Development and Characterization of Terbinafine-Loaded Nanoemulgel for Effective Management of Dermatophytosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Excipients
2.1.1. Screening of Oils
2.1.2. Screening of Surfactants
2.1.3. Screening of Co-Surfactants
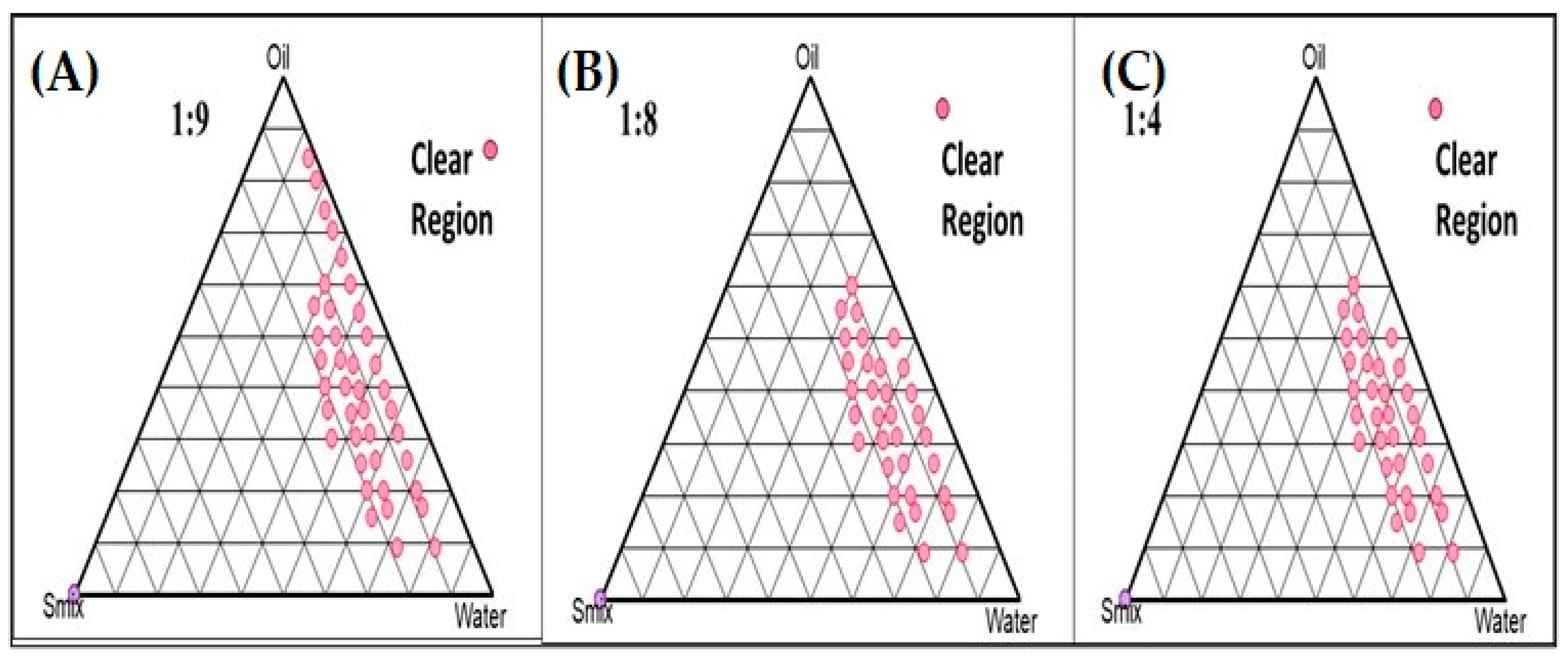
2.2. Pseudo-Phase Ternary Diagrams
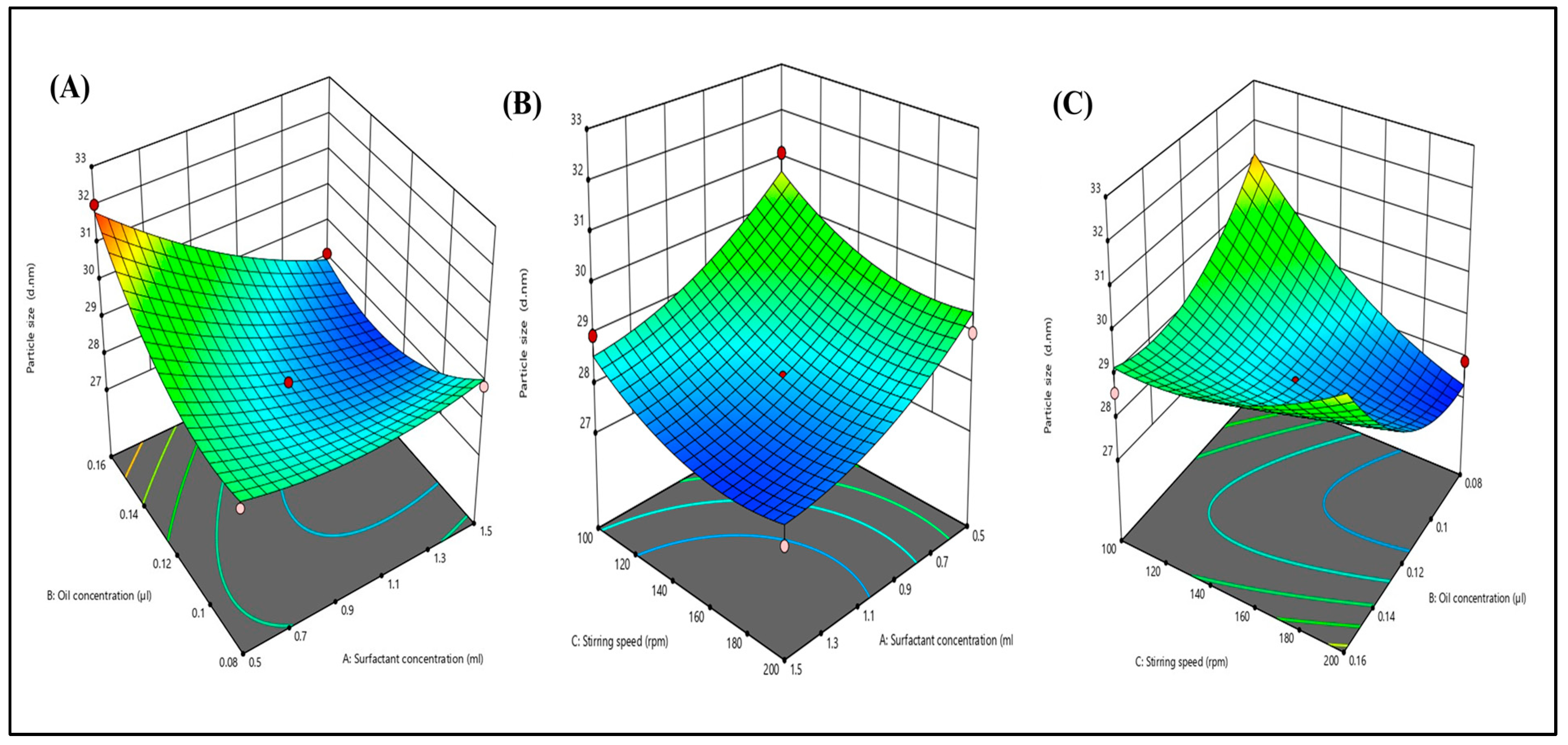
2.3. Box–Behnken Design (BBD) Mathematical Model Fitting and Optimization of TH-NE
2.3.1. Effects of Independent Variables on Particle Size
2.3.2. Effects of Independent Variables on PDI
2.3.3. Effects of Independent Variables on Zeta Potential
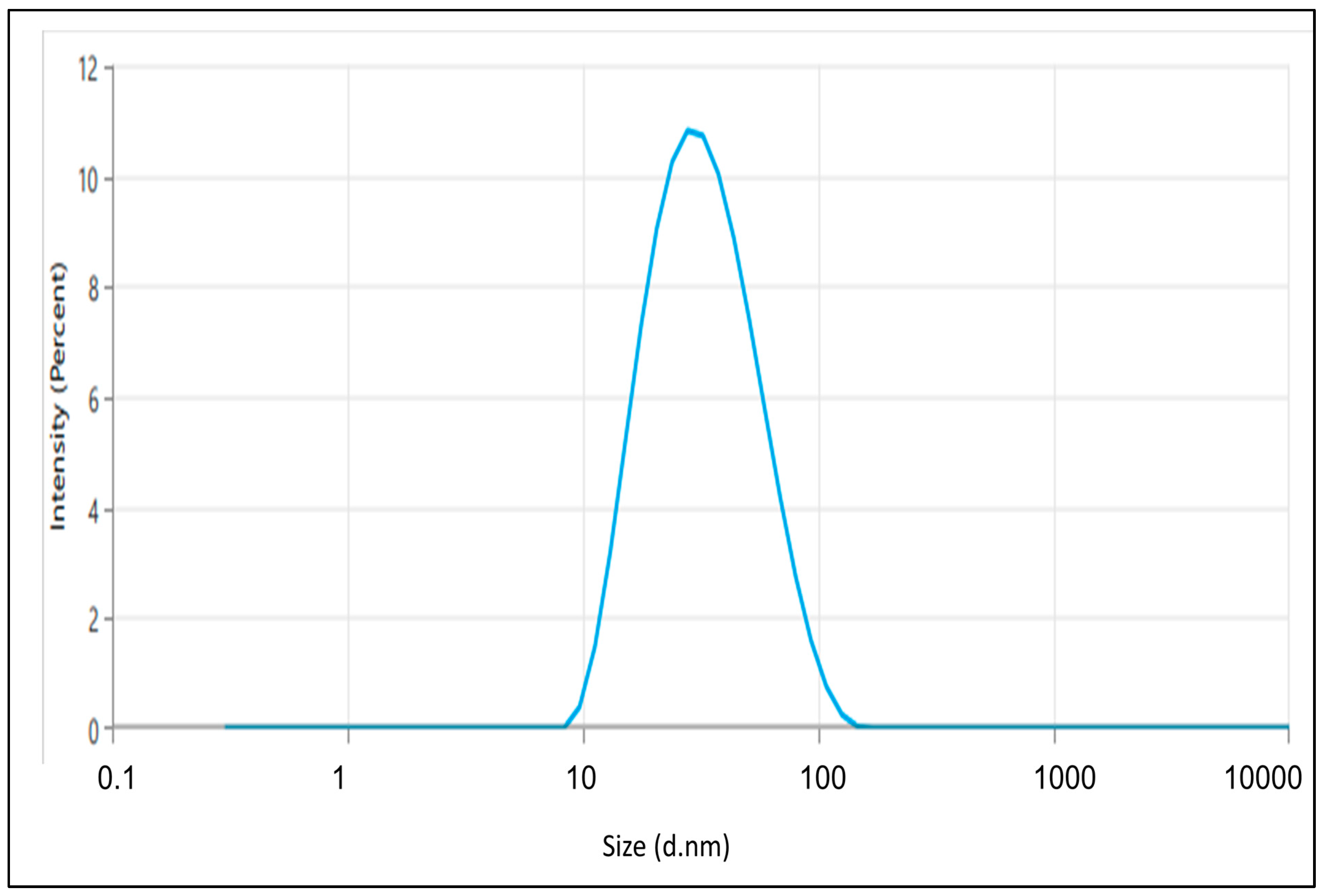
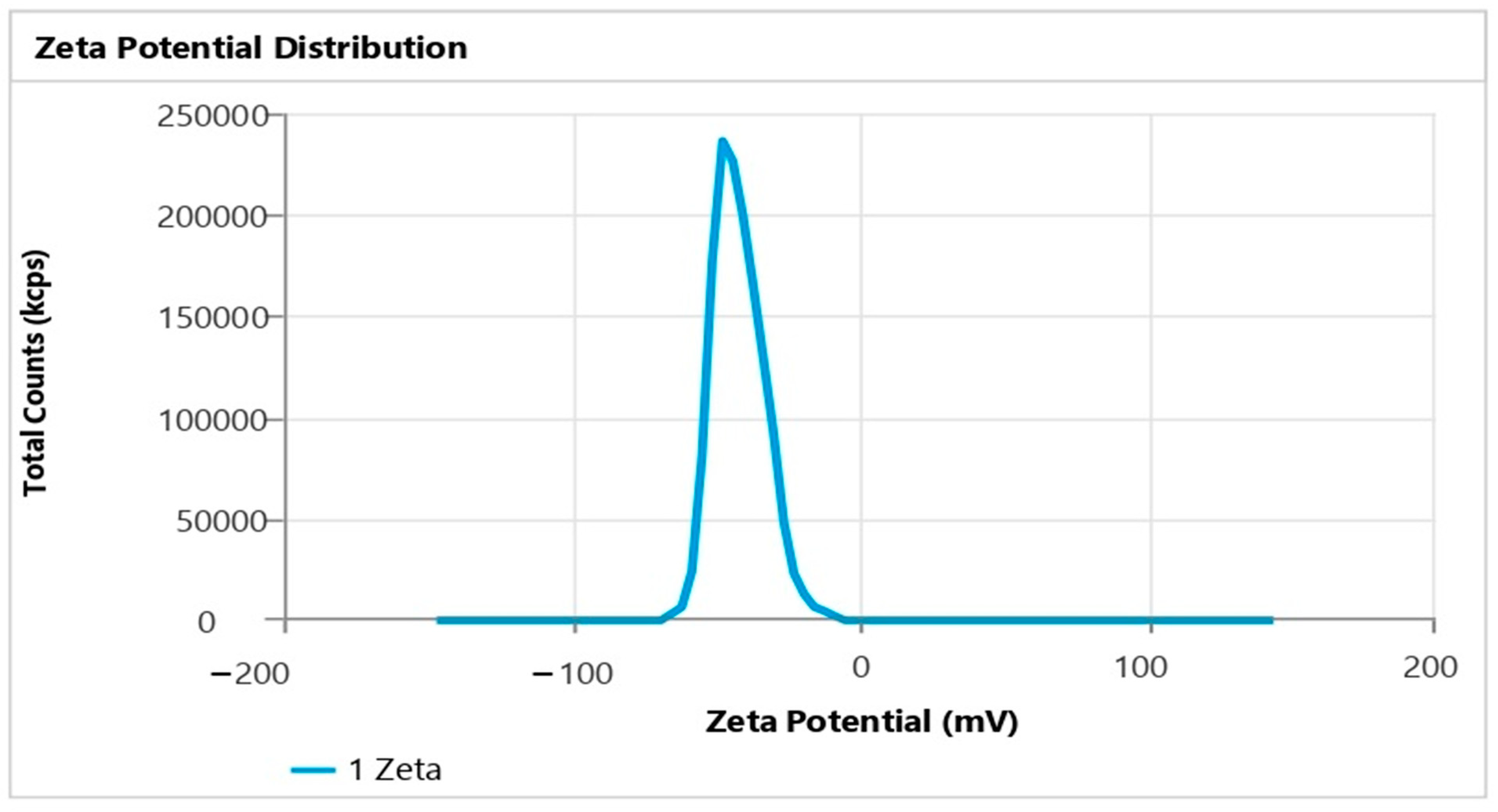
2.4. Particle Size, Polydispersity Index (PDI), and Zeta Potential
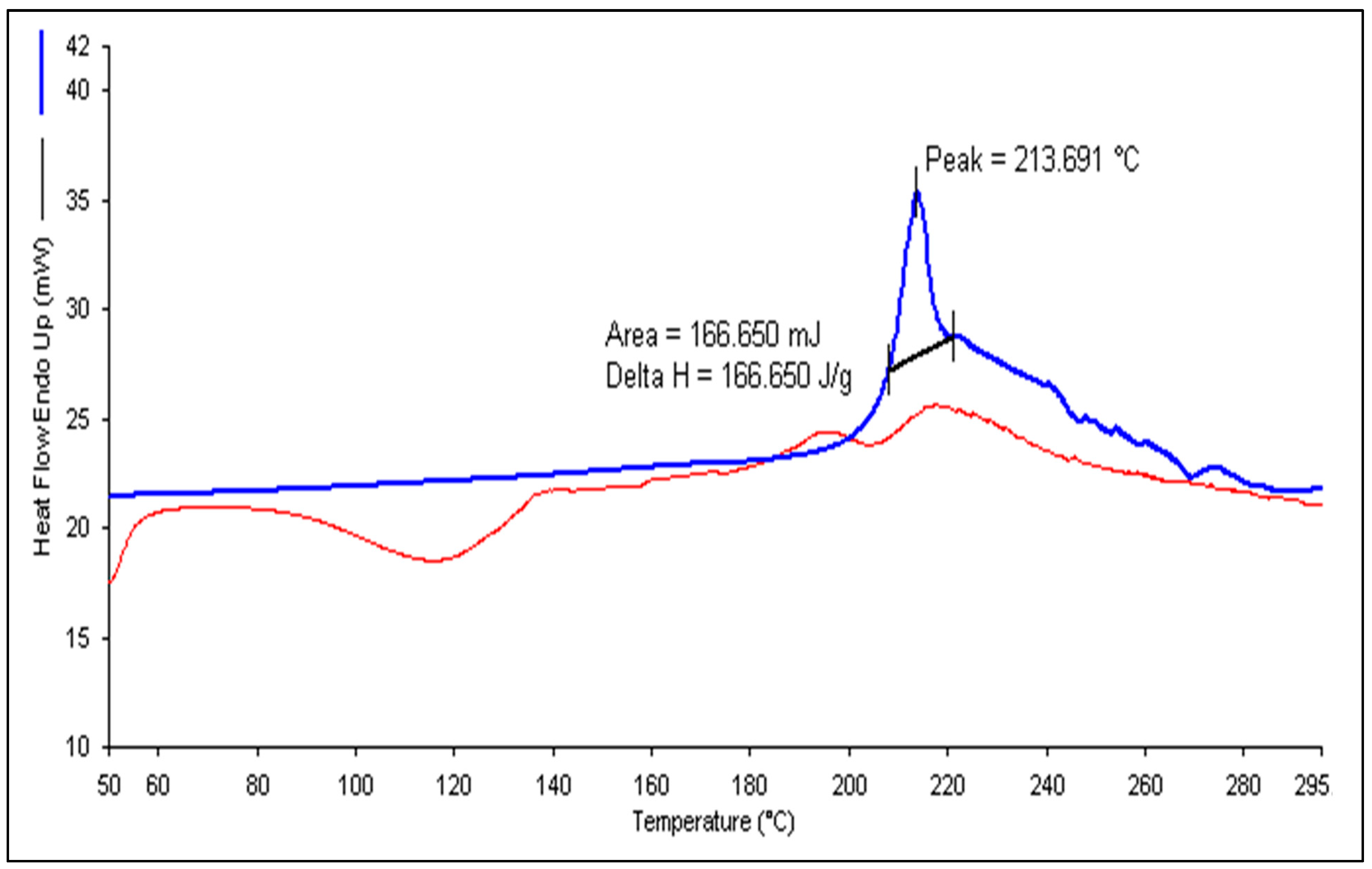
2.5. Differential Scanning Calorimetry (DSC)
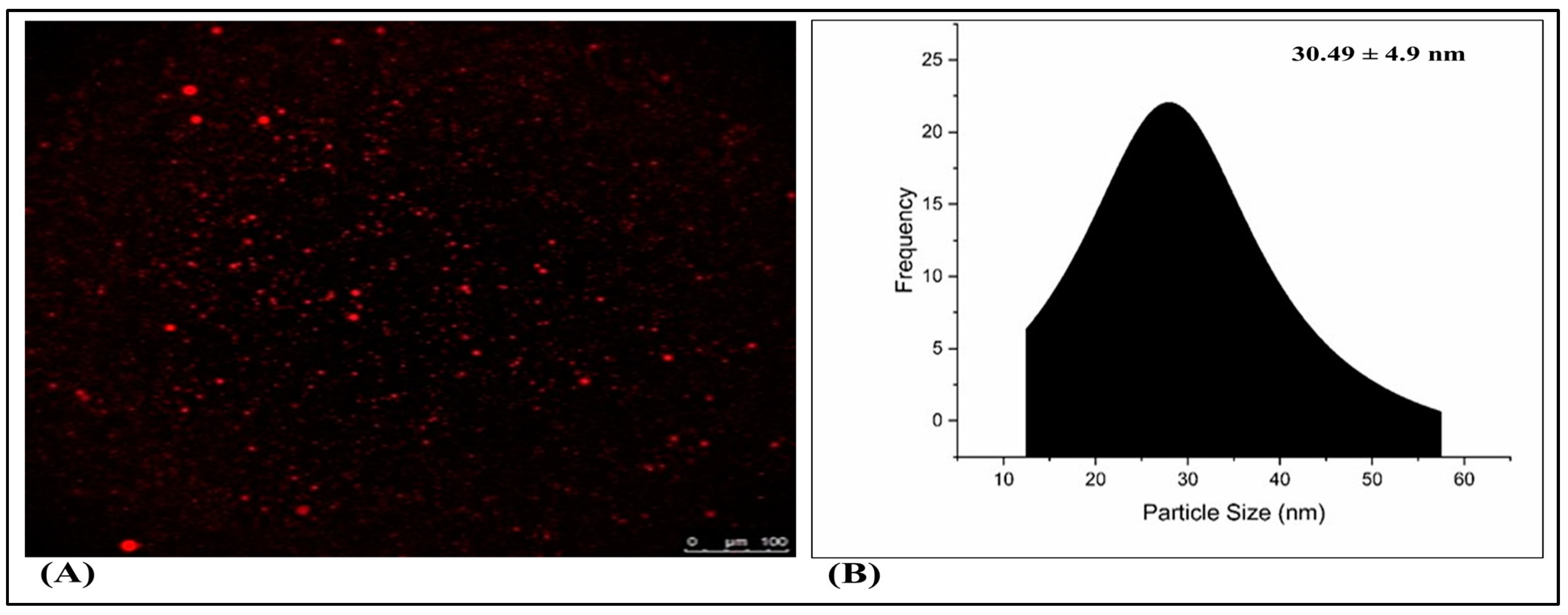
2.6. Fluorescence Microscopy
2.7. Preparation of Drug-Loaded Nanoemulgel Using Optimized Terbinafine HCl Nanoemulsion (TH-NEG)
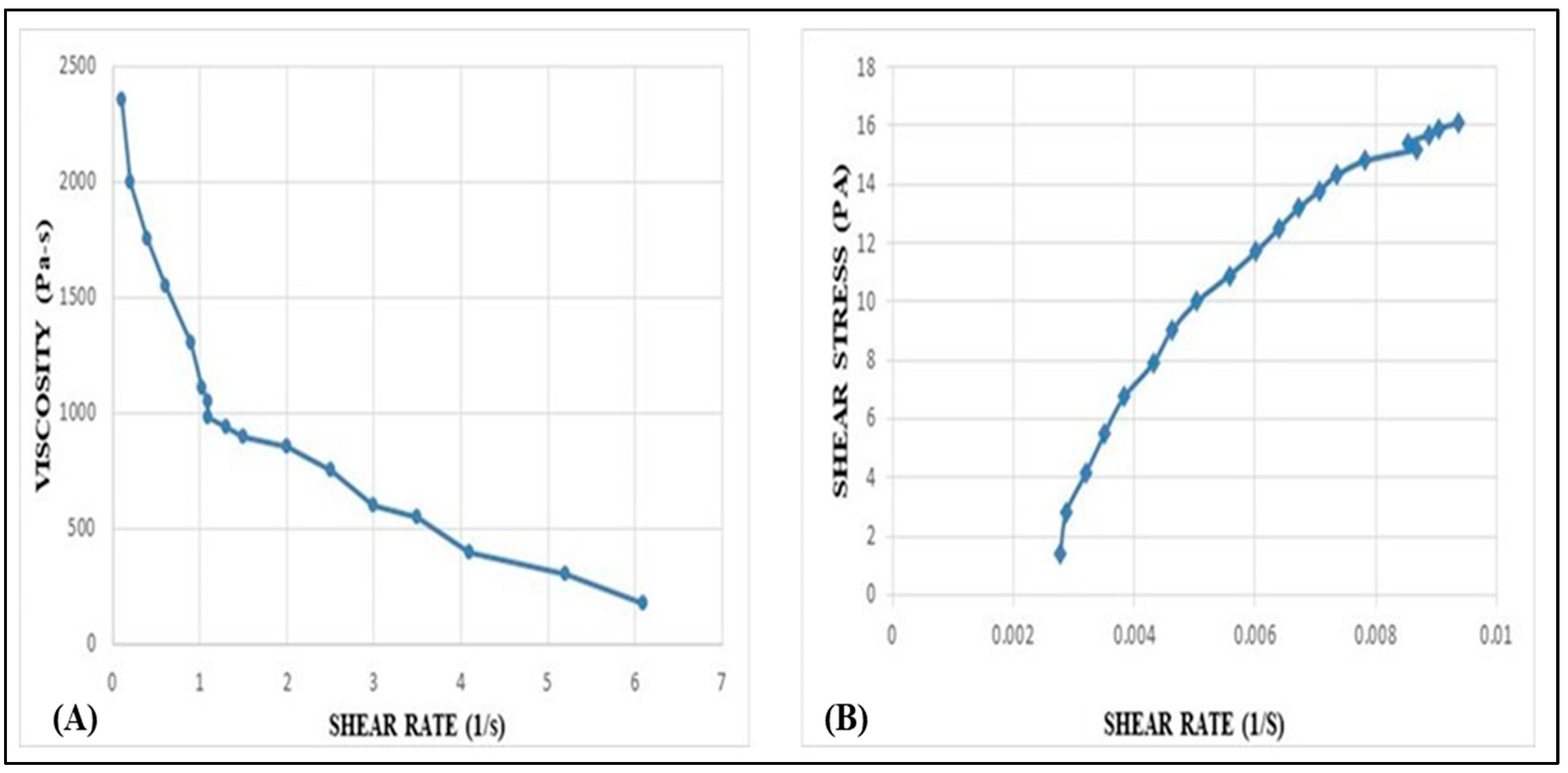
2.8. Rheological Behavior Studies
2.9. Spreadability and Extrudability
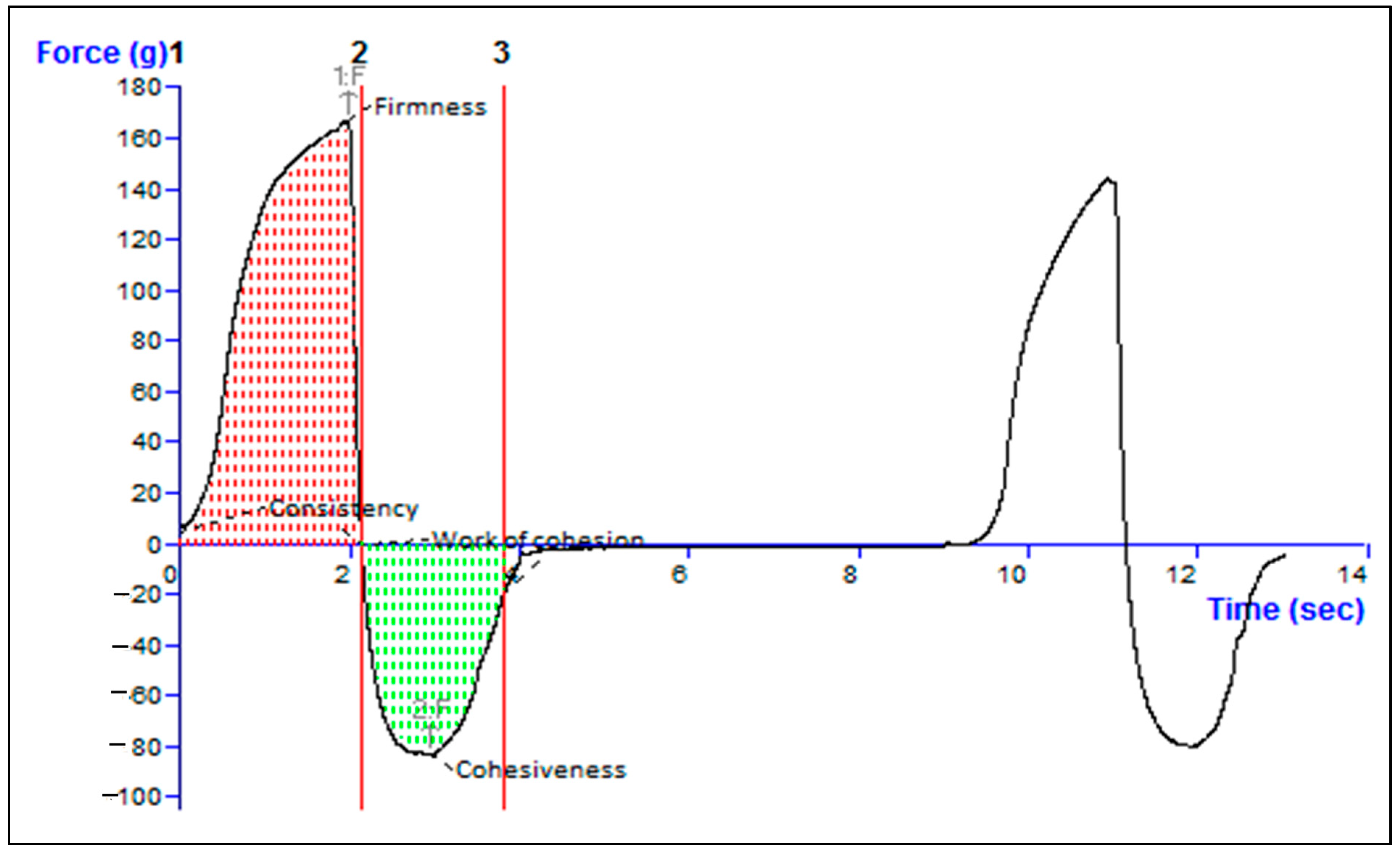
2.10. Texture Analysis of Formulation
2.11. Homogeneity and pH
2.12. In Vitro Drug Release Study
2.13. Ex Vivo Drug Release Study
2.14. Confocal Laser Scanning Microscopy (CLSM)
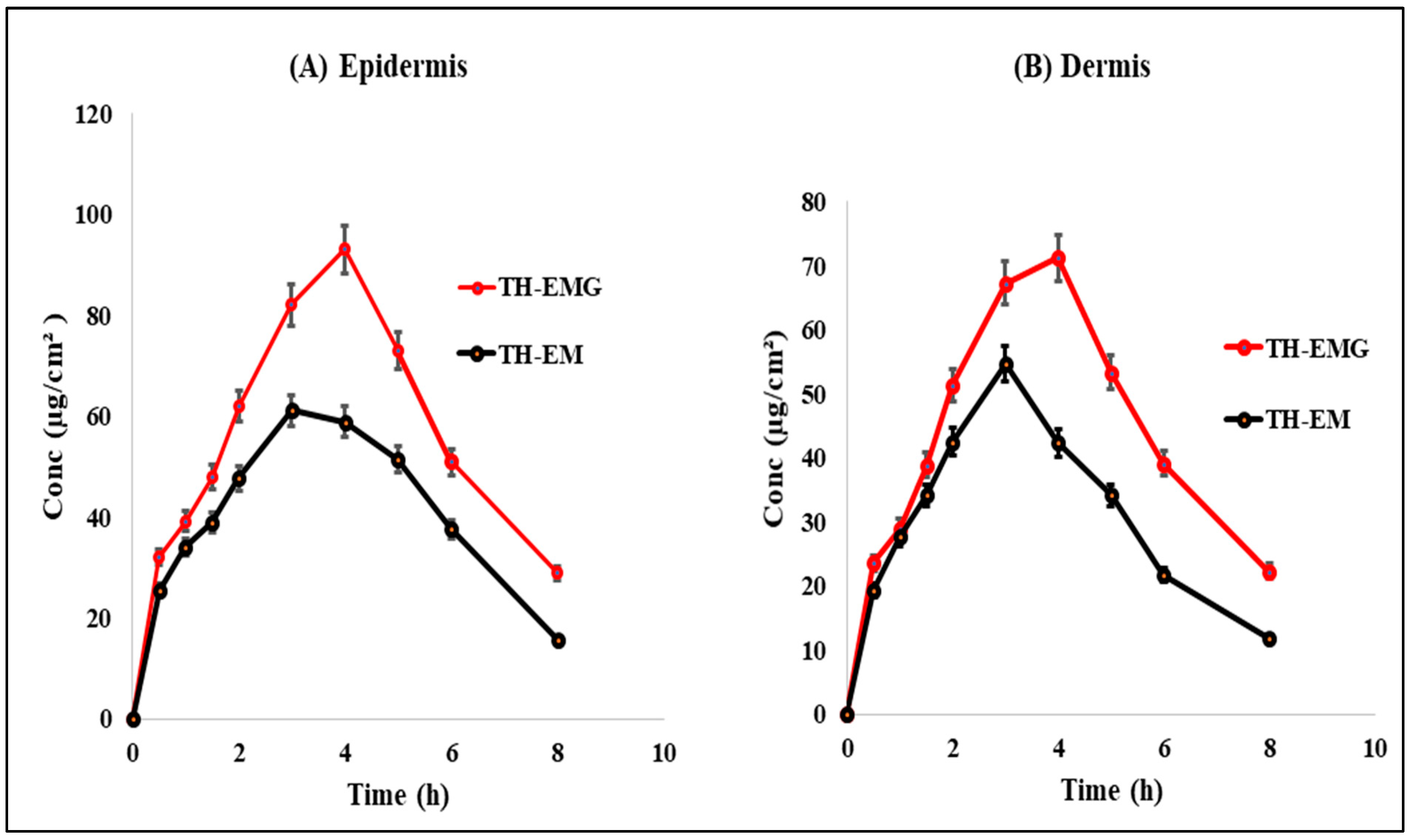
