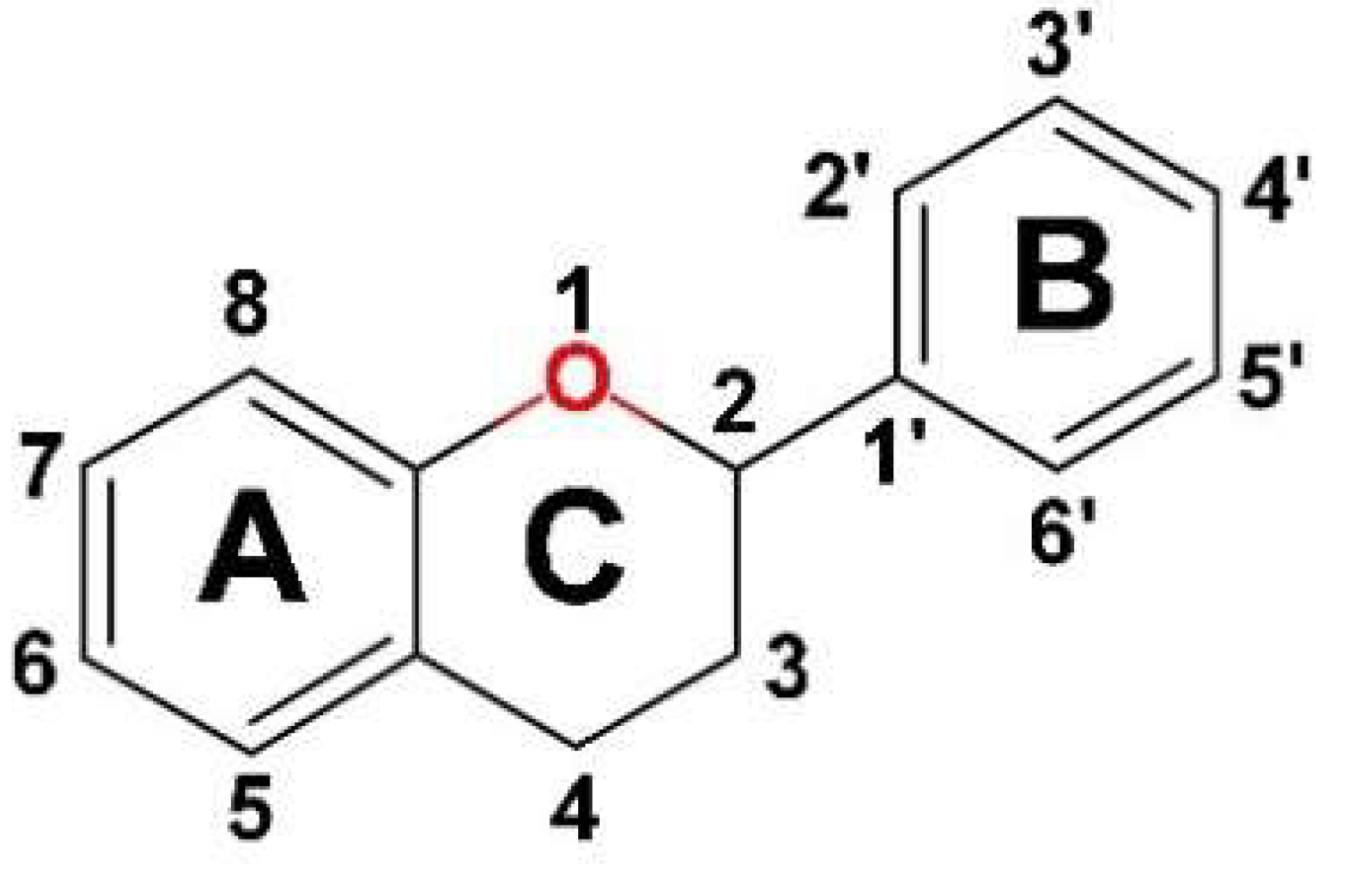
3.1.1. Flavonoids
3.1.2. Non-Flavonoid Compounds
3.1.3. Other Plant Bioactive Compounds
| Polyphenols Class | Compound | Activity | Reference |
|---|---|---|---|
| Flavonoids | Luteolin | In vivo decrease in blood glucose levels, accelerated skin wounds’ re-epithelization in diabetic rats by inhibiting the inflammatory cell infiltration, decreasing IL1-β, IL-6, TNF-α expression, and reducing oxidative stress, down-regulated NF-kB and up-regulated SOD1 and glutathione peroxidase (GSH-Px) expression mediated by p-Nrf2; In vivo intraperitoneal administration treated diabetes-associated wounds by targeting NF-kB/MMP-9 axis and Nrf2-mediated antioxidant system. | [46] |
| Quercetin | In vivo modulated fibroblast activity, up-regulated VEGF and TGF-β1 in diabetic scars; In vivo oral application increased COL synthesis, deposition and orientation and decreased inflammatory cytokines (IL1-β and TNF-α) in rat diabetic wounds; In vivo topical administration modulated cytokines and GFs, and inhibited inflammatory reactions in rat diabetic wounds; promoted macrophages’ M1-M2 phenotype switch during wound healing in diabetic mice. | [33,35,46] | |
| Rutin (quercetin-3-O-rutoside | In vivo prevented oxidative stress and inflammatory response, improving wound healing in hyperglycemic rats; In vivo decreased the number of inflammatory cells; stimulated Nrf-2 activity and antioxidant enzymes (SOD1 and GSH-Px) expression; In vivo down-regulated IL-1β, IL-6, TNF-α, NF-kB, MMP-2, MMP-9, TGF-β1, VEGF expression levels; In vivo intraperitoneal administration elevated neurogenic-related protein expression. | [46] | |
| Myricetin | In vitro prevented cellular oxidative stress by regulating antioxidant enzymes; In vitro enhanced pro-COL I and III levels, inhibited MMP-1, MMP-2 and MMP-9 synthesis, increased TIMP1/MMPs ratio by enhancing TIMP-1 mRNA expression, suppressed catalase (CAT) and SOD1 in diabetic fibroblasts. | [46,47] | |
| Icariin | In vivo anti-inflammatory and pro-angiogenic activities in diabetic rats by down-regulating NF-kB, TNF-α, MMP-2, MMP-9 levels and increasing IL-10 and CD31 levels; In vivo topical administration stimulated normal ECM formation in the healing tissue by increasing the relative COL deposition. | [46,48] | |
| Vicenin-2 (VCN-2) | In vivo inhibited oxidative and inflammatory stress in a dose-dependent manner, stimulating wound healing in STZ-induced DM rats; In vitro increased cell proliferation, reduced inflammatory cells, down-regulated proinflammatory cytokines (IL-1β, IL-6, TNF-α), mediators (iNOS, COX2) and nitric oxide (NO) expression via NF-kB pathway; improved epithelialization and remodeling; stimulated fibroblast proliferation and migration, neoangiogenesis and wound contraction, down-regulated MMP-9, VEGF and TGF-1β levels via HIF-1α pathway; In vivo topical administration reduced food and fluid intakes, decreased blood glucose level and increased insulin level, body weight and percentage of wound closure. | [46,49] | |
