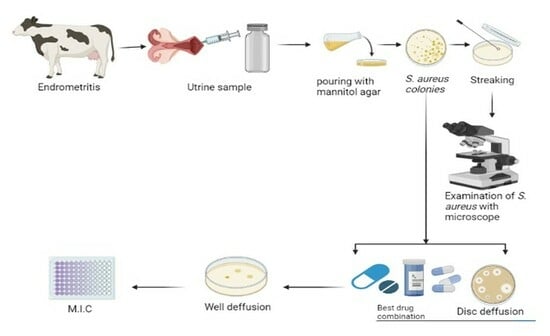
Recent Trends in S. aureus and E. coli-Based Endometritis, and the Therapeutic Evaluation of Sodium Alginate-Based Antibiotics and Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Nanoparticles
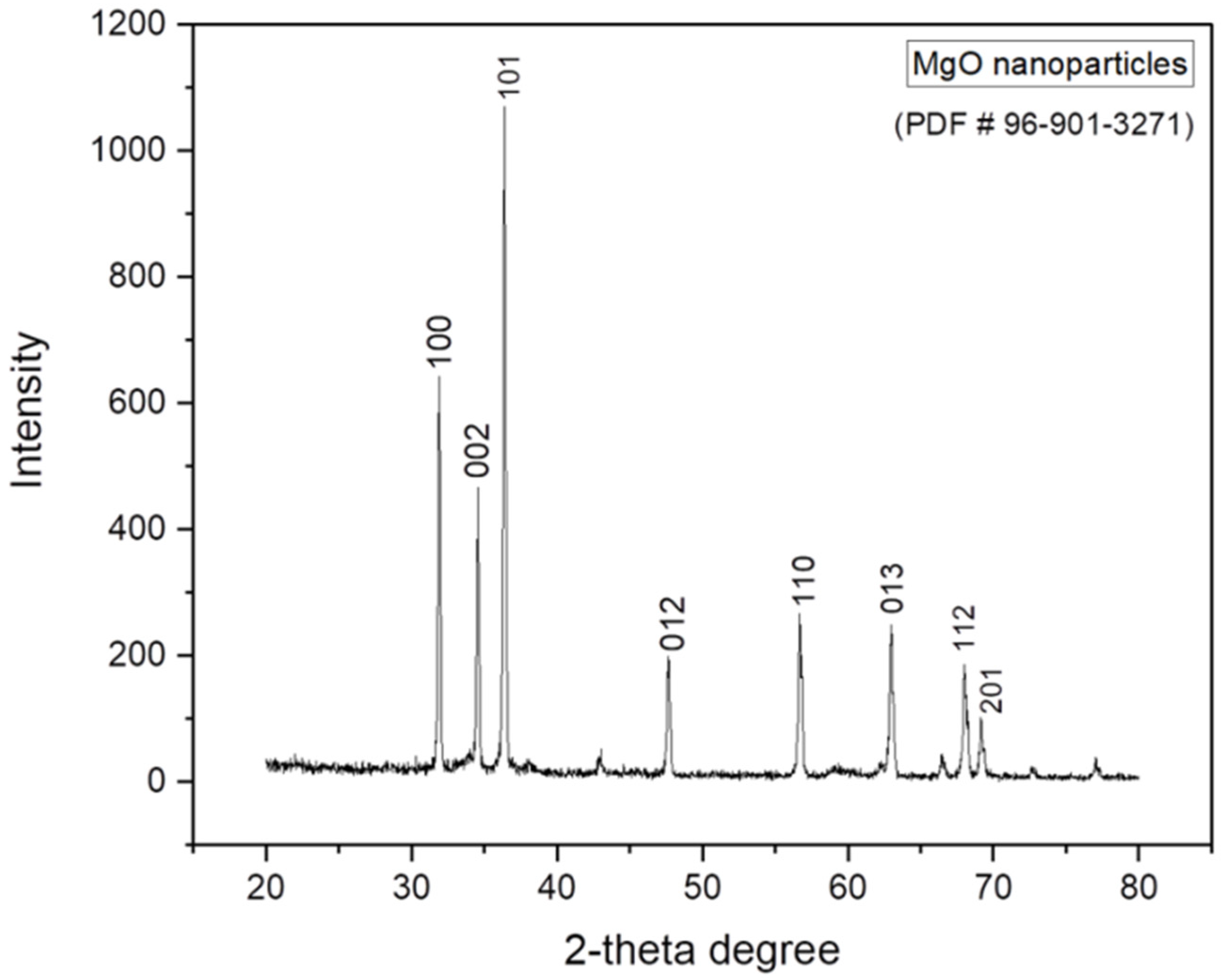
2.1.1. XRD Analysis of MgO Nanoparticles
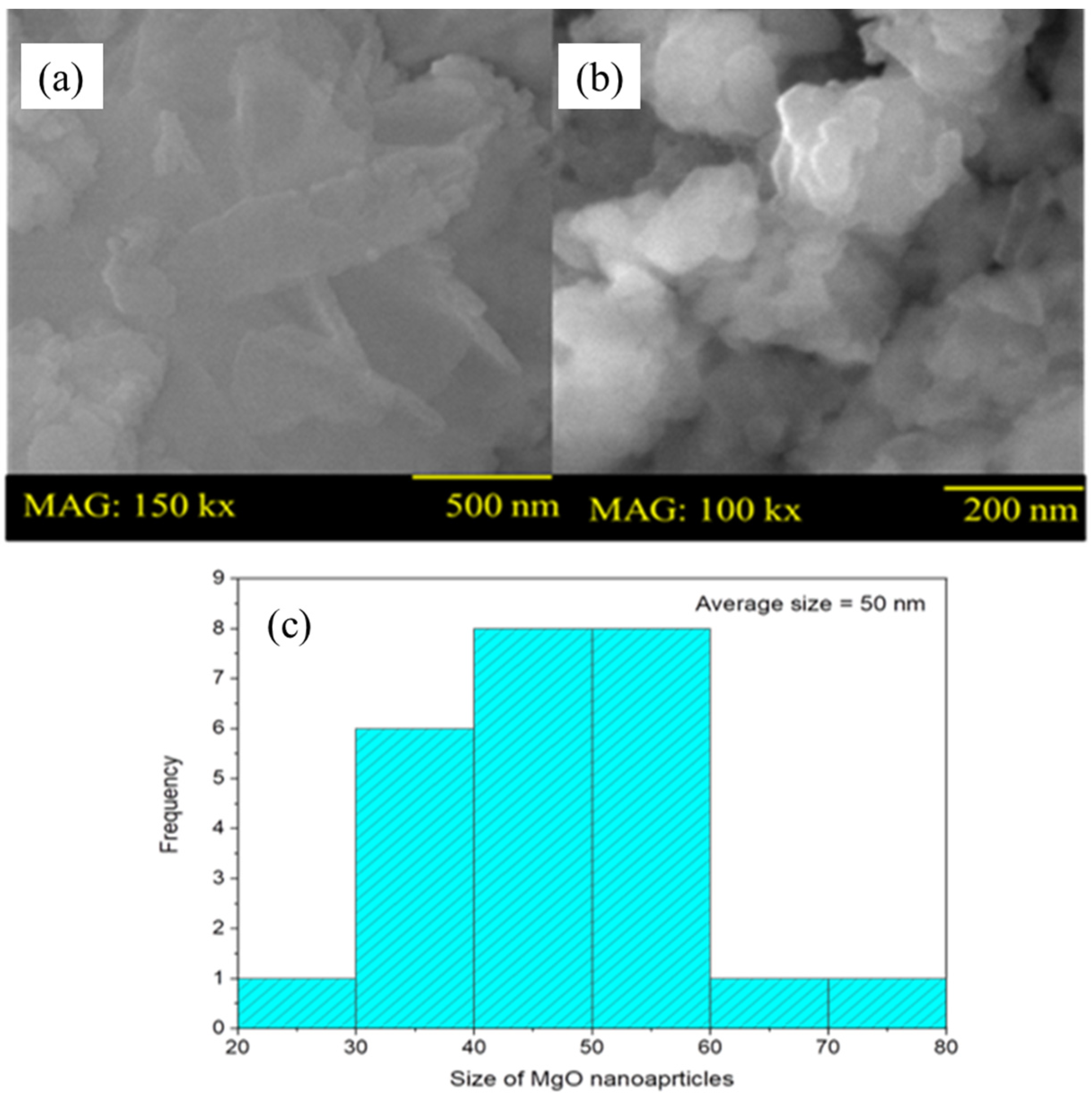
2.1.2. SEM Analysis
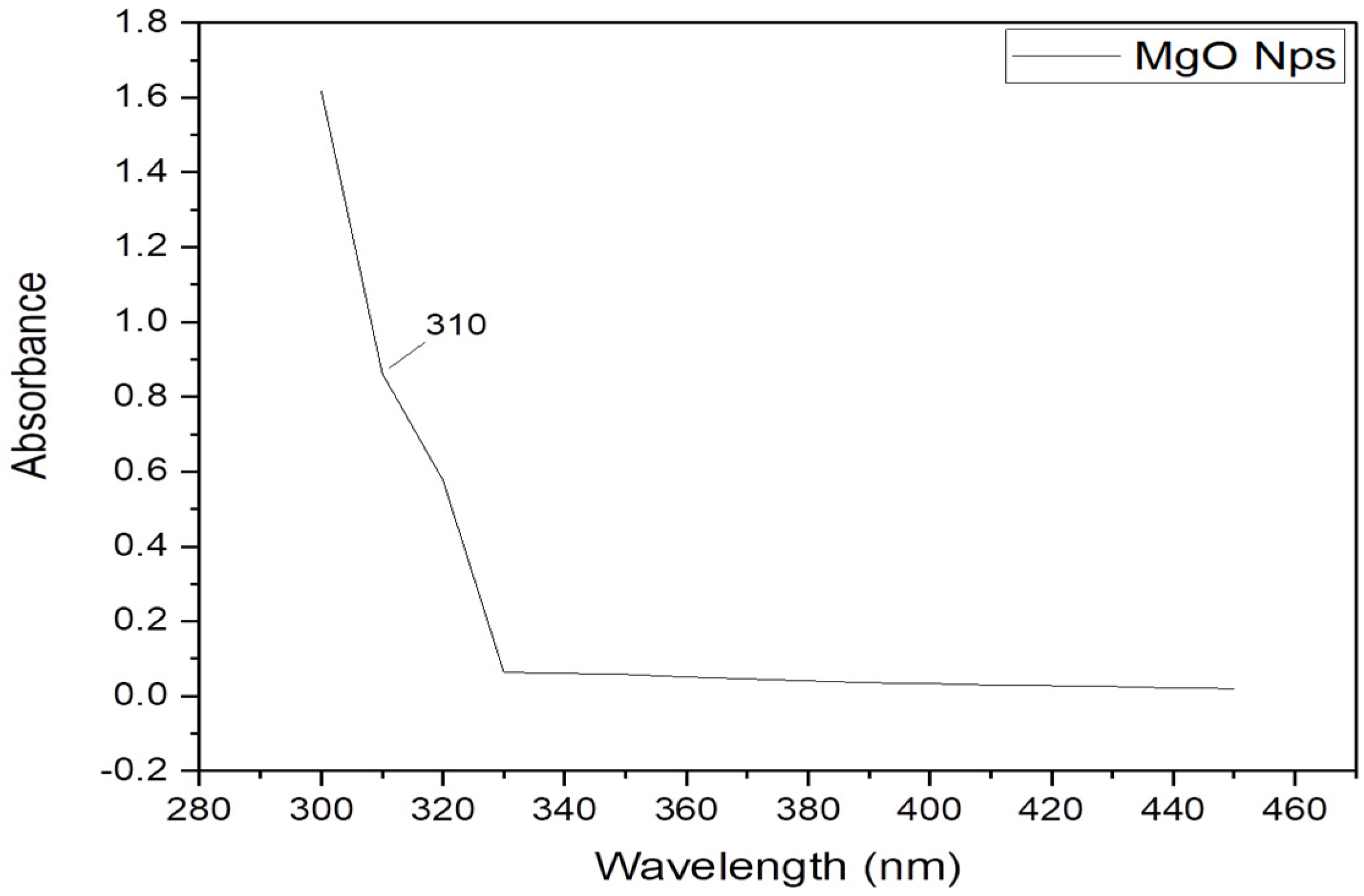
2.1.3. UV–Visible
2.1.4. Raman Analysis
2.2. Prevalence and Risk Factors of Endometritis S. aureus and E. coli
2.3. Antibiotic Susceptibility of S. aureus and E. coli against Antibiotics
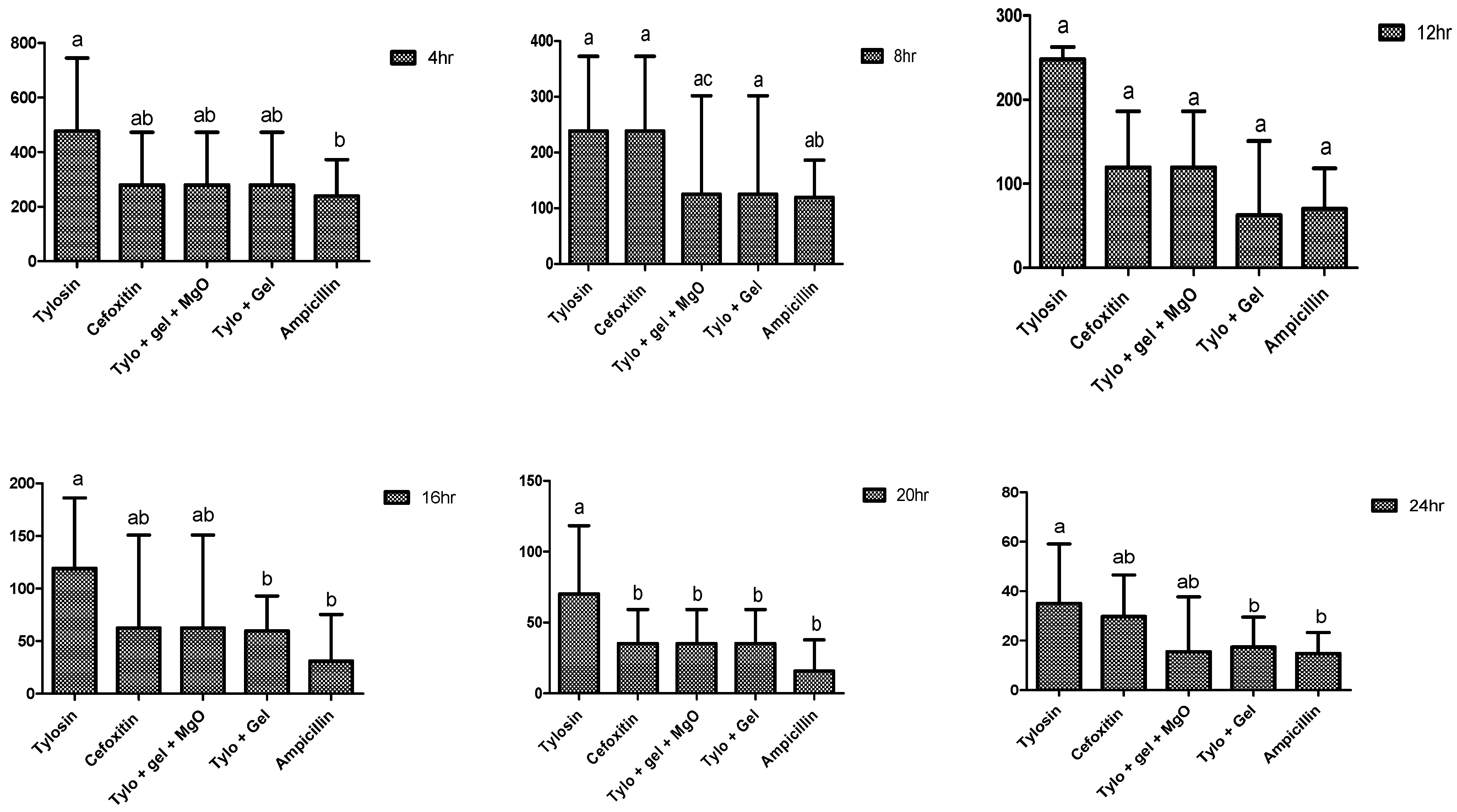
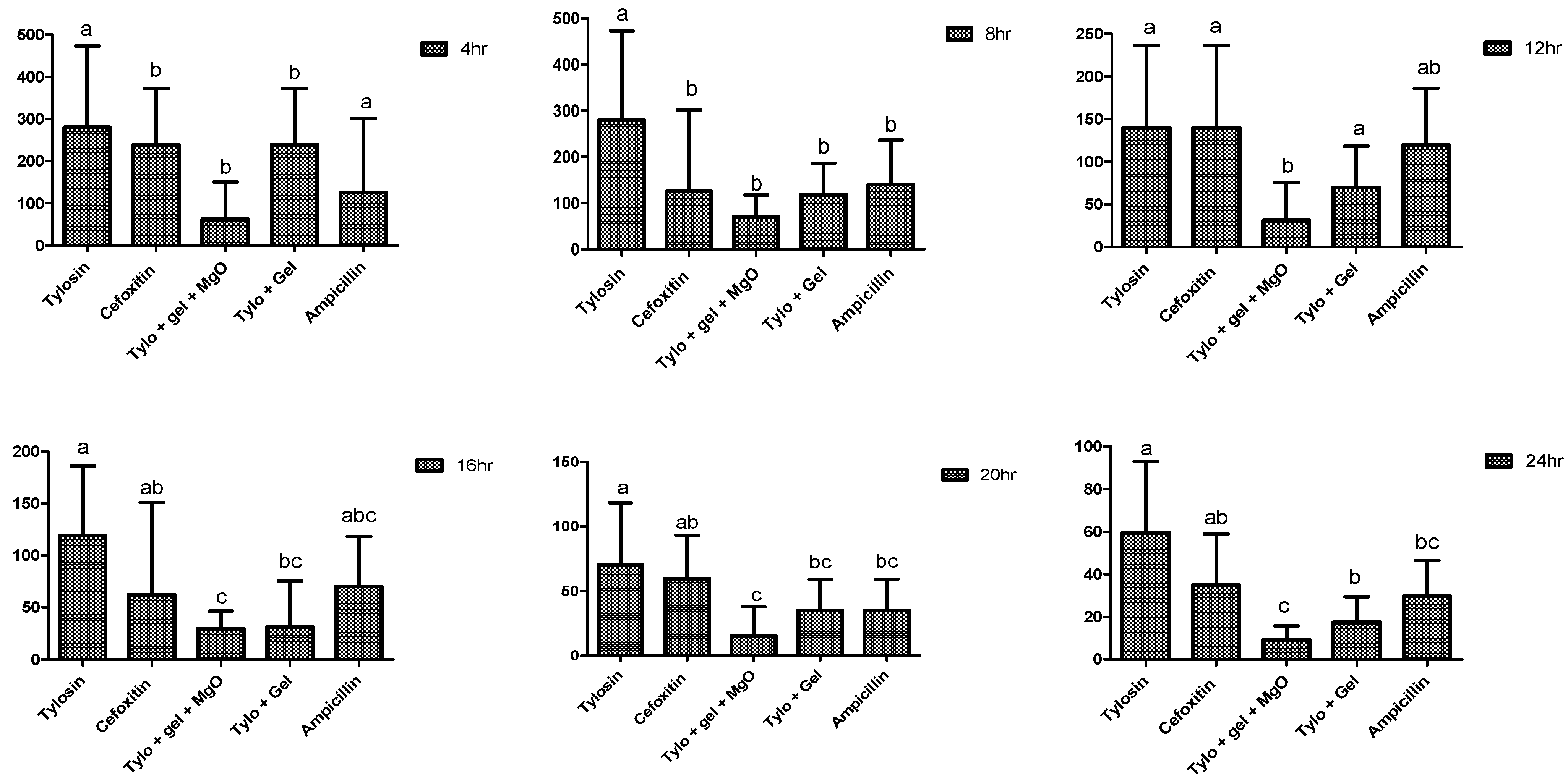
2.4. Antibacterial Potential of Gel-Based Nanoparticles and Antibiotics
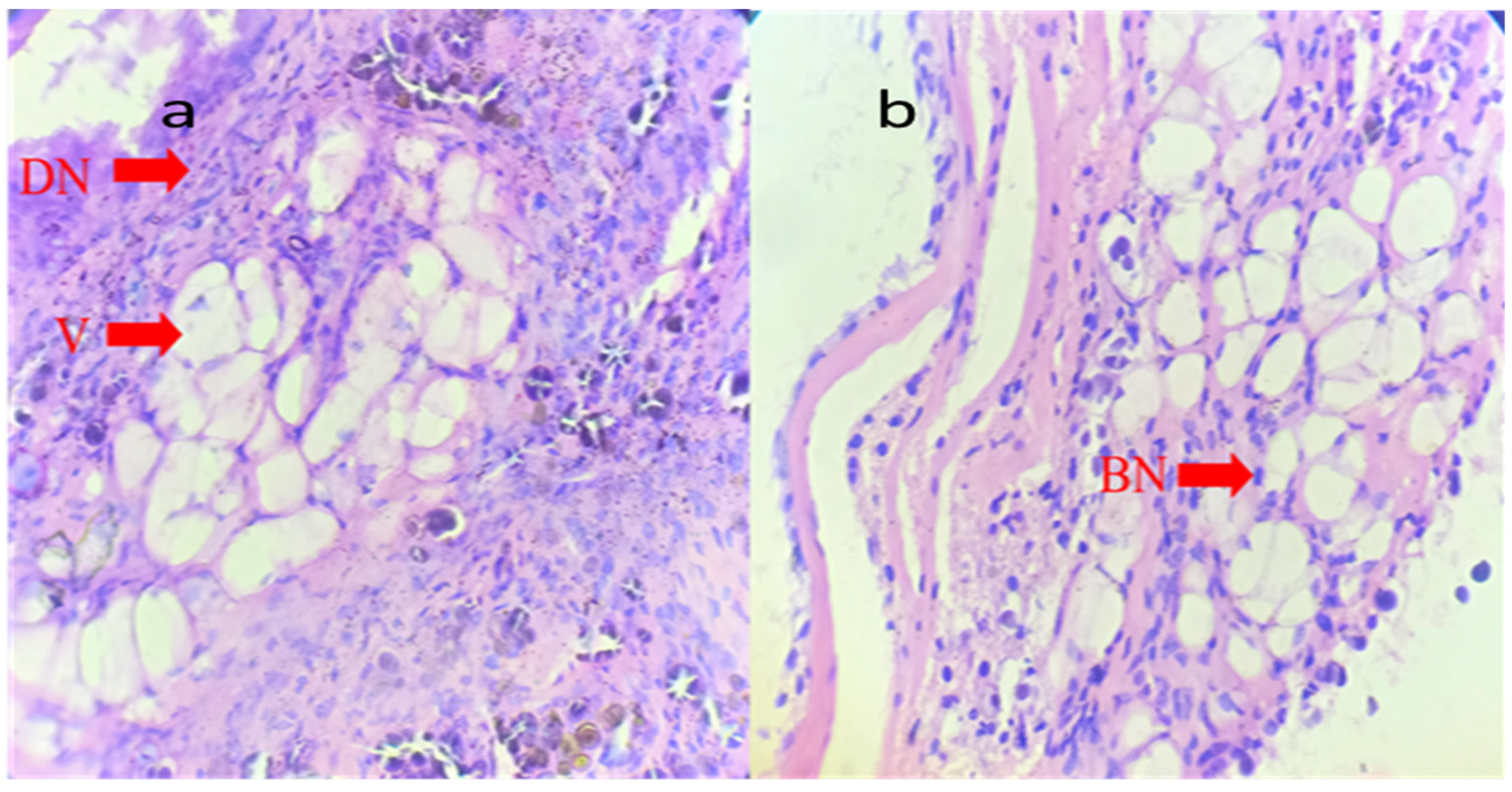
2.5. Histopathology
2.6. Toxicity Evaluation
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Nanoparticles
4.2. Characterization of Nanoparticles
4.3. SEM Analysis
4.4. Toxicity Analysis
4.5. Histopathology
4.6. Uterine Sample Collection
4.6.1. Tracking Bovine Endometritis
4.6.2. Isolation of S. aureus and E. coli
4.6.3. Antibiotic Susceptibility
4.7. Evaluation of Gel Based and Nongel Based Preprations
4.7.1. Well Diffusion Assay
4.7.2. Minimum Inhibitory Concentration
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, G.; Nakao, T.; Yusuf, M.; Koike, K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim. Reprod. Sci. 2009, 116, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Samardžija, M.; Bačić, G. Oxidative stress and reproductive disorders in dairy cows. In Dairy Cows: Nutrition, Fertility and Milk Production; Marek, E.R., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 57–98. [Google Scholar]