2.15. Dermatokinetic Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methodology
4.2.1. Selection of Excipients
4.2.2. Pseudo-Phase Ternary Diagrams
4.2.3. Formulation of Terbinafine HCl Nanoemulsion (TH-NE)
4.2.4. Thermodynamic Stability Studies
4.2.5. Optimization of Terbinafine HCL-Nanoemulsion (TH-NE) Using Design of Expert (DoE)
4.2.6. Particle Size, Polydispersity Index (PDI), and Zeta Potential
4.2.7. Differential Scanning Calorimetry (DSC) of the Lyophilized Formulation
4.2.8. Fluorescent Microscopy
4.2.9. Preparation of Drug-Loaded Nanoemulgel Using Optimized Terbinafine HCl Nanoemulsion (TH-NEG)
4.2.10. Rheological Behavior
4.2.11. Spreadability
4.2.12. Extrudability
4.2.13. Texture Analyzer
4.2.14. Homogeneity and pH
4.2.15. In Vitro Drug Release Study
4.2.16. Ex Vivo Release Study
4.2.17. Confocal Laser Scanning Microscopy
4.2.18. Dermatokinetic Study
4.2.19. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misra, S.K.; Pandey, H.; Patil, S.; Virmani, T.; Virmani, R.; Kumar, G.; Alhalmi, A.; Noman, O.M.; Alshahrani, S.S.; Mothana, R.A. Graphene Scaffolds: A Striking Approach to Combat Dermatophytosis. Nanomaterials 2023, 13, 2305. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.M.; Badawi, N.M.; Tartor, Y.H.; Sobhy, N.M.; Swidan, S.A. Development, Optimization, and In Vitro/In Vivo Evaluation of Azelaic Acid Transethosomal Gel for Antidermatophyte Activity. Antibiotics 