| Mangiferin | In vivo topical administration inhibited oxidative stress, decreased the wound area and increased skin thickness; enhanced EGF, FGF, Nrf-2, TGF-β, VEGF and PI3K expression and decreased MMP-2, TNF-α and NF-kB p65 expression in diabetic wound; reduced the inflammatory phase in hyperglycemic conditions. | [46,50] | |
| Curcumin | In vivo topical administration accelerated re-epithelialization rate, accelerated wound closure through down-regulation of TNF-α, IL-1β and MMP-9 levels, up-regulation of IL-10 level and elevation of SOD, CAT and GSH-Px activity, improved thick granulation tissue formation, COL synthesis, deposition and orientation in rat models of diabetic ulcer and wound. | [46,51] | |
| Kaempferol | In vivo topical agent with 92.12% wound healing rate in diabetic rats. | [37] | |
| Epigallocatechin-3 gallate | In vivo enhanced wound healing through acceleration of re-epithelization and angiogenesis, reduced cytokines level and inhibited macrophage accumulation, inflammation response and Notch signaling in diabetic mouse wounds. | [52] | |
| Hesperidin | In vivo accelerated angiogenesis and vasculogenesis via up-regulation of VEGF-C, TGF-β, Ang-1/Tie-2 and Smad-2/3 mRNA expression, increased COL deposition and suppressed IL-6 and TNF-α inflammatory mediators, enhancing wound healing of chronic DFUs in STZ-induced Sprague Dawley rats. | [53] | |
| Genistein | In vivo subcutaneous administration modulated oxidative stress, improved angiogenesis by FoxO1 and iNOS suppression; oral administration supported wound healing, lowered oxidative stress and inflammation in diabetic mice wounds. | [35] | |
| Puerarin | In vitro down-regulated inflammatory cytokines expression by inhibition of MAPK and NF-κB inflammatory signaling pathways in high-glucose cell culture; improved polarization to M2 macrophages at cellular level. | [54] | |
| Stilbenes | Resveratrol | In vitro exerted antioxidant effect and inhibited TNF-α and NF-kB. | [55] |
| Phenolic acids | Ferulic acid | In vivo inhibited lipid peroxidation, increased CAT, SOD and glutathione expression, elevated NO and serum zinc and copper, improving the healing process in diabetic ulcers. | [56] |
| Syringic acid | In vivo topical administration improved wound healing by promoting cell migration and proliferation in STZ-induced diabetic rats; significantly reduced MMP-2, MMP-8 and MMP-9 levels, up-regulated TIMP-1 and TIMP-2 levels; elevated COL I, CD31, CD68, α-SMA, TGF-β1 and VEGF content in diabetic wounds. | [46,56,57] | |
