Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings
Abstract
:1. Introduction
2. Results and Discussion
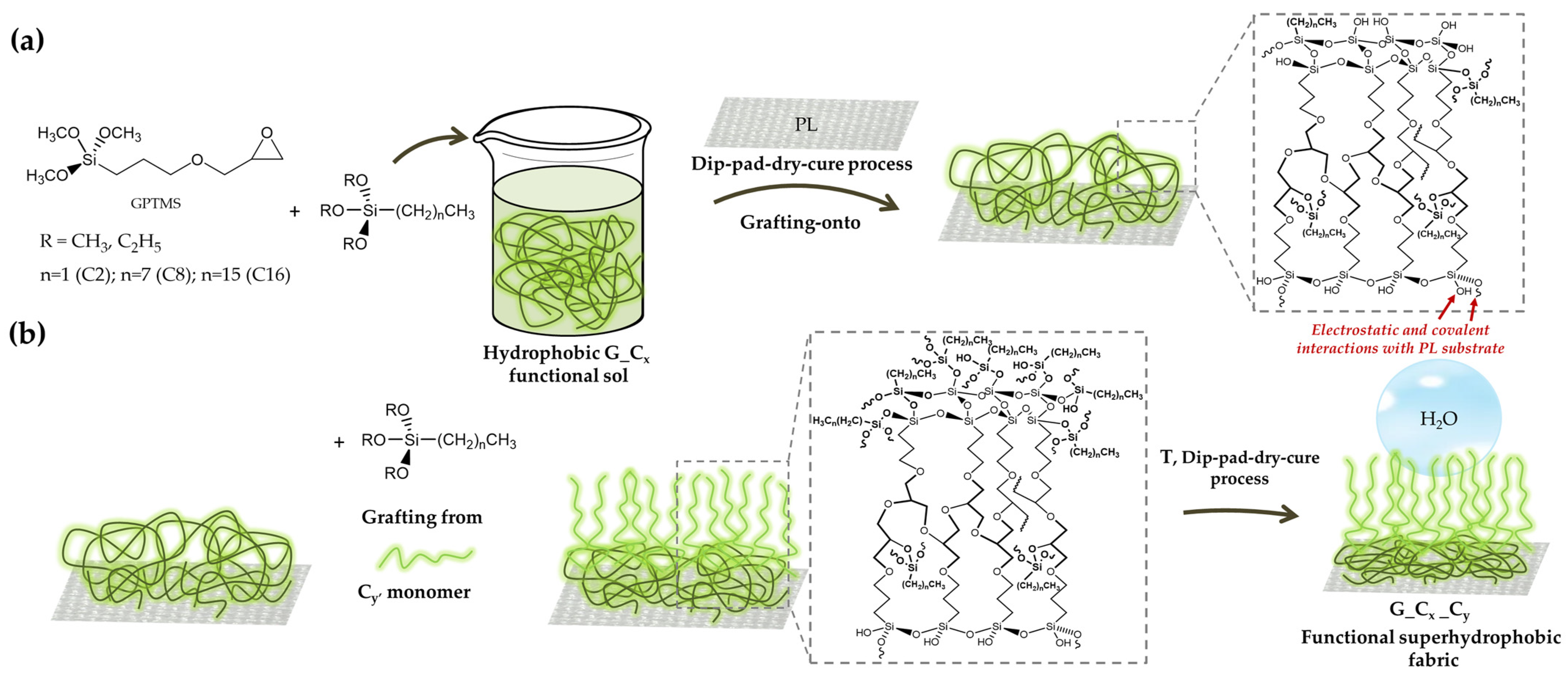