2023, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Masih, A.; Chowdhary, A.; Sardana, K.; Borker, S.; Gupta, A.; Gautam, R.K.; Sharma, P.K.; Jain, D. Correlation of in Vitro Susceptibility Based on MICs and Squalene Epoxidase Mutations with Clinical Response to Terbinafine in Patients with TINEa Corporis/Cruris. Antimicrob. Agents Chemother. 2018, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.; Ganavalli, A.; Kikkeri, N.N. Antifungal Resistance in Dermatology. Indian J. Dermatol. 2018, 63, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Haghani, I.; Akhtari, J.; Yahyazadeh, Z.; Espahbodi, A.; Kermani, F.; Javidnia, J.; Hedayati, M.T.; Shokohi, T.; Badali, H.; Rezaei-Matehkolaei, A.; et al. Potential Inhibitory Effect of Miltefosine against Terbinafine-Resistant Trichophyton Indotineae. Pathogens 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Tanrıverdi, S.T.; Özer, Ö. Novel Topical Formulations of Terbinafine-HCl for Treatment of Onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Hemrajani, C.; Negi, P.; Parashar, A.; Gupta, G.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; Dua, K. Overcoming Drug Delivery Barriers and Challenges in Topical Therapy of Atopic Dermatitis: A Nanotechnological Perspective. Biomed. Pharmacother. 2022, 147, 112633. [Google Scholar] [CrossRef]