| Chlorogenic acid | In vivo stimulated COL production, reduced the level of oxidative and inflammation markers (MDA/NO), increased GSH level, maintained SOD/CAT level, accelerating wound healing in STZ-induced diabetic rats. | [58] | |
| Gallic acid | In vivo ROS scavenger, exerted antioxidant activity, promoting wound healing in a diabetic mouse model. | [59] | |
| Tannins | Tannic acid | In vivo antioxidant, hemostatic, anti-inflammatory, antimicrobial activity useful in skin wounds and ulcers. | [58] |
| Terpenes | Kirenol | In vivo exerted anti-inflammatory, antioxidant and wound healing activity by regulation of MMP-2 and MMP-9 expression, inhibition of NF-kB, COX-2 and iNOS expression and MDA content, elevated antioxidant enzymes activity, favoring angiogenesis and formation of granulation tissue in STZ-induced diabetic rats. | [46] |
| Alkaloids | Berberine | In vivo topical application accelerated novel ECM synthesis and wound healing process through modulation of TrxR1 and its downstream JNK signaling, expression of MMP9 and TIMP1, up-regulation of TGF-β1, resulting in promotion of fibroblast proliferation and inhibition of oxidative stress and apoptosis in HFD- and STZ-induced diabetic rats. | [46,60] |
3.2. Natural Polymers
3.2.1. Cellulose
3.2.2. Alginate
3.2.3. Chitosan (CS)
3.2.4. Hyaluronic Acid (HA)
3.2.5. Other Polysaccharides
3.2.6. Collagen
3.2.7. Gelatin
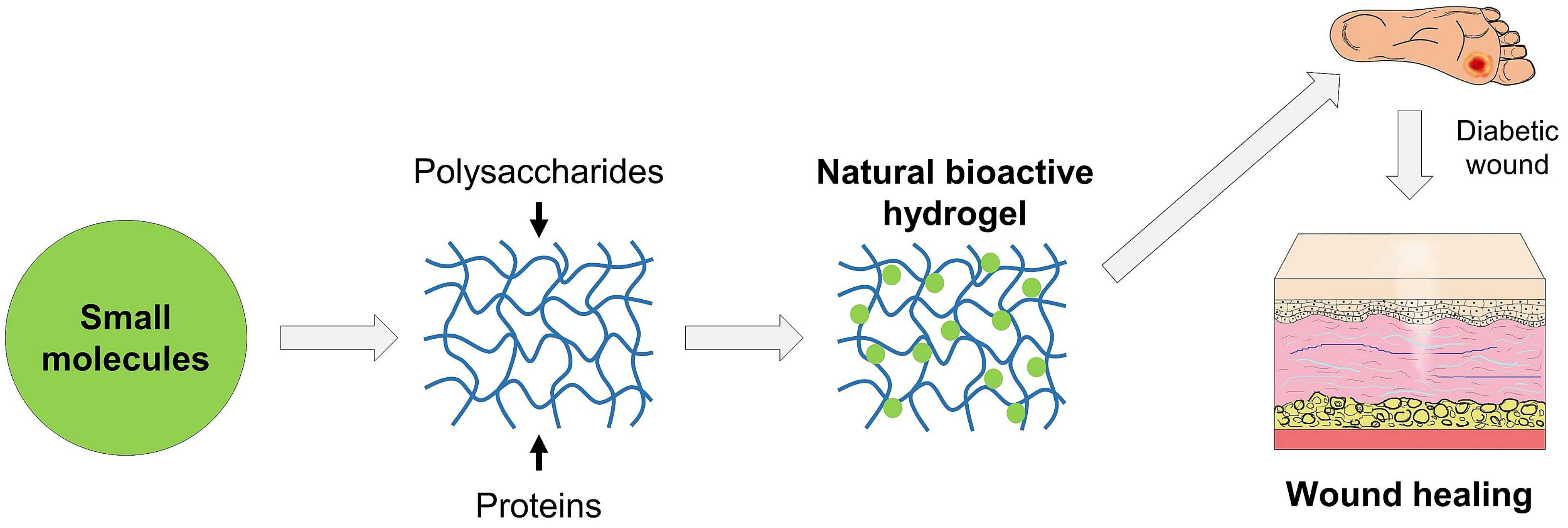
4. Recent Development of Hydrogels Encapsulating Small Molecules for Healing of Diabetic Wounds
- -
- Antibacterial hydrogels loaded with antibiotics or drug-like components to control bacterial growth;
- -
- Antifouling hydrogels by creating superhydrophilic or more rarely superhydrophobic surfaces, which can reduce bacterial attachment and biofilm formation [22];
- -
- Antioxidant hydrogels loaded with antioxidant scavengers or drugs, such as curcumin and gallic and tannic acid, to improve the inherent antioxidant properties of the constituent macromolecules against ROS formation [73].
4.1. Polysaccharide-Based Hydrogels
4.1.1. Cellulose-Based Hydrogels
4.1.2. Alginate-Based Hydrogels
4.1.3. CS-Based Hydrogels
| Hydrogel Type | Experiment Type | Activity | Reference |
|---|---|---|---|
| Quaternized CS/tannic acid hydrogels | STZ-induced diabetic rat model | Good injectability and self-healing, cytocompatibility, hemostatic capability and biodegradability, radical scavenging activity, COL deposition, no scar formation, skin regeneration. | [88] |
| CS-puerarin hydrogel | STZ-induced diabetic rat model | Promoted diabetic wound healing and accelerated angiogenesis, inhibition of the miR-29 mediated inflammation response. | [1,89] |