- Pérez-Báez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic cost of metritis in dairy herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Wu, Y.; Meng, Q.; Qiao, J.; Zhang, X.; Ma, S.; Cai, K.; Zhang, J.; Cheng, Z.; Zhang, Z.; et al. Antimicrobial resistance, virulence gene profile and molecular typing of Staphylococcus aureus isolates from dairy cows in Xinjiang Province, northwest China. J. Glob. Antimicrob. Resist. 2019, 16, 98–104. [Google Scholar]
- Gautam, G.; Nakao, T.; Koike, K.; Long, S.T.; Yusuf, M.; Ranasinghe, R.M.S.B.K.; Hayashi, A. Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows. Theriogenology 2010, 73, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Vercouteren, M.M.A.A.; Bittar, J.H.J.; Pinedo, P.J.; Risco, C.A.; Santos, J.E.P.; Vieira-Neto, A.; Galvão, K.N. Factors associated with early cyclicity in postpartum dairy cows. J. Dairy Sci. 2015, 98, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, F.G.; Hartanto, A.; Bintang, M.; Jamilah, I.; Priyani, N.; Putra, E.D. GC-MS analysis and antimicrobial activity of the aqueous extract from the bulbs of Allium chinense G. Don. cultivated in North Sumatra, Indonesia. Asian J. Agric. Biol. 2021, 2, 201912562. [Google Scholar] [CrossRef]
- Ahmed, A.; Ijaz, M.; Khan, J.A.; Anjum, A.A. Molecular characterization and therapeutic insights into biofilm positive Staphylococcus aureus isolated from bovine subclinical mastitis. Pak. Vet. J. 2022, 42, 584–590. [Google Scholar] [CrossRef]
- Mansoor, A.; Mehmood, M.; Hassan, S.M.U.; Ali, M.I.; Badshah, M.; Jamal, A. Anti-bacterial effect of titanium-oxide nanoparticles and their application as alternative to antibiotics. Pak. Vet. J. 2023, 43, 269–275. [Google Scholar] [CrossRef]
