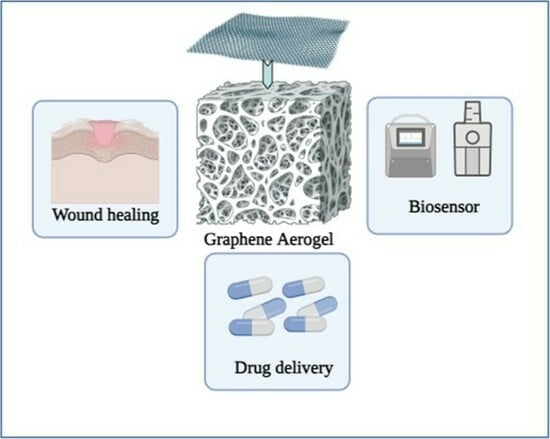
Graphene-Based Aerogels for Biomedical Application
Abstract
:1. Introduction
2. Preparation of Graphene-Based Aerogel
2.1. Sol–Gel Method
2.2. Freeze Casting
2.3. Hydrothermal Reduction
3. Graphene Aerogel in Biomedical Applications
3.1. Applications of Graphene-Based Aerogels in Wound Healing and Hemostat Application
3.2. Applications of Graphene-Based Aerogels in Bilirubin Adsorption
3.3. Applications of Graphene-Based Aerogels in Drug Delivery
3.4. Applications of Graphene-Based Aerogels in Bone Regeneration
3.5. Applications of Graphene-Based Aerogels in Biosensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, K.; Chen, J.; Xia, M.; Li, M.; Liu, K.; Wang, D.; Wu, C.; Xie, Y. Recent advances in novel aerogels through the hybrid aggregation of inorganic nanomaterials and polymeric fibers for thermal insulation. Aggregate 2021, 2, e30. [Google Scholar] [CrossRef]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, J.; Chen, S.; Varley, R.J.; Pan, K. 1D/2D nanomaterials synergistic, compressible, and response rapidly 3D graphene aerogel for piezoresistive sensor. Adv. Funct. Mater. 2020, 30, 2003618. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef]
- Trembecka-Wójciga, K.; Sobczak, J.J.; Sobczak, N. A comprehensive review of graphene-based aerogels for biomedical applications: The impact of synthesis parameters onto material microstructure and porosity. Arch. Civ. Mech. Eng. 2023, 23, 133. [Google Scholar] [CrossRef]
- Nassar, G.; Daou, E.; Najjar, R.; Bassil, M.; Habchi, R. A review on the current research on graphene-based aerogels and their applications. Carbon Trends 2021, 4, 100065. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Wang, Q.; Mao, H. A one-step hydrothermal route to fabricate a ZnO nanorod/3D graphene aerogel-sensitized structure with enhanced photoelectrochemistry performance and self-powered photoelectrochemical biosensing of parathion-methyl. RSC Adv. 2021, 11, 35644–35652. [Google Scholar] [CrossRef]
- Jiang, X.F.; Li, R.; Hu, M.; Hu, Z.; Golberg, D.; Bando, Y.; Wang, X.B. Zinc-tiered synthesis of 3D graphene for monolithic electrodes. Adv. Mater. 2019, 31, 1901186. [Google Scholar] [CrossRef]
- Zou, X.; Wu, D.; Mu, Y.; Xing, L.; Zhang, W.; Gao, Z.; Xu, F.; Jiang, K. Boron and nitrogen Co-doped holey graphene aerogels with rich B–N motifs for flexible supercapacitors. Carbon 2020, 159, 94–101. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Qin, L.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. A novel robust adsorbent for efficient oil/water separation: Magnetic carbon nanospheres/graphene composite aerogel. J. Hazard. Mater. 2020, 392, 122499. [Google Scholar] [CrossRef]
- Riaz, M.A.; Hadi, P.; Abidi, I.H.; Tyagi, A.; Ou, X.; Luo, Z. Recyclable 3D graphene aerogel with bimodal pore structure for ultrafast and selective oil sorption from water. RSC Adv. 2017, 7, 29722–29731. [Google Scholar] [CrossRef]