| Sulfated chitosan (SCS)-doped COL type I (Col I/SCS) hydrogel | STZ-induced diabetic rat model | Reduced inflammation through minimizing macrophages’ polarization into M1 phenotype, decreased production of pro-inflammatory IL-6 and increased production of anti-inflammatory cytokines IL-4, TGF-β1 in chronic diabetic wounds; stimulated COL synthesis, angiogenesis and cell migration for wound closure in diabetic wounds. | [1,90] |
| Apigenin loaded gellan gum-CS (GGCH) hydrogel | STZ-induced diabetic rat model | Increased level of SOD, GSH, CAT, protein content in granuloma tissue; biocompatibility, biodegradability, moist nature, antioxidant effectiveness; increased hydroxyproline level and collagen turnover; decreased epithelialization period; higher wound healing in diabetics. | [91] |
| CS/ HA-based hydrogel with MOF-loaded lipoic acid | In vitro and in vivo analysis in diabetic Sprague Dawley rats | Antibacterial activity and antioxidant performance, promoted cell proliferation and migration, wound healing process, better granulation tissue formation and more COL deposition. | [92] |
| Bio-multifunctional benzaldehyde-terminated 4-arm PEG (4-arm-PEG-CHO)/carboxymethyl CS (CMCS)/basic fibroblast growth factor (bFGF) hydrogels (BP/CS-bFGF) | STZ-induced diabetic rat model | Strong wet-tissue adhesion, self-mending fast hemostasis capacity, excellent biocompatibility, antibacterial property, increased production of Ki67, promoted the generation of epithelialization and COL, induced formation of hair follicles, enhanced neovascularization by up-regulating the production of CD31 and CD34. | [93] |
| CS hydrogels functionalized with either unfractionated heparin or bemiparin (a low molecular weight heparin, LMWH) | STZ-induced diabetic rat model | Accelerated inflammation, improved the epithelization process, formation of high-quality cicatricial tissue, improved diabetes-associated impaired wound healing. | [94] |
4.1.4. HA-Based Hydrogels
4.2. Protein Hydrogels
4.2.1. Collagen-Based Hydrogels
4.2.2. Gelatin-Based Hydrogels
4.2.3. Other Protein Hydrogels
5. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Hu, Q.; Wei, Z.; Ou, Y.; Cao, Y.; Zhou, H.; Wang, M.; Yu, K.; Liang, B. Advanced polymer hydrogels that promote diabetic ulcer healing: Mechanisms, classifications, and medical applications. Biomater. Res. 2023, 2023, 27. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Oyebode, O.A.; Jere, S.W.; Houreld, N.N. Current Therapeutic Modalities for the Management of Chronic Diabetic Wounds of the Foot. J. Diabetes Res. 2023, 2023, 1359537. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 2021, 10th ed.; IDF Diabetes Atlas: Brusseles, Belgium, 2021; Available online: http://www.diabetesatlas.org/ (accessed on 6 August 2023).