- Khan, R.; Mirza, M.A.; Aqil, M.; Alex, T.S.; Raj, N.; Manzoor, N.; Naseef, P.P.; Saheer Kuruniyan, M.; Iqbal, Z. In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis. Gels 2023, 9, 112. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Resveratrol Anchored Nanostructured Lipid Carrier Loaded in Situ Gel via Nasal Route: Formulation, Optimization and in Vivo Characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Hajare, A.; Dol, H.; Patil, K. Design and Development of Terbinafine Hydrochloride Ethosomal Gel for Enhancement of Transdermal Delivery: In Vitro, in Vivo, Molecular Docking, and Stability Study. J. Drug Deliv. Sci. Technol. 2021, 61, 102280. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in Vitro Evaluation of Topical Nanosponge-Based Gel Containing Butenafine for the Treatment of Fungal Skin Infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Ghose, A.; Nabi, B.; Rehman, S.; Md, S.; Alhakamy, N.A.; Ahmad, O.A.A.; Baboota, S.; Ali, J. Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers 2020, 12, 2903. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Amin, S.; Beg, S.; Al-Salahi, R.; Mir, S.R.; Kohli, K. Formulation and Optimization of Naringin Loaded Nanostructured Lipid Carriers Using Box-Behnken Based Design: In Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 74, 103590. [Google Scholar] [CrossRef]