2.1. Water Repellent Nanohybrid Cross-Linked Polyalkylsiloxanes Sol-Gel Coating
2.1.1. Aqueous Liquid Repellency Tests (Water/Alcohol Solution)
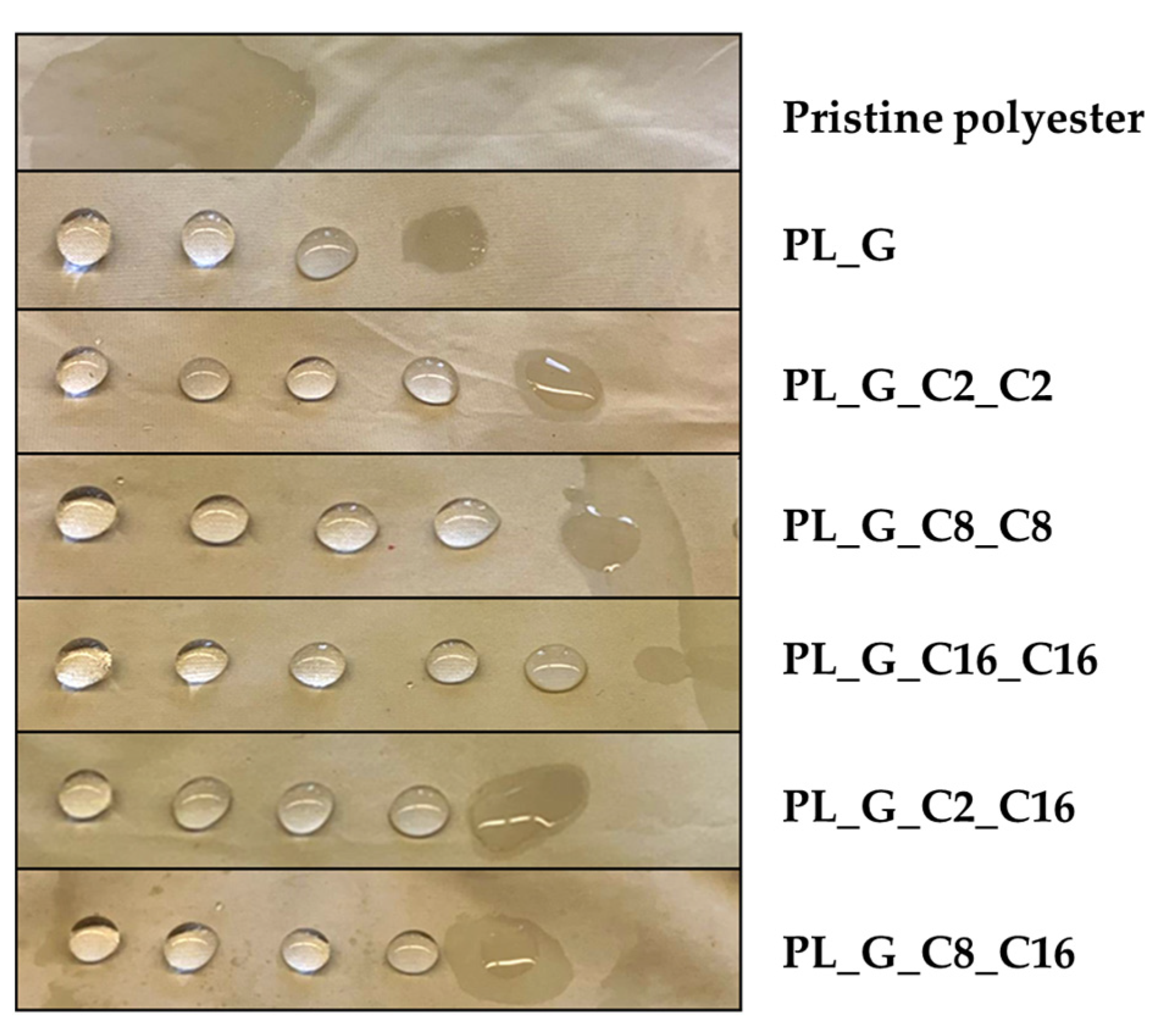
2.1.2. Sessile Drop Method
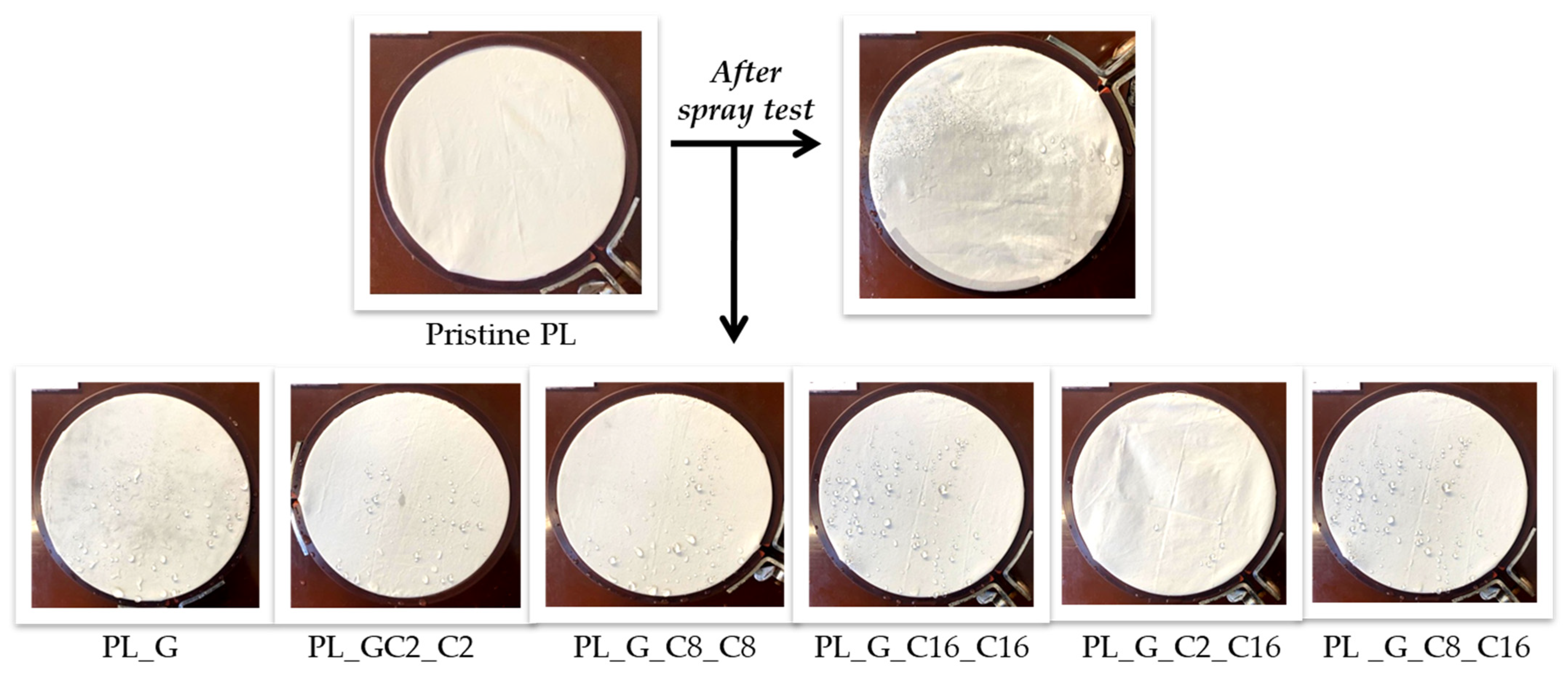
2.1.3. Spray Test
2.1.4. Self-Cleaning Behavior of the Fabric