- Zhang, J.; Liu, H.; Che, T.; Zheng, Y.; Nan, X.; Wu, Z. Nanomaterials for diabetic wound healing: Visualization and bibliometric analysis from 2011 to 2021. Front. Endocrinol. 2023, 14, 1124027. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound bed preparation: TIME for an update. Int. Wound J. 2016, 13 (Suppl. S3), 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent advances in responsive hydrogels for diabetic wound healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective are nano-based dressings in diabetic wound healing? A comprehensive review of literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar]
- Guo, S.; DiPietro, L. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Huang, C.; Yuan, W.; Chen, J.; Wu, L.-P.; You, T. Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules 2023, 28, 1110. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Thakur, V.K.; Gupta, M. Polysaccharide-based hydrogels: New insights and futuristic prospects in wound healing. Int. J. Biol. Macromol. 2022, 223, 1586–1603. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.; Pintado, M.; Tavaria, F. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.M.; Préat, V.; Gallez, B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wu, Y.; Liu, J.; Yuan, X.; Gao, J.A. Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int. J. Nanomed. 2022, 17, 6007–6029. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef]
- A Elafros, M.; Andersen, H.; Bennett, D.L.; Savelieff, M.G.; Viswanathan, V.; Callaghan, B.C.; Feldman, E.L. Towards prevention of diabetic peripheral neuropathy: Clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022, 21, 922–936. [Google Scholar] [CrossRef]
- Zulkefli, N.; Zahari, C.N.M.C.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Wu, X.; Hui, B.; Han, S.; Gao, J.; Li, Y.; Shi, J.; Zhu, H.; Zhao, B.; et al. Simultaneous deactivation of FAK and Src improves the pathology of hypertrophic scar. Sci. Rep. 2016, 6, 26023. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. Int. 2022, 29, 35518–35541. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef] [PubMed]
- Falbo, F.; Spizzirri, U.G.; Restuccia, D.; Aiello, F. Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics 2023, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; A Haddad, M.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Z.; Khachemoune, A. Flavonoids and their therapeutic applications in skin diseases. Arch. Dermatol. Res. 2023, 315, 321–331. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, I.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Rakotondrabe, T.F.; Fan, M.-X.; Muema, F.W.; Guo, M.-Q. Modulating inflammation-mediated diseases via natural phenolic compounds loaded in nanocarrier systems. Pharmaceutics 2023, 15, 699. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, H.; Gong, W.; Wang, R.; He, W.; Wang, X.; Hu, J. Co-Assembling of Natural Drug-Food Homologous Molecule into Composite Hydrogel for Accelerating Diabetic Wound Healing. Biomat. Adv. 2022, 140, 213034. [Google Scholar] [CrossRef]