- Al Hossain, A.S.M.M.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Nikumbh, K.V.; Sevankar, S.G.; Patil, M.P. Formulation Development, in Vitro and in Vivo Evaluation of Microemulsion-Based Gel Loaded with Ketoprofen. Drug Deliv. 2015, 22, 509–515. [Google Scholar] [CrossRef]
- Khan, R.; Mirza, M.A.; Aqil, M.; Hassan, N.; Zakir, F.; Ansari, M.J.; Iqbal, Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels 2022, 8, 733. [Google Scholar] [CrossRef]
- Yeo, E.; Yew Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-Rich Naringenin Nanoemulgel for the Management of Diabetic Wound: Fabrication, Characterization and Comparative in Vitro Evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Pathak, K.; Kamaly, O.A.; Saleh, A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2022, 15, 1343. [Google Scholar] [CrossRef]
- Park, H.H.; Srisombat, L.; Jamison, A.C.; Liu, T.; Marquez, M.D.; Park, H.; Lee, S.; Lee, T.-C.; Lee, T.R. Temperature-Responsive Hydrogel-Coated Gold Nanoshells. Gels 2018, 4, 28. [Google Scholar] [CrossRef]
- Mahmood, S.; Chatterjee, B.; Mandal, U.K. Pharmacokinetic Evaluation of the Synergistic Effect of Raloxifene Loaded Transfersomes for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102545. [Google Scholar] [CrossRef]
- Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D.A. The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef]
- Burki, I.K.; Khan, M.K.; Khan, B.A.; Uzair, B.; Braga, V.A.; Jamil, Q.A. Formulation Development, Characterization, and Evaluation of a Novel Dexibuprofen-Capsaicin Skin Emulgel with Improved In Vivo Anti-Inflammatory and Analgesic Effects. AAPS PharmSciTech 2020, 21, 211. [Google Scholar] [CrossRef]