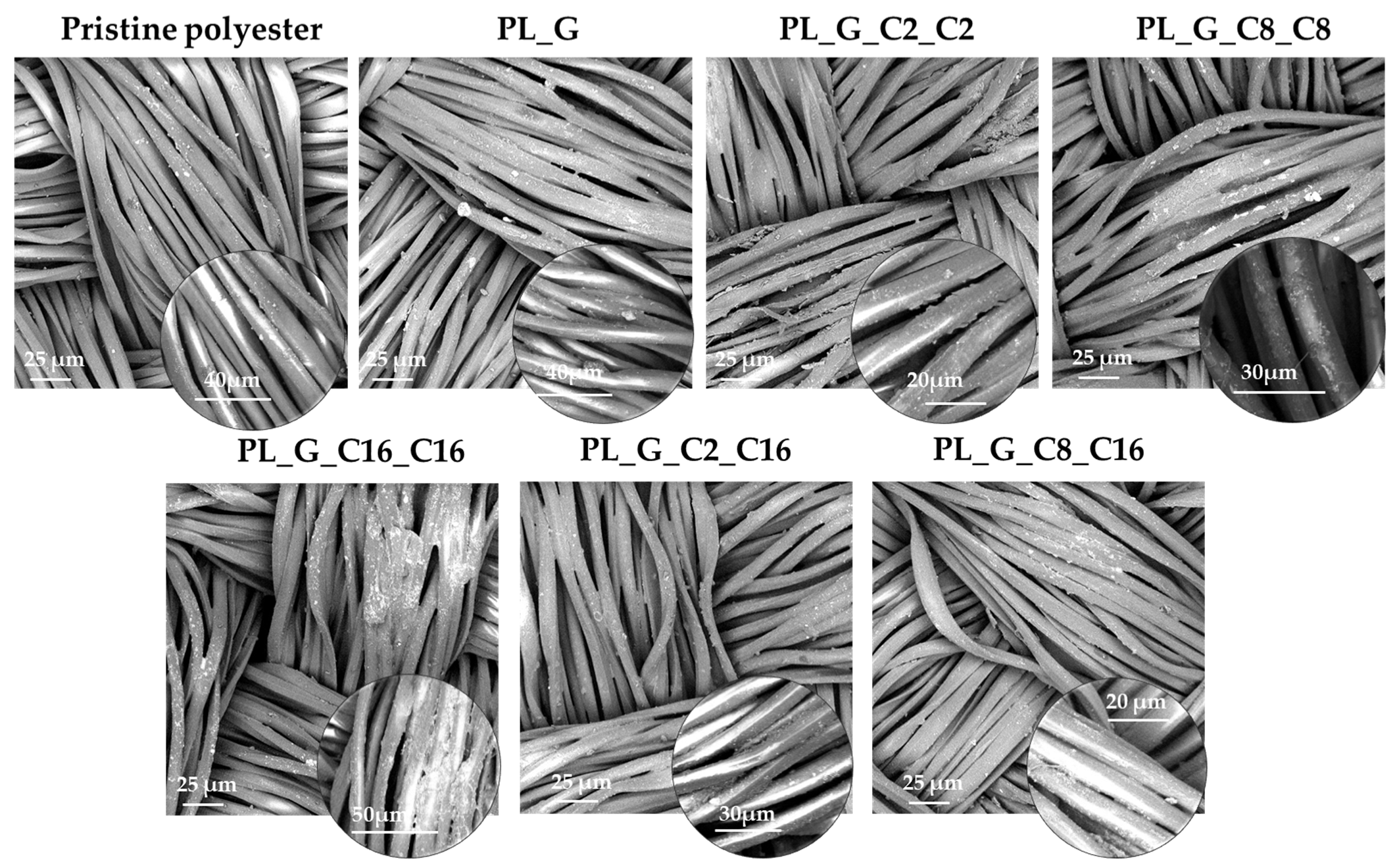
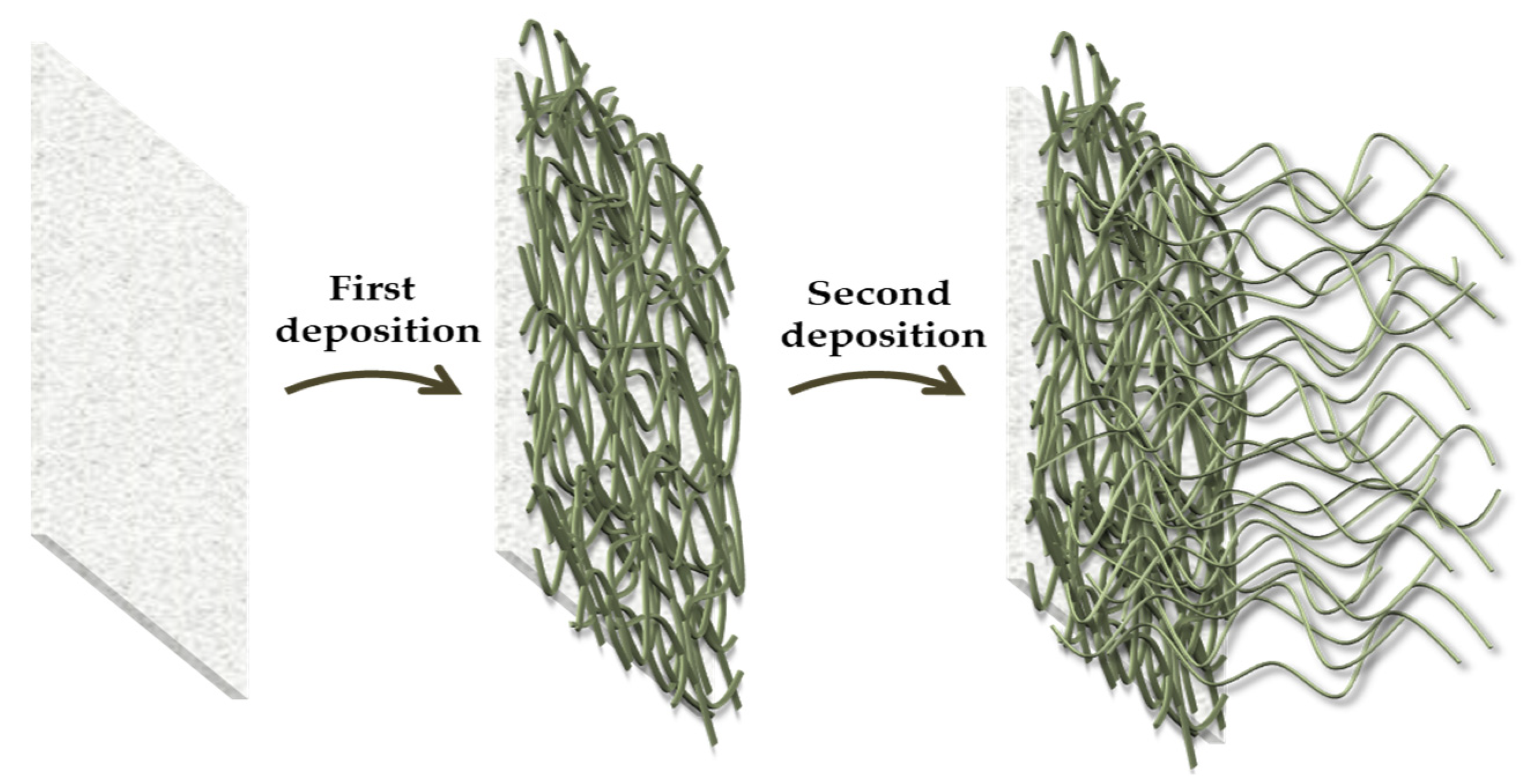
2.2. Micro-Nano/Morphology Analysis by Scanning Electron Microscopy (SEM)