- Clarke, T.H.; Gomez, A.; Singh, H.; Nelson, K.E.; Brinkac, L.M. Integrating the microbiome as a resource in the forensics toolkit. Forensic Sci. Int. Genet. 2017, 30, 141–147. [Google Scholar] [CrossRef]
- Feng, Y.; Hodiamont, C.J.; Van Hest, R.M.; Brul, S.; Schultsz, C.; Kuile, B.H.T. Development of antibiotic resistance during simulated treatment of Pseudomonas aeruginosa in chemostats. PLoS ONE 2016, 11, e0149310. [Google Scholar] [CrossRef]
- Lippolis, J.D.; Holman, D.B.; Brunelle, B.W.; Thacker, T.C.; Bearson, B.L.; Reinhardt, T.A.; Sacco, R.E.; Casey, T.A. Genomic and transcriptomic analysis of Escherichia coli strains associated with persistent and transient bovine mastitis and the role of colanic acid. Infect. Immun. 2018, 86, e00566-17. [Google Scholar] [CrossRef] [PubMed]
- Mackeen, A.D.; Packard, R.E.; Ota, E.; Speer, L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst. Rev. 2015, 2015, CD001067. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, M.T.; Rahman, S.M.E.; Khan, M.R.I. Impacts of COVID-19 pandemic on livestock industry and food security: A review. Asian J. Agric. Biol. 2022, 3, 202104142. [Google Scholar] [CrossRef]
- Muneer, A.; Kumar, S.; Aqib, A.I.; Khan, S.R.; Shah, S.Q.A.; Zaheer, I.; ur Rehman, T.; Abbas, A.; Hussain, K.; Rehman, A.; et al. Evaluation of Sodium Alginate Stabilized Nanoparticles and Antibiotics against Drug Resistant Escherichia coli Isolated from Gut of Houbara Bustard Bird. Oxidative Med. Cell. Longev. 2022, 2022, 7627759. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Naz, S.; Hussain, R.; Chatha, A.M.M.; Ahmad, F.; Yamin, A.; Akram, R.; Naz, H.; Shaheen, A. Exposure to heavy metals causes histopathological changes and alters antioxidant enzymes in fresh water fish (Oreochromis niloticus). Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.; Rao, K.V.; Chakra, C.S. Structural properties of MgO nanoparticles: Synthesized by co-precipitation technique. Int. J. Sci. Res. 2014, 3, 43–46. [Google Scholar]
- Mendoza-Martinez, N.L.; Cadena-Galeana, A.D.; Villanueva-Sanchez, F.G.; Perez-Cornejo, N.; Avelar-Juarez, K.M.; Ramos-Baena, J.D.; Cruz-Monroy, E.A.; Vazquez-Zuniga, U.; Garcia-Contreras, R. Efficacy of Antineoplastic Nanocarriers on 3D Oral Cancer Spheroids. In Vivo 2023, 37, 1658–1665. [Google Scholar] [CrossRef]
- Almontasser, A.; Parveen, A.; Azam, A. Synthesis, Characterization and antibacterial activity of Magnesium Oxide (MgO) nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577, 012051. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, F.; Zhang, J.; Xia, L. Converting layered zinc acetate nanobelts to one-dimensional structured ZnO nanoparticle aggregates and their photocatalytic activity. Nanoscale Res. Lett. 2008, 3, 201–204. [Google Scholar] [CrossRef]
- Morozov, I.G.; Sathasivam, S.; Belousova, O.; Parkin, I.; Kuznetcov, M. Effect of synthesis conditions on room-temperature ferromagnetic properties of Mg-O nanoparticles. J. Alloys Compd. 2018, 765, 343–354. [Google Scholar] [CrossRef]
- Prathap, M.A.; Kaur, B.; Srivastava, R. Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J. Colloid Interface Sci. 2012, 370, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Fujima, N.; Komura, H. First-order Raman scattering in MgO microcrystals. J. Appl. Phys. 1985, 57, 973–975. [Google Scholar] [CrossRef]