- Mundada, V.P.; Patel, M.H.; Mundada, P.K.; Sawant, K.K. Enhanced Bioavailability and Antihypertensive Activity of Nisoldipine Loaded Nanoemulsion: Optimization, Cytotoxicity and Uptake across Caco-2 Cell Line, Pharmacokinetic and Pharmacodynamic Studies. Drug Dev. Ind. Pharm. 2020, 46, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured Lipid Carriers for Oral Bioavailability Enhancement of Exemestane: Formulation Design, In Vitro, Ex Vivo, and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef]
- Alam, P.; Imran, M.; Gupta, D.K.; Akhtar, A. Formulation of Transliposomal Nanocarrier Gel Containing Strychnine for the Effective Management of Skin Cancer. Gels 2023, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and Optimization of Sulforaphane- Loaded Nanostructured Lipid Carriers by the Box- Behnken Design for Improved Oral Efficacy against Cancer: In Vitro, Ex Vivo and in Vivo Assessments. Artif. Cells, Nanomedicine, Biotechnol. 2018, 46, S15–S31. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Husain, M.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]






| S.NO. | Factor 1 A: Surfactant Concentration (mL) | Factor 2 B: Oil Concentration (µL) | Factor 3 C: Stirring Speed (rpm) | Response 1 Particle Size (nm) | Response 2 PDI | Response 3 Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 1.5 | 0.16 | 150 | 28.09 | 0.1911 | −40.9 |