- Li, C.-B.; Li, Y.-J.; Zhao, Q.; Luo, Y.; Yang, G.-Y.; Hu, Y.; Jiang, J.-J. Electromagnetic interference shielding of graphene aerogel with layered microstructure fabricated via mechanical compression. ACS Appl. Mater. Interfaces 2020, 12, 30686–30694. [Google Scholar] [CrossRef]
- Berrio, M.E.; Oñate, A.; Salas, A.; Fernández, K.; Meléndrez, M.F. Synthesis and applications of graphene oxide aerogels in bone tissue regeneration: A review. Mater. Today Chem. 2021, 20, 100422. [Google Scholar] [CrossRef]
- Mellado, C.; Figueroa, T.; Báez, R.; Castillo, R.; Melendrez, M.; Schulz, B.; Fernández, K. Development of Graphene Oxide Composite Aerogel with Proanthocyanidins with Hemostatic Properties As a Delivery System. ACS Appl. Mater. Interfaces 2018, 10, 7717–7729. [Google Scholar] [CrossRef] [PubMed]
- Guzel Kaya, G.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Aerogels as promising materials for antibacterial applications: A mini-review. Biomater. Sci. 2021, 9, 7034–7048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Y.; Liu, Y.-J.; Sui, G.-X. Lightweight spongy bone-like graphene@SiC aerogel composites for high-performance microwave absorption. Chem. Eng. J. 2018, 337, 522–531. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B Biointerfaces 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Guo, P.; Pang, S.; Hu, C.; Zhao, R.; Tang, S.; Cheng, H.-M. Tailoring microstructures of carbon fiber reinforced carbon aerogel-like matrix composites by carbonization to modulate their mechanical properties and thermal conductivities. Carbon 2022, 196, 807–818. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Peng, Z.; Liu, Y.; Zhang, J.; Liu, Z.; Li, D. 3D free-standing nitrogen-doped reduced graphene oxide aerogel as anode material for sodium ion batteries with enhanced sodium storage. Sci. Rep. 2017, 7, 4886. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible graphene oxide-collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110137. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, I.; Bajpai, V.K.; Lee, H.; Kim, T.; Upadhyay, A.; Huh, Y.S.; Han, Y.-K.; Tripathi, K.M. Sustainable Graphene Aerogel as an Ecofriendly Cell Growth Promoter and Highly Efficient Adsorbent for Histamine from Red Wine. ACS Appl. Mater. Interfaces 2019, 11, 18165–18177. [Google Scholar] [CrossRef] [PubMed]
- Gudkov Maksim, V.; Valery Pavlovich, M. Graphene Oxide/Reduced Graphene Oxide Aerogels. In Graphene Oxide; Ganesh, K., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 4. [Google Scholar]
- Bai, H.; Li, C.; Wang, X.; Shi, G. On the Gelation of Graphene Oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Bosch-Navarro, C.; Coronado, E.; Martí-Gastaldo, C.; Sánchez-Royo, J.F.; Gómez, M.G. Influence of the pH on the synthesis of reduced graphene oxide under hydrothermal conditions. Nanoscale 2012, 4, 3977–3982. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Abidli, A.; Behzadfar, E.; Rezaei, S.; Naguib, H.E.; Park, C.B. The effect of graphene-nanoplatelets on gelation and structural integrity of a polyvinyltrimethoxysilane-based aerogel. RSC Adv. 2019, 9, 11503–11520. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Żelechowska, K.; Nadolska, M.; Jażdżewska, A.; Gazda, M. Comprehensive study on graphene hydrogels and aerogels synthesis and their ability of gold nanoparticles adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2017, 528, 65–73. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Effect of synthesis pH on the structure of carbon xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Ortiz, A.; Ortiz, I. Overview on the use of surfactants for the preparation of porous carbon materials by the sol-gel method: Applications in energy systems. Rev. Chem. Eng. 2020, 36, 771–787. [Google Scholar] [CrossRef]
- Worsley, M.A.; Olson, T.Y.; Lee, J.R.I.; Willey, T.M.; Nielsen, M.H.; Roberts, S.K.; Pauzauskie, P.J.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. High Surface Area, sp2-Cross-Linked Three-Dimensional Graphene Monoliths. J. Phys. Chem. Lett. 2011, 2, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Gante, N.; Sacchi, M. Reduction of NO on chemically doped, metal-free graphene. Carbon Trends 2021, 5, 100111. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Wu, P.; Ma, Z.; Shang, Y.; Bai, G.; Liu, X.; Chang, G.; Li, N.; Dai, J.; et al. Precise control of versatile microstructure and properties of graphene aerogel via freezing manipulation. Nanoscale 2020, 12, 4882–4894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Z. Ultralight, highly compressible, hydrophobic and anisotropic lamellar carbon aerogels from graphene/polyvinyl alcohol/cellulose nanofiber aerogel as oil removing absorbents. J. Hazard. Mater. 2020, 388, 121804. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Sang, X.; Liu, C.; Luo, T.; Peng, L.; Han, B.; Tan, X.; Ma, X.; Wang, D.; et al. Cellular graphene aerogel combines ultralow weight and high mechanical strength: A highly efficient reactor for catalytic hydrogenation. Sci. Rep. 2016, 6, 25830. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, N.; Yao, W.; Xu, Z.; Bai, H.; Gao, C. Effect of flake size on the mechanical properties of graphene aerogels prepared by freeze casting. RSC Adv. 2017, 7, 33600–33605. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Hu, K.; Xie, X.; Szkopek, T.; Cerruti, M. Understanding Hydrothermally Reduced Graphene Oxide Hydrogels: From Reaction Products to Hydrogel Properties. Chem. Mater. 2016, 28, 1756–1768. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhang, F.; Yu, S.; Zhang, R.; Zhou, Y. Hydrothermal formation of graphene aerogel for oil sorption: The role of reducing agent, reaction time and temperature. New J. Chem. 2016, 40, 3040–3046. [Google Scholar] [CrossRef]
- Hu, K.; Szkopek, T.; Cerruti, M. Tuning the aggregation of graphene oxide dispersions to synthesize elastic, low density graphene aerogels. J. Mater. Chem. A 2017, 5, 23123–23130. [Google Scholar] [CrossRef]