2.3. Functionalized Fabric Performances
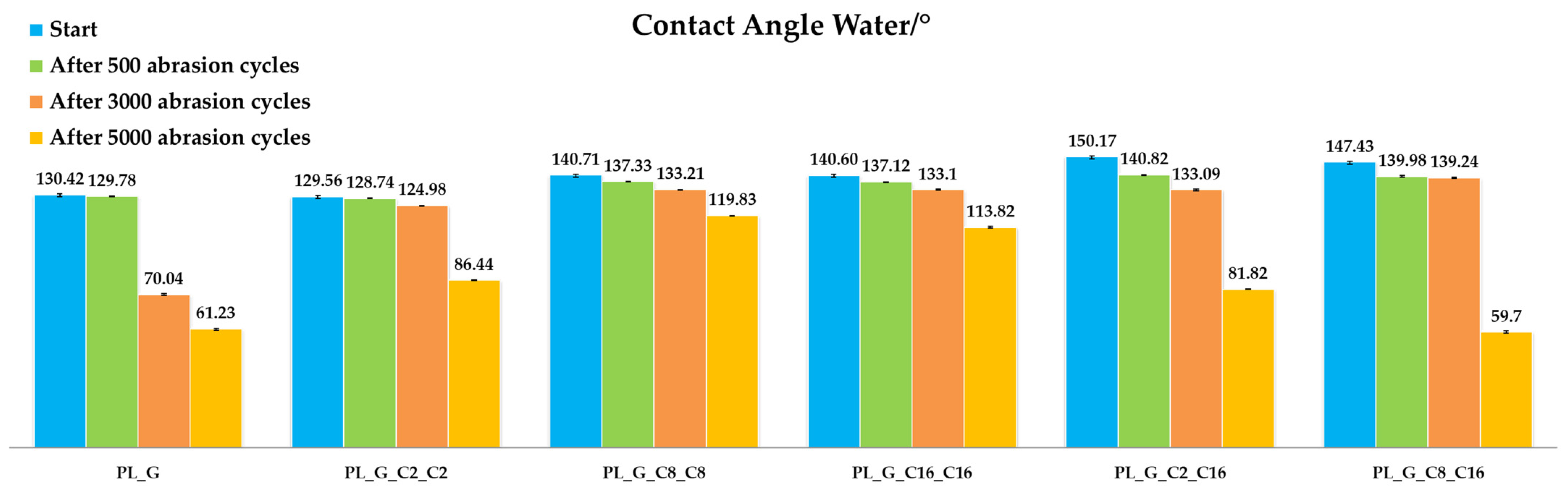
2.3.1. Abrasion Resistance
2.3.2. Moisture-Adsorption Analysis