- Baran, W.; Zdun, S.; Janowski, T. The diagnosis and prevalence of subclinical endometritis in cows evaluated by different cytologic thresholds. Theriogenology 2012, 78, 1939–1947. [Google Scholar]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Denis-Robichaud, J.; Dubuc, J. Determination of optimal diagnostic criteria for purulent vaginal discharge and cytological endometritis in dairy cows. J. Dairy Sci. 2015, 98, 6848–6855. [Google Scholar] [CrossRef]
- Nyabinwa, P.; Kashongwe, O.B.; Habimana, J.P.; Hirwa, A.; Bebe, B.O. Estimating prevalence of endometritis in smallholder zero-grazed dairy cows in Rwanda. Trop. Anim. Health Prod. 2020, 52, 3135–3145. [Google Scholar] [CrossRef]
- Kumar, D.B.C.; Chacko, L.; Promod, K.; Sumod, K. Detection of Escherichia coli in Postpartum Clinical Endometritis of Dairy Cattle by PCR. Asian J. Dairy Food Res. 2021, 40, 193–196. [Google Scholar] [CrossRef]
- Udhayavel, S.; Malmarugan, S.; Palanisamy, K.; Rajeswar, J. Antibiogram pattern of bacteria causing endometritis in cows. Vet. World 2013, 6, 100. [Google Scholar] [CrossRef]
- Shafique, L.; Wu, S.; Aqib, A.I.; Ali, M.M.; Ijaz, M.; Naseer, M.A.; Sarwar, Z.; Ahmed, R.; Saleem, A.; Qudratullah Ahmad, A.S.; et al. Evidence-based tracking of MDR E. coli from bovine endometritis and its elimination by effective novel therapeutics. Antibiotics 2021, 10, 997. [Google Scholar] [CrossRef]
- Koba, I.S.; Lysenko, A.A.; Koshchaev, A.G.; Rodin, I.A.; Shantyz, A.U. Effective treatment of chronic endometritis in cows by Florinazol preparation. Indian Vet. J. 2017, 94, 15–18. [Google Scholar]