- Serrapede, M.; Fontana, M.; Gigot, A.; Armandi, M.; Biasotto, G.; Tresso, E.; Rivolo, P. A Facile and Green Synthesis of a MoO2-Reduced Graphene Oxide Aerogel for Energy Storage Devices. Materials 2020, 13, 594. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced mechanical, thermal, and electric properties of graphene aerogels via supercritical ethanol drying and high-temperature thermal reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef] [PubMed]
- García-Bordejé, E.; Víctor-Román, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef]
- Guajardo, S.; Figueroa, T.; Borges, J.; Aguayo, C.; Fernández, K. Graphene oxide-gelatin aerogels as wound dressings with improved hemostatic properties. Mater. Today Chem. 2021, 20, 100418. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Aguayo, C.; Fernández, K. Improved hemocompatibility for gelatin-graphene oxide composite aerogels reinforced with proanthocyanidins for wound dressing applications. Colloids Surf. B Biointerfaces 2021, 206, 111941. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Poblete, J.; Gajardo, F.; Aguayo, C.; Fernández, K. Graphene oxide/polyethylene glycol aerogel reinforced with grape seed extracts as wound dressing. J. Mater. Sci. 2021, 56, 16082–16096. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Carmona, S.; Mellado, C.; Meléndrez, M.; Aguayo, C.; Fernández, K. Novel and effective hemostats based on graphene oxide-polymer aerogels: In vitro and in vivo evaluation. Biomater. Adv. 2022, 139, 213007. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Khan, I.; Kang, S.-M.; Haldorai, Y.; Tripathi, K.M.; Jung, S.; Chen, L.; Kim, T.; Huh, Y.S.; et al. A Sustainable Graphene Aerogel Capable of the Adsorptive Elimination of Biogenic Amines and Bacteria from Soy Sauce and Highly Efficient Cell Proliferation. ACS Appl. Mater. Interfaces 2019, 11, 43949–43963. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Li, Z.; Li, Z.; Wu, K.; Jiao, Y.; Zhou, C. Construction of blood compatible chitin/graphene oxide composite aerogel beads for the adsorption of bilirubin. Carbohydr. Polym. 2019, 207, 704–712. [Google Scholar] [CrossRef]
- Li, Z.; Song, X.; Cui, S.; Jiao, Y.; Zhou, C. Fabrication of macroporous reduced graphene oxide composite aerogels reinforced with chitosan for high bilirubin adsorption. RSC Adv. 2018, 8, 8338–8348. [Google Scholar] [CrossRef]
- Zhang, G.; Shao, D.; Yu, H.; Wan, Y.; Jiao, Y.; Li, L.; Tian, J.; Zhou, C.; Lu, L. MXene Nanosheet-Enhanced Chitin Aerogel Spheres for Bilirubin Adsorption. ACS Appl. Nano Mater. 2022, 5, 17293–17303. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, G.; Shao, D.; Ding, S.; Li, L.; Li, H.; Zhou, C.; Luo, B.; Lu, L. Preparation of chitin/MXene/poly(L-arginine) composite aerogel spheres for specific adsorption of bilirubin. Int. J. Biol. Macromol. 2023, 243, 125140. [Google Scholar] [CrossRef]