| 2 | 1 | 0.12 | 150 | 28.21 | 0.182 | −40.3 |
| 3 | 1 | 0.16 | 100 | 28.6 | 0.191 | −40.2 |
| 4 | 1 | 0.12 | 150 | 28.12 | 0.195 | −40.5 |
| 5 | 1 | 0.12 | 150 | 28.07 | 0.1922 | −41.87 |
| 6 | 0.5 | 0.12 | 100 | 31.2 | 0.298 | −38.9 |
| 7 | 1 | 0.08 | 200 | 27.9 | 0.189 | −37.02 |
| 8 | 1.5 | 0.12 | 200 | 27.25 | 0.184 | −37.07 |
| 9 | 1 | 0.16 | 200 | 31.01 | 0.295 | −39.9 |
| 10 | 1.5 | 0.08 | 150 | 28.9 | 0.211 | −39.7 |
| 11 | 0.5 | 0.16 | 150 | 32.04 | 0.302 | −38.7 |
| 12 | 1.5 | 0.12 | 100 | 28.97 | 0.219 | −39.5 |
| 13 | 1 | 0.08 | 100 | 31 | 0.28 | −38.65 |
| 14 | 0.5 | 0.08 | 150 | 29 | 0.215 | −39.6 |
| 15 | 0.5 | 0.12 | 200 | 29 | 0.227 | −39.5 |
| Responses | R2 | Adjusted R2 | Predicted R2 | S.D. | C.V.% | Adequate Precision |
|---|---|---|---|---|---|---|
| R1 = Particle Size | 0.9452 | 0.8467 | 0.1286 | 0.5663 | 1.94 | 9.8076 |
| R2 = PDI | 0.9237 | 0.7864 | −0.1757 | 0.0209 | 9.30 | 7.5459 |
| R3 = Zeta Potential | 0.7583 | 0.3233 | −2.0193 | 1.07 | 2.71 | 3.8354 |
| Firmness [g] Force 1 | Consistency [g/s] Area F-T 1:2p | Cohesiveness [g] Force 2 | Work of Cohesion [g/s] Area F-T 2:3 |
|---|---|---|---|
| 168.00 | 229.81 | −83.36 | −107.02 |
| Variables | Constraints | ||
|---|---|---|---|
| Lower Limit | Upper Limit | Middle Limit | |