- Nalini Mohanty, N.; Das, P.; Subhadarsini Pany, S.; Narayan Sarangi, L.; Ranabijuli, S.; Kumar Panda, H. Isolation and antibiogram of Staphylococcus, Streptococcus and Escherichia coli isolates clinical and subclinical cases of bovine mastitis. Vet. World 2013, 6, 739–743. [Google Scholar] [CrossRef]
- Haftu, R.; Taddele, H.; Gugsa, G.; Kalayou, S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, biology and medicine. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with antibiotic resistance: Combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti-Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of single-iron-atom nanocatalysts for highly efficient catalytic antibiotics. Small 2019, 15, 1901834. [Google Scholar] [CrossRef] [PubMed]
- Manan, A.; Aqib, A.I.; Shahbaz, A.; Khan, S.R.; Akram, K.; Majeed, H.; Muneer, A.; Murtaza, M.; Afrasiab, M.; Merola, C.; et al. Modification of the drug resistance of emerging milk-borne pathogens through sodium alginate-based antibiotics and nanoparticles. Front. Vet. Sci. 2023, 10, 1130130. [Google Scholar] [CrossRef]
- Aqib, A.I.; Akram, K.; Majeed, H.; Murtaza, M.; Muneer, A.; Alsayeqh, A.F. Resistance Modulation of Dairy Milk Borne Streptococcus agalactiae and Klebsiella pneumoniae through Metallic Oxide Nanoparticles. Pak. Vet. J. 2022, 42, 424–428. [Google Scholar]
- Otludil, B.; Ayaz, S. Effect of copper sulphate (CuSO 4) on freshwater snail, Physa acuta Draparnaud, 1805: A histopathological evaluation. Bull. Environ. Contam. Toxicol. 2020, 104, 738–747. [Google Scholar] [CrossRef]