- Ayazi, H.; Akhavan, O.; Raoufi, M.; Varshochian, R.; Hosseini Motlagh, N.S.; Atyabi, F. Graphene aerogel nanoparticles for in-situ loading/pH sensitive releasing anticancer drugs. Colloids Surf. B Biointerfaces 2020, 186, 110712. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, Z.; Namazi, H. Nontoxic double-network polymeric hybrid aerogel functionalized with reduced graphene oxide: Preparation, characterization, and evaluation as drug delivery agent. J. Polym. Res. 2022, 29, 37. [Google Scholar] [CrossRef]
- Lim, H.; Kim, J.Y.; Yoon, M.G.; Kang, Y.-M.; Park, Y.M.; Lee, H.-N.; Moon, S.-H.; Koh, W.-G.; Kim, H.-J. Facile control of porous structure of graphene aerogel via two-step drying process and its effect on drug release. J. Porous Mater. 2023, 30, 1725–1734. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, X.; An, J.; Chen, Z.; Zhang, X. Highly sensitive, flexible, green synthesized graphene/biomass aerogels for pressure sensing application. Compos. Sci. Technol. 2021, 207, 108730. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, Y.; Cui, C.; Li, A.; Wang, W.; Guo, R.; Qin, W.; Ren, E.; Xiao, H.; Zhou, M. Flexible Waterborne Polyurethane/Cellulose Nanocrystal Composite Aerogels by Integrating Graphene and Carbon Nanotubes for a Highly Sensitive Pressure Sensor. ACS Sustain. Chem. Eng. 2021, 9, 14029–14039. [Google Scholar] [CrossRef]
- Li, G.; Chu, Z.; Gong, X.; Xiao, M.; Dong, Q.; Zhao, Z.; Hu, T.; Zhang, Y.; Wang, J.; Tan, Y.; et al. A Wide-Range Linear and Stable Piezoresistive Sensor Based on Methylcellulose-Reinforced, Lamellar, and Wrinkled Graphene Aerogels. Adv. Mater. Technol. 2022, 7, 2101021. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, C.; Feng, S.; Zhang, H.; Liu, Y.; Zhu, Y.; Wang, J.; Xiang, X.; Xie, H. Highly Compressible, Light-Weight and robust Nitrogen-Doped graphene composite aerogel for sensitive pressure sensors. Chem. Eng. J. 2023, 471, 144790. [Google Scholar] [CrossRef]
- Luo, R.; Li, Z.; Wu, X.; Liu, H.; Ma, L.; Wu, J.; Qin, G.; Wang, J.; Yang, S. Super durable graphene aerogel inspired by deep-sea glass sponge skeleton. Carbon 2022, 191, 153–163. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Luan, D.; Yuan, Q.; Tao, L.; Wang, X. Black hemostatic sponge based on facile prepared cross-linked graphene. Colloids Surf. B Biointerfaces 2015, 132, 27–33. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Aguayo, C.; Fernández, K. The Effect on Hemostasis of Gelatin-Graphene Oxide Aerogels Loaded with Grape Skin Proanthocyanidins: In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 14, 1772. [Google Scholar] [CrossRef]
- Xu, C.; Tang, X.; Niu, Z.; Li, Z. Studies of adsorbents for hemoperfusion in artificial liver support: I. Preparation and in vitro studies of cross-linked agarose beads entrapped activated charcoal (CAAC). Int. J. Artif. Organs 1981, 4, 200–204. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Rana, J.; Pundir, C.S. Construction of an amperometric bilirubin biosensor based on covalent immobilization of bilirubin oxidase onto zirconia coated silica nanoparticles/chitosan hybrid film. Biosens. Bioelectron. 2013, 44, 64–69. [Google Scholar] [CrossRef]