| Independent variables | |||
| Stirring speed (rpm) | 100 | 200 | 150 |
| Surfactant concentration (mL) | 0.5 | 1.5 | 1 |
| Oil concentration (µL) | 0.08 | 0.16 | 0.12 |
| Dependent variables | |||
| Particle size (nm) | Minimum | ||
| PDI | Minimum | ||
| Zeta potential (mV) | −40 to +40 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phagna, M.; Badhwar, R.; Singh, M.; Alhalmi, A.; Khan, R.; Noman, O.M.; Alahdab, A. Development and Characterization of Terbinafine-Loaded Nanoemulgel for Effective Management of Dermatophytosis. Gels 2023, 9, 894. https://doi.org/10.3390/gels9110894
Phagna M, Badhwar R, Singh M, Alhalmi A, Khan R, Noman OM, Alahdab A. Development and Characterization of Terbinafine-Loaded Nanoemulgel for Effective Management of Dermatophytosis. Gels. 2023; 9(11):894. https://doi.org/10.3390/gels9110894
Chicago/Turabian StylePhagna, Mayank, Reena Badhwar, Manvi Singh, Abdulsalam Alhalmi, Rahmuddin Khan, Omar M. Noman, and Ahmad Alahdab. 2023. "Development and Characterization of Terbinafine-Loaded Nanoemulgel for Effective Management of Dermatophytosis" Gels 9, no. 11: 894. https://doi.org/10.3390/gels9110894
APA StylePhagna, M., Badhwar, R., Singh, M., Alhalmi, A., Khan, R., Noman, O. M., & Alahdab, A. (2023). Development and Characterization of Terbinafine-Loaded Nanoemulgel for Effective Management of Dermatophytosis. Gels, 9(11), 894. https://doi.org/10.3390/gels9110894