- Caixeta, M.B.; Araújo, P.S.; Gonçalves, B.B.; Silva, L.D.; Grano-Maldonado, M.I.; Rocha, T.L. Toxicity of engineered nanomaterials to aquatic and land snails: A scientometric and systematic review. Chemosphere 2020, 260, 127654. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.M.; Mohammed, A.N.; El-Ela, F.I.A. Acaricidal efficacy of deltamethrin-zinc oxide nanocomposite on Rhipicephalus (Boophilus) annulatus tick. Vet. Parasitol. 2019, 268, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Support for the safe use of zinc oxide nanoparticle sunscreens: Lack of skin penetration or cellular toxicity after repeated application in volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, P.P.; Tkachenko, E.A.; Kuznetsov, S.V.; Voronov, V.V.; Lavrishchev, S.V. Preparation of MgO nanoparticles. Inorg. Mater. 2007, 43, 502–504. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Hosseini, A.; Gheisari, H.R.; Yavari, M. Preliminary trial in treatment of postpartum endometritis with intrauterine application of hyperimmune serum in dairy cows. Asian Pac. J. Trop. Dis. 2014, 4, S360–S365. [Google Scholar] [CrossRef]
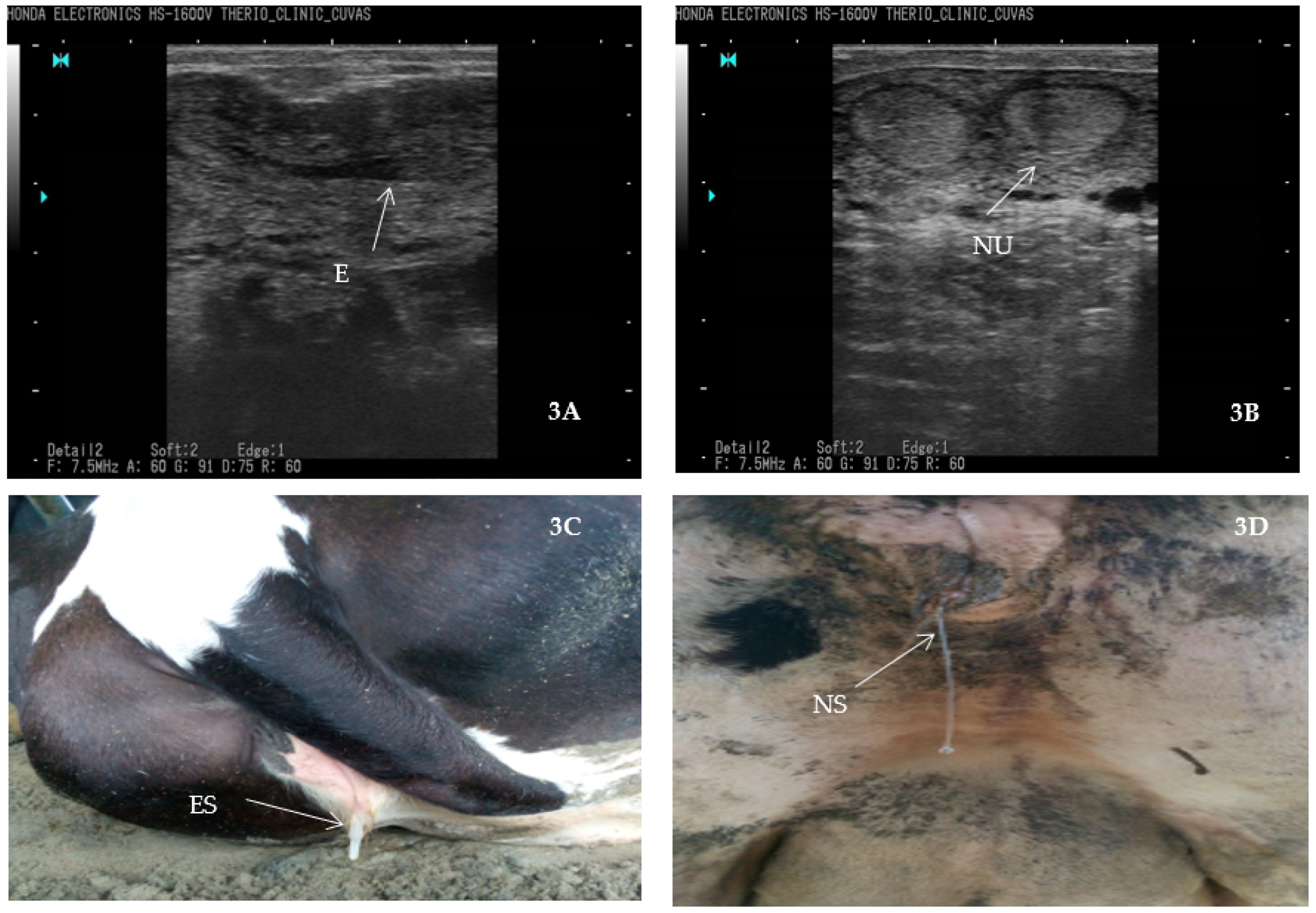
- Fissore, R.A.; Edmondson, A.J.; Pashen, R.L.; Bondurant, R.H. The use of ultrasonography for the study of the bovine reproductive tract. II. Non-pregnant, pregnant and pathological conditions of the uterus. Anim. Reprod. Sci. 1986, 12, 167–177. [Google Scholar] [CrossRef]
- Ihnatsenka, B.; Boezaart, A.P. Ultrasound: Basic understanding and learning the language. Int. J. Shoulder Surg. 2010, 4, 55. [Google Scholar]
- Bergey, D.H.; Holt, J.G. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- M100-S25; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement; Clinical and Laboratory Standard Institute: Wayne, NY, USA, 2015; Volume 35, pp. 1–240.