- Gautam, R.; Marriwala, N.; Devi, R. A review: Study of Mxene and graphene together. Meas. Sens. 2023, 25, 100592. [Google Scholar] [CrossRef]
- Sang, Y.; Miao, P.; Chen, T.; Zhao, Y.; Chen, L.; Tian, Y.; Han, X.; Gao, J. Fabrication and Evaluation of Graphene Oxide/Hydroxypropyl Cellulose/Chitosan Hybrid Aerogel for 5-Fluorouracil Release. Gels 2022, 8, 649. [Google Scholar] [CrossRef]
- Wang, R.; Shou, D.; Lv, O.; Kong, Y.; Deng, L.; Shen, J. pH-Controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int. J. Biol. Macromol. 2017, 103, 248–253. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, N.; Liu, B.; Song, L.; Wen, B.; Yang, D. Aligned porous chitosan/graphene oxide scaffold for bone tissue engineering. Mater. Lett. 2018, 233, 78–81. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Pandele, A.M.; Galateanu, B.; Iovu, H.; Ardelean, A.; Costache, M.; Hermenean, A. In vitro cytocompatibility evaluation of chitosan/graphene oxide 3D scaffold composites designed for bone tissue engineering. Biomed. Mater. Eng. 2014, 24, 2249–2256. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Vasile, E.; Iovu, H. Novel Chitosan-poly(vinyl alcohol)/graphene oxide biocomposites 3D porous scaffolds. Compos. Part B Eng. 2017, 126, 81–87. [Google Scholar] [CrossRef]
- Asha, S.; Ananth, A.N.; Jose, S.P.; Rajan, M.A.J. Reduced graphene oxide aerogel networks with soft interfacial template for applications in bone tissue regeneration. Appl. Nanosci. 2018, 8, 395–405. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, D.; Srivastava, P.; Singh, B.N.; Mishra, A. Generation of graphene oxide and nano-bioglass based scaffold for bone tissue regeneration. Biomed. Mater. 2022, 17, 065012. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. Development of a porous 3D graphene-PDMS scaffold for improved osseointegration. Colloids Surf. B Biointerfaces 2017, 159, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Baheiraei, N.; Salehnia, M. Fabrication and characterization of PHEMA–gelatin scaffold enriched with graphene oxide for bone tissue engineering. J. Orthop. Surg. Res. 2022, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017, 17, 1600397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ran, W.; Wang, L.; Shen, G. Interlocked MXene/rGO aerogel with excellent mechanical stability for a health-monitoring device. J. Semicond. 2022, 43, 082601. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, X.; Zhu, Z.; Xu, L.; Chen, X.; Luo, L.; Liu, X.; Qin, J. Flexible pressure sensors with high pressure sensitivity and low detection limit using a unique honeycomb-designed polyimide/reduced graphene oxide composite aerogel. RSC Adv. 2021, 11, 11760–11770. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wu, N.; Yang, W.; Xu, H.; Liao, Y.; Li, C.; Luković, M.; Yang, Y.; Zhao, S.; Su, Z.; et al. Sustainable-Macromolecule-Assisted Preparation of Cross-linked, Ultralight, Flexible Graphene Aerogel Sensors toward Low-Frequency Strain/Pressure to High-Frequency Vibration Sensing. Small 2022, 18, 2202047. [Google Scholar] [CrossRef] [PubMed]