- Kaltalioglu, K. Sinapic acid-loaded gel accelerates diabetic wound healing process by promoting re-epithelialization and attenuating oxidative stress in rats. Biomed. Pharmacother. 2023, 163, 114788. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- Hu, W.; Yu, H.; Zhou, X.; Li, M.; Xiao, L.; Ruan, Q.; Huang, X.; Li, L.; Xie, W.; Guo, X.; et al. Topical administration of pterostilbene accelerates burn wound healing in diabetes through activation of the HIF1α signaling pathway. Burns 2022, 48, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Dang, M.; Lin, Y.; Xue, F. Evaluation of wound healing activity of plumbagin in diabetic rats. Life Sci. 2019, 231, 116422. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Liu, S. Targeting matrix metalloproteases in diabetic wound healing. Front. Immunol. 2023, 14, 1089001. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, X.; Gong, M.; Li, Y. Myricetin, a potent natural agent for treatment of diabetic skin damage by modulating TIMP/MMPs balance and oxidative stress. Oncotarget 2016, 7, 71754–71760. [Google Scholar] [CrossRef]
- Yan, T.; Cheng, F.; Wei, X.; Huang, Y.; He, J. Biodegradable collagen sponge reinforced with chitosan/calcium pyrophosphate nano flowers for rapid hemostasis. Carbohydr. Polym. 2021, 170, 271–280. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.-F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef]
- Lwin, O.M.; Giribabu, N.; Kilari, E.K.; Salleh, N. Topical administration of mangiferin promotes healing of the wound of streptozotocin-nicotinamide-induced type-2 diabetic male rats. J. Dermatol. Treat. 2020, 32, 1039–1048. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.W.; Zhu, Q.Q.; Yang, X.Y.; Xu, H.H.; Sun, B.; Wang, X.J.; Sheng, J. Wound healing can be improved by (−)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018, 17, 399–419. [Google Scholar] [PubMed]
- Li, S.; Yang, P.; Ding, X.; Zhang, H.; Ding, Y.; Tan, Q. Puerarin improves diabetic wound healing via regulation of macrophage M2 polarization phenotype. Burn. Trauma 2022, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Zhou, R.; Xiang, C.; Cao, G.; Xu, H.; Zhang, Y.; Yang, H. Berberine accelerated wound healing by restoring TrxR1/JNK in diabetes. Clin. Sci. 2021, 135, 613–627. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Lin, S.-C.; Wu, Y.-H.; Hu, C.-Y.; Chen, Y.-T.; Chen, Y.-C. The Antimicrobial Effects of Bacterial Cellulose Produced by Komagataeibacter intermedius in Promoting Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 5456. [Google Scholar] [CrossRef]
- Moradpoor, H.; Mohammadi, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Gorji, P.; Sulong, A.B.; Muhamad, N.; Ebadi, M. Recent Advances on Bacterial Cellulose-Based Wound Management: Promises and Challenges. Int. J. Polym. Sci. 2023, 2022, 1214734. [Google Scholar] [CrossRef]
- García-Sánchez, M.E.; de Guadalajara, U.; Robledo-Ortiz, J.R.; Jiménez-Palomar, I.; González-Reynoso, O.; González-García, Y. Production of bacterial cellulose by Komagataeibacter xylinus using mango waste as alternative culture medium. Rev. Mex. Ing. Quim. 2020, 19, 851–865. [Google Scholar] [CrossRef]
- Abazari, M.; Akbari, T.; Hasani, M.; Sharifikolouei, E.; Raoufi, M.; Foroumadi, A.; Sharifzadeh, M.; Firoozpour, L.; Khoobi, M. Polysaccharide-based hydrogels containing herbal extracts for wound healing applications. Carbohydr. Polym. 2022, 294, 119808. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Escárcega-Galaz, A.A.; De La Cruz-Mercado, J.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Brito-Zurita, O.R.; Ornelas-Aguirre, J.M. Chitosan treatment for skin ulcers associated with diabetes. Saudi J. Biol. Sci. 2018, 25, 130–135. [Google Scholar] [CrossRef]