| Parameter | Categories | Chi-Square Analysis | Regression Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency | % Age | p-Value | ODD’s Ration | CI (95%) for Odds Ratio | p-Value | |||
| Lower | Upper | |||||||
| Calving history | Dystocia | 12 | 40 | <0.001 | 0.444 | 0.158 | 1.249 | 0.124 |
| Abortion | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Eutocia | 18 | 60 | - | - | - | - | ||
| Milk yield/day | 10–20 | 4 | 13.33 | <0.001 | 0.103 | 0.028 | 0.369 | 0.001 |
| 21–30 | 18 | 60 | 0.615 | 0.155 | 2.450 | 0.491 | ||
| 31–40 | 6 | 20 | 2.154 | 0.363 | 12.764 | 0.398 | ||
| 41–50 | 2 | 6.67 | - | - | - | - | ||
| Days in milk | 1–100 | 18 | 60 | <0.001 | 3.500 | 1.201 | 10.196 | 0.022 |
| 101–200 | 9 | 30 | 13.500 | 3.333 | 54.673 | 0.001 | ||
| 201–300 | 3 | 10 | - | - | - | - | ||
| Parity | 1–3 | 19 | 63.33 | 0.039 | 2.983 | 1.044 | 8.527 | 0.041 |
| 4–6 | 11 | 36.67 | - | - | - | - | ||
| Feeding regime | Silage + Concentrate | 2 | 6.67 | <0.001 | 56.00 | 10.327 | 303.682 | <0.001 |
| Silage + Hay + Concentrate | 4 | 13.33 | 26.00 | 6.532 | 103.498 | 0.001 | ||
| E. coli | Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Categories | Frequency | % Age | p-Value | ODD’s Ration | Lower | Upper | p-Value |
| Calving history | Dystocia | 12 | 42.86 | <0.001 | 1.538 | 0.536 | 4.416 | 0.423 |
| Abortion | 1 | 3.57 | 31.154 | 3.704 | 262.059 | 0.002 | ||
| Eutocia | 15 | 53.57 | - | - | - | - | ||
| Milk yield/day | 10–20 | 5 | 17.86 | 0.002 | 1.533 | 0.422 | 5.577 | 0.516 |
| 21–30 | 14 | 50 | 0.33 | 0.108 | 1.034 | 0.057 | ||
| 31–40 | 7 | 25 | 1.000 | 0.298 | 3.353 | 1.000 | ||
| 41–50 | 2 | 7.14 | - | - | - | - | ||
| Days in milk | 1–100 | 16 | 57.14 | 0.001 | 0.09 | 0.022 | 0.369 | 0.001 |
| 101–200 | 9 | 32.14 | 0.253 | 0.060 | 1.065 | 0.061 | ||
| 201–300 | 3 | 10.72 | - | - | - | - | ||
| Parity | 1–3 | 19 | 67.86 | <0.001 | 4.457 | 1.452 | 13.681 | 0.009 |
| 4–6 | 9 | 32.14 | 0.106 | - | - | - | ||
| Feeding regime | Silage + Concentrate | 4 | 14.29 | 0.593 | 78.000 | 13.078 | 465.196 | 0.000 |
| Silage + Hay + Concentrate | 4 | 14.28 | 36.000 | 8.057 | 160.849 | <0.001 | ||
| Silage + Concentrate + Fresh fodder | - | - | - | - | ||||
| Combination | 15 | 53.57 | 0.185 | 0.066 | ||||
| Antibiotics | Potency | S. aureus | E. coli | ||||
|---|---|---|---|---|---|---|---|
| Resistant(R) % | Intermediate(I) % | Sensitive(S) % | Resistant(R) % | Intermediate(I) % | Sensitive(S) % | ||
| Fusidic acid | 10 µg | 60 | 20 | 20 | 60 | 20 | 20 |
| Enrofloxacin | 10 µg | 20 | 20 | 60 | 10 | 10 | 80 |
| Ciprofloxacin | 5 µg | 10 | 20 | 70 | 10 | 20 | 70 |
| Levofloxacin | 5 µg | 30 | 30 | 40 | 30 | 30 | 40 |
| Chloramphenicol | 30 µg | 20 | 30 | 50 | 50 | 30 | 20 |
| Vancomycin | 30 µg | 10 | 20 | 70 | 10 | 20 | 70 |
| Gentamicin | 10 µg | 10 | 10 | 80 | 60 | 10 | 30 |
| Linezolid | 30 µg | 30 | 10 | 60 | 30 | 10 | 60 |
| Cefoxitin | 30 µg | 50 | 10 | 40 | 50 | 10 | 40 |
| 500 Preparation Name | Concentration Stock | No. of Snails Tested | Mortality Until Day 1 | Mortality Until Day 3 | Mortality Until Day 5 | |||
|---|---|---|---|---|---|---|---|---|
| Ratio | % | Ratio | % | Ratio | % | |||
| MgO (Group 1) | 10 mg/mL | 5 | 1/5 | 20 | 3/5 | 60 | 4/5 | 80 |
| 1 mg/mL | 5 | 1/5 | 20 | 2/5 | 40 | 3/5 | 60 | |
| 0.1 mg/mL | 5 | 0/5 | 0 | 1/5 | 20 | 2/5 | 40 | |
| 0.01 mg/mL | 5 | 0/5 | 0 | 1/5 | 20 | 1/5 | 20 | |
| Control (Group 2) | - | 10 | 0 | 0 | 0 | 0 | 2/10 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, M.; Nabeel, M.A.; Haq, S.U.; Waqas, M.S.; Jamil, H.; Aqib, A.I.; Muneer, A.; Fouad, D.; Ataya, F.S. Recent Trends in S. aureus and E. coli-Based Endometritis, and the Therapeutic Evaluation of Sodium Alginate-Based Antibiotics and Nanoparticles. Gels 2023, 9, 955. https://doi.org/10.3390/gels9120955
Talib M, Nabeel MA, Haq SU, Waqas MS, Jamil H, Aqib AI, Muneer A, Fouad D, Ataya FS. Recent Trends in S. aureus and E. coli-Based Endometritis, and the Therapeutic Evaluation of Sodium Alginate-Based Antibiotics and Nanoparticles. Gels. 2023; 9(12):955. https://doi.org/10.3390/gels9120955
Chicago/Turabian StyleTalib, Muzammil, Muhammad Ashir Nabeel, Shahbaz Ul Haq, Muhammad Salman Waqas, Huma Jamil, Amjad Islam Aqib, Afshan Muneer, Dalia Fouad, and Farid Shokry Ataya. 2023. "Recent Trends in S. aureus and E. coli-Based Endometritis, and the Therapeutic Evaluation of Sodium Alginate-Based Antibiotics and Nanoparticles" Gels 9, no. 12: 955. https://doi.org/10.3390/gels9120955