- Afroze, J.D.; Tong, L.; Abden, M.J.; Chen, Y. Multifunctional hierarchical graphene-carbon fiber hybrid aerogels for strain sensing and energy storage. Adv. Compos. Hybrid Mater. 2022, 6, 18. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, Y.; Gao, Q.; Wang, X.; Li, A.; Yuan, Y.; Guan, S. Biocompatible graphene/chitosan hybrid aerogel reinforced polydimethylsiloxane nanocomposite with excellent dielectric properties. J. Appl. Polym. Sci. 2023, 140, e53261. [Google Scholar] [CrossRef]
- Yang, G.; Qin, X.; Chen, T.; Wang, J.; Ma, L.; Yang, S. Ultralight, superelastic pure graphene aerogel for piezoresistive sensing application. J. Mater. Sci. 2023, 58, 850–863. [Google Scholar] [CrossRef]
- Figueroa, T.; Carmona, S.; Guajardo, S.; Borges, J.; Aguayo, C.; Fernández, K. Synthesis and characterization of graphene oxide chitosan aerogels reinforced with flavan-3-ols as hemostatic agents. Colloids Surf. B Biointerfaces 2021, 197, 111398. [Google Scholar] [CrossRef]
- Shi, C.; Tang, Y.; Yang, H.; Yang, J.; Wu, Y.; Sun, H.; Yin, S.; Wang, G. Capture and detection of Escherichia coli with graphene aerogels. J. Mater. Chem. B 2022, 10, 8211–8217. [Google Scholar] [CrossRef]







| Controlled Factor | Effect on Pore Size | References |
|---|---|---|
| Precursor concentration | The higher the graphene concentration, the smaller the pores | [29] |
| pH | The lower the pH, the smaller the pores | [30,31] |
| Surfactant | Depends on the surfactant used | [32] |
| Resorcinol–formaldehyde | The less the resorcinol–formaldehyde, the smaller the pores | [33] |
| Controlled Factor | Effect on Pore Size | Reference |
|---|---|---|
| Temperature | The lower the freezing temperature, the smaller the pores | [35] |
| Precursor concentration | The higher the precursor concentration, the smaller the pores | [38] |
| Flake size | The larger the graphene flakes, the smaller the pores | [39] |
| Suspension viscosity | The higher the viscosity, the smaller the pores | [39] |
| Controlled Factor | Effect on Pore Size | Reference |
|---|---|---|
| Temperature | The higher the temperature, the smaller the pores | [44] |
| pH | The higher the pH, the larger the pores | [28] |
| GO dispersion | The more π−π interactions between GO sheets in the primary solution, the larger the pores | [45] |
| Reducing agent | Depends on reducing the agent | [46] |
| Post-treatment | If annealing the aerogel, the pore size decreases | [47] |
| Time | The longer the hydrothermal reduction time, the smaller the pores | [48] |
| Types of GA | Fabrication Method | Pore Size/Volume | Electrical Conductivity /Resistivity | Surface Area | Application | Ref |
|---|---|---|---|---|---|---|
| GO/G | Physical interaction (GO oxygenated groups and G amine groups) | Basic aerogels: 42.17 ± 12.54 μm Acid aerogels: 25.30 ± 10.38 μm | - | - | Hemostatic /Wound healing | [49] |