- Bollyky, P.L.; Falk, B.A.; Long, S.A.; Preisinger, A.; Braun, K.R.; Wu, R.P.; Evanko, S.P.; Buckner, J.H.; Wight, T.N.; Nepom, G.T. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-β. J. Immunol. 2009, 183, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-L.; Lin, J.-A.; Chen, S.-Y.; Lin, J.-H.; Lin, H.-T.; Chen, Y.-Y.; Yen, G.-C. Effects of Hsian-tsao (Mesona procumbens Hemsl.) extracts and its polysaccharides on the promotion of wound healing under diabetes-like conditions. Food Funct. 2021, 12, 119–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Qi, R.; Yuan, H. Recent advances of natural-polymer-based hydrogels for wound antibacterial therapeutics. Polymers 2023, 15, 3305. [Google Scholar] [CrossRef]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef]
- Ghosal, K.; Chakraborty, D.; Roychowdhury, V.; Ghosh, S.; Dutta, S. Recent Advancement of Functional Hydrogels toward Diabetic Wound Management. ACS Omega 2022, 7, 43364–43380. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liao, Q.; Wu, Y.; Wang, X.; Fu, C.; Geng, F.; Qu, Y.; Zhang, J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int. J. Biol. Macromol. 2020, 164, 3846–3857. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Aziz, K.K.A.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Wang, P.; Yang, R.; Tan, X.; Shi, T.; Ma, J.; Xue, W.; Chi, B. Bio-fabricated nanocomposite hydrogel with ROS scavenging and local oxygenation accelerates diabetic wound healing. J. Mater. Chem. B 2022, 10, 4083–4095. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Madni, A.; Raza, B.; Islam, M.U.; Hassan, A.; Ahmad, F.; Ali, H.; Khan, T.; Wahid, F. Multiwalled carbon nanotubes functionalized bacterial cellulose as an efficient healing material for diabetic wounds. Int. J. Biol. Macromol. 2022, 203, 256–267. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved drug delivery and accelerated diabetic wound healing by chondroitin sulfate grafted alginate-based thermoreversible hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112169. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Paggiaro, A.O.; de Carvalho, V.F.; Isaac, C.; Gemperli, R. Effects of Hydrogel with Enriched Sodium Alginate in Wounds of Diabetic Patients. Plast. Surg. Nurs. 2020, 40, 110–115. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, Q.; Ma, J.; Xu, M.; Zhang, S.-J.; Qiu, B.-S.; Xia, B.; Gu, H.-F.; Hong, J.-F.; Zhao, C.; et al. Effect of combination therapy with alginate dressing and mouse epidermal growth factor on epidermal stem cells in patients with refractory wounds. Chin. Med. J. Engl. Chin. Med. J. Engl. 2012, 125, 257–261. [Google Scholar]
- Casado-Díaz, A.; La Torre, M.; Priego-Capote, F.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Berenguer Pérez, M.; Tunez, I. EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation. J. Clin. Med. 2022, 11, 1229. [Google Scholar] [CrossRef]
- Soares, R.D.; Campos, M.G.; Ribeiro, G.P.; Salles, B.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. A 2022, 108, 654–662. [Google Scholar] [CrossRef]
- Ferreira, M.O.G.; Leite, L.L.R.; de Lima, I.S.; Barreto, H.M.; Nunes, L.C.C.; Ribeiro, A.B.; Osajima, J.A.; Filho, E.C.d.S. Chitosan hydrogel in combination with nerolidol for healing wounds. Carbohydr. Polym. 2016, 152, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Velazco, G.; Gonzalez, A.; Ortiz, R. Chitosan films for the diabetic foot treatment. Avan. Biomed. 2012, 1, 38–41. [Google Scholar]
- Available online: https://chitotech.com/page/627/ChitoHeal-Gel (accessed on 10 August 2023).