| G/GO G/GO/PA (5%) G/GO/PA (10%) | Microwave-assisted synthesis | 25.0 ± 1.9 µm 24.9 ± 1.6 µm 24.7 ± 1.8 µm | - | - | Hemostatic /Wound healing | [50] |
| GO/PEG/PA | 20.5 ± 6.3 µm | - | - | Hemostatic | [51] | |
| GO/CS GO/GEL GO/PVA | Microwave-assisted synthesis | 32.4~36.8 μm | - | - | Hemostatic | [52] |
| GA from Pyrus pyrifolia biomass | Hydrothermal and post-pyrolysis process | 0.27 cm3/g | - | Wound healing | [25] | |
| GA | One-step pyrolysis of glucose and ammonium chloride | 2.24 cm3/g | - | Wound healing | [53] | |
| Ch/GO-5 Ch/GO-10 Ch/GO-20 | Using hybrid aqueous solution system of NaOH and urea blending with chitin and GO using H2SO4 as a coagulation bath | 0.8307~1.0270 cm3/g | - | 159.81~186.98 m2/g | Bilirubin adsorption | [54] |
| CS/rGO | Chemical reduction method | cm3/g | - | m2/g | Bilirubin adsorption | [55] |
| Ch/MX aerogel sphere | Supercritical technology | 0~37 nm | - | m2/g | Bilirubin adsorption | [56] |
| Ch/MX/PLA | Supercritical technology | - | m2/g | Bilirubin adsorption | [57] | |
| GA NPs | Reduction/aggregation of GO sheets in the presence of vitamin C | 30∼300 μm | - | - | Drug delivery | [58] |
| SA/K-CG/rGO | Sol–gel technique | 1~6 nm | - | 204.90 m2/g | Drug delivery | [59] |
| Gn aerogel | Hydrothermal reaction and controlled drying process | 30~500 μm | - | 88.3~147.7 m2/g | Drug delivery | [60] |
| GO/COL | Sol–gel process | 100~160 μm | - | - | Bone regeneration | [24] |
| Bacteria cellulose /rGO | Reduction, mixing, and freeze-drying technique | 40~300 μm | - | - | Biosensors | [61] |
| CNTs/Gn/WPU /CNC | Facile solution mixing and freeze-drying technique | 110~180 μm | - | - | Biosensors | [62] |
| MC/GA | Stirring, freeze-drying, steaming | - | Resistance normal to the lamellae: 158 kΩ at thickness of 2.0 mm, parallel to the lamellae: 394 kΩ at a length of 10.0 mm | - | Biosensors | [63] |
| GO/DA/PANI combined nitrogen-doped aerogel | Crosslinking at 90 °C, freeze-drying, thermal annealing | - | Without doping: 11.0 S/m GDA: 16.9 S/m GPA: 82.3 S/m GDPA: 56.7 S/m | - | Biosensors | [64] |
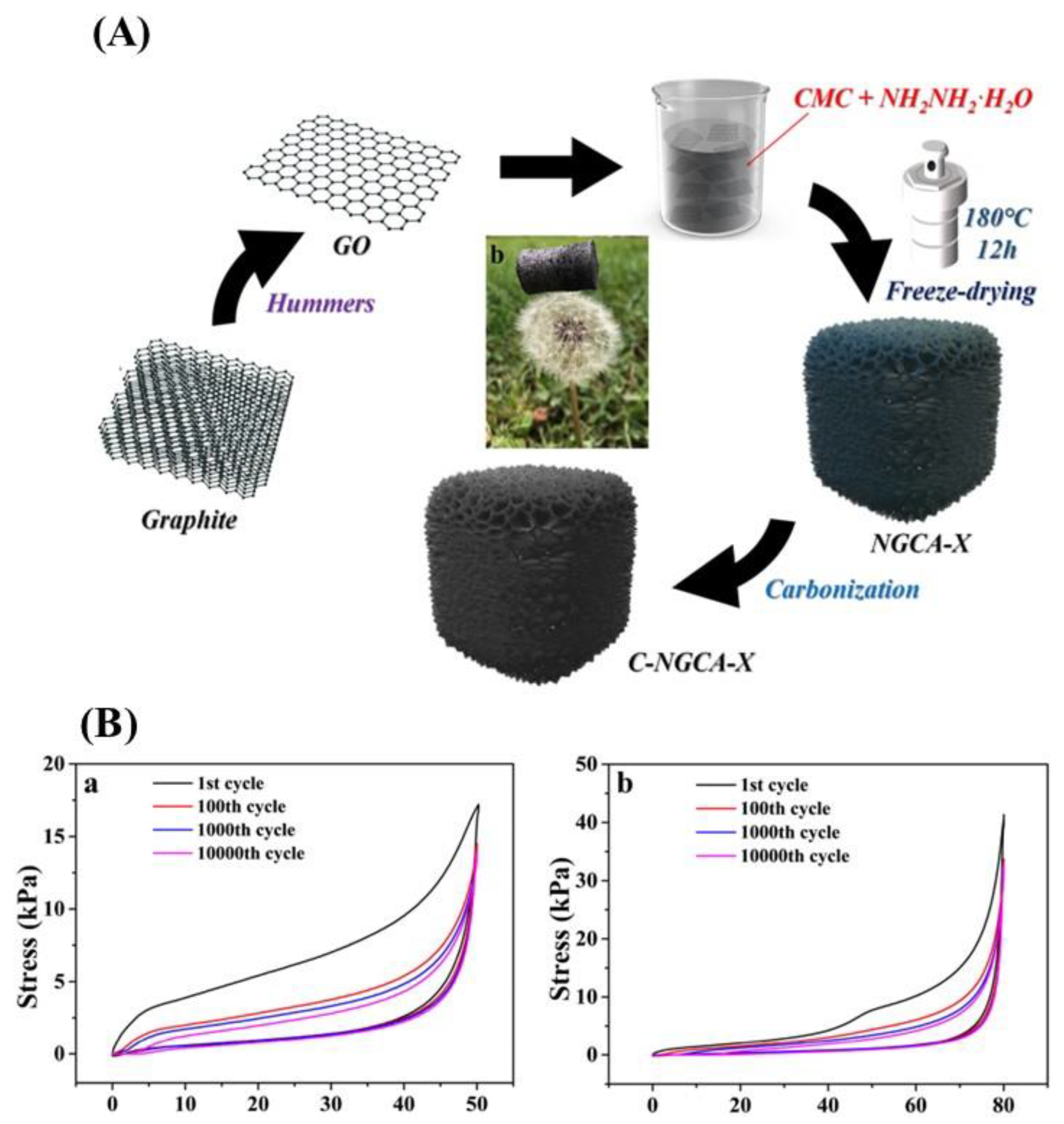
| NGCA-X aerogels | Facile hydrothermal self-assembling strategy | - | C-NGCA-10: 42.7 S/m | - | Biosensors | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Patel, R.; Kulkarni, C.V.; Patel, M. Graphene-Based Aerogels for Biomedical Application. Gels 2023, 9, 967. https://doi.org/10.3390/gels9120967
Kim Y, Patel R, Kulkarni CV, Patel M. Graphene-Based Aerogels for Biomedical Application. Gels. 2023; 9(12):967. https://doi.org/10.3390/gels9120967
Chicago/Turabian StyleKim, Yeongsang, Rajkumar Patel, Chandrashekhar V. Kulkarni, and Madhumita Patel. 2023. "Graphene-Based Aerogels for Biomedical Application" Gels 9, no. 12: 967. https://doi.org/10.3390/gels9120967
APA StyleKim, Y., Patel, R., Kulkarni, C. V., & Patel, M. (2023). Graphene-Based Aerogels for Biomedical Application. Gels, 9(12), 967. https://doi.org/10.3390/gels9120967