- Pan, W.; Qi, X.; Xiang, Y.; You, S.; Cai, E.; Gao, T.; Tong, X.; Hu, R.; Shen, J.; Deng, H. Facile formation of injectable quaternized chitosan/tannic acid hydrogels with antibacterial and ROS scavenging capabilities for diabetic wound healing. Int. J. Biol. Macromol. 2022, 195, 190–197. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, B.; Wang, L.; Sun, Y.; Jin, Z.; Liu, X.; Ouyang, L.; Liao, Y. Chitosan@Puerarin hydrogel for accelerated wound healing in diabetic subjects by miR-29ab1 mediated inflammatory axis suppression. Bioact. Mater. 2023, 19, 653–665. [Google Scholar] [CrossRef]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef]
- Sanaye, P.M.; Mojaveri, M.R.; Ahmadian, R.; Jahromi, M.S.; Bahramsoltani, R. Apigenin and its dermatological applications: A comprehensive review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and self-healing chitosan-based hydrogel with MOF-loaded α-lipoic acid promotes diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112519. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Cifuentes, A.; Gómez-Gil, V.; Ortega, M.A.; Asúnsolo, Á.; Coca, S.; Román, J.S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Chitosan hydrogels functionalized with either unfractionated heparin or bemiparin improve diabetic wound healing. Biomed. Pharmacother. 2020, 129, 110498. [Google Scholar] [CrossRef]
- Singh, S.K.; Dwivedi, S.D.; Yadav, K.; Shah, K.; Chauhan, N.S.; Pradhan, M.; Singh, M.R.; Singh, D. Novel Biotherapeutics Targeting Biomolecular and Cellular Approaches in Diabetic Wound Healing. Biomedicines 2023, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater. Today Bio 2021, 12, 100139. [Google Scholar] [CrossRef]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic acid-based glucose-responsive antioxidant hydrogel platform for enhanced diabetic wound repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M. European and Australian Apligraf Diabetic Foot Ulcer Study Group Apligraf in the treatment of neuropathic diabetic foot ulcers. Int. J. Low. Extremity Wounds 2009, 8, 11–18. [Google Scholar] [CrossRef]
- Sharma, A.D.B.; Jarman, E.H.B.; Fox, P.M. Scoping Review of Hydrogel Therapies in the Treatment of Diabetic Chronic Wounds. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4984. [Google Scholar] [CrossRef]
- Djavid, G.E.; Tabaie, S.M.; Tajali, S.B.; Totounchi, M.; Farhoud, A.; Fateh, M.; Ghafghazi, M.; Koosha, M.; Taghizadeh, S. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomised control trial. J. Wound Care 2020, 29, S13–S18. [Google Scholar] [CrossRef]
- Narisepalli, S.; Salunkhe, S.A.; Chitkara, D.; Mittal, A. Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: In-vitro and in-vivo evaluation. Int. J. Pharm. 2023, 631, 122508. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Weng, T.; Jin, R.; Yang, M.; Yu, M.; Zhang, W.; Wang, X.; Han, C. Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burn. Trauma 2022, 10, tkac001. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, X.; Li, X.; Wang, S.; Zhang, Y.; Xu, G. Impact of a Novel Hydrogel with Injectable Platelet-Rich Fibrin in Diabetic Wound Healing. J. Diabetes Res. 2023, 2023, 7532637. [Google Scholar] [CrossRef] [PubMed]
| Hydrogel Type | Experiment Type | Activity | Reference |
|---|---|---|---|
| Paeniflorin-loaded HA-based hydrogel | Ex vivo and in vivo experimental approaches in diabetic mice model | Stimulated transition of macrophages from M1 pro-inflammatory phenotype to M2 anti-inflammatory/pro-healing phenotype, lowered inflammation and promoted COL synthesis, new blood vessel formation, re-epithelialization of cutaneous wounds. | [96] |
| Hyaluronan/COL-based hydrogels containing high-sulfated hyaluronan | In vitro and in vivo studies in diabetic db/db mice | Reduced inflammation, augmented pro-regenerative macrophage activation, increased vascularization, accelerated new tissue formation and wound closure. | [97] |
| Nanotechnologically-modified curcumin and EGF encapsulated into HA and CS-based hydrogel | In vitro and in vivo studies in STZ-induced diabetic mice | High antioxidant, anti-inflammatory and migration-promoting effects, improved wound healing by granulation tissue formation, re-epithelialization and skin regeneration. | [98] |
| Glucose-responsive HA derivate (HAMA-PBA)/catechin (HMPC) hydrogel | In vitro and in vivo studies in diabetic wound model | High antioxidant capability, increased expression of VEGF and CD31, stimulated angiogenesis, decreased inflammatory responses by low IL-6 level and high IL-10 level, fast wound repair in three weeks. | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprita, E.I.; Iosageanu, A.; Craciunescu, O. Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing. Gels 2023, 9, 867. https://doi.org/10.3390/gels9110867
Oprita EI, Iosageanu A, Craciunescu O. Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing. Gels. 2023; 9(11):867. https://doi.org/10.3390/gels9110867
Chicago/Turabian StyleOprita, Elena Iulia, Andreea Iosageanu, and Oana Craciunescu. 2023. "Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing" Gels 9, no. 11: 867. https://doi.org/10.3390/gels9110867
APA StyleOprita, E. I., Iosageanu, A., & Craciunescu, O. (2023). Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing. Gels, 9(11), 867. https://doi.org/10.3390/gels9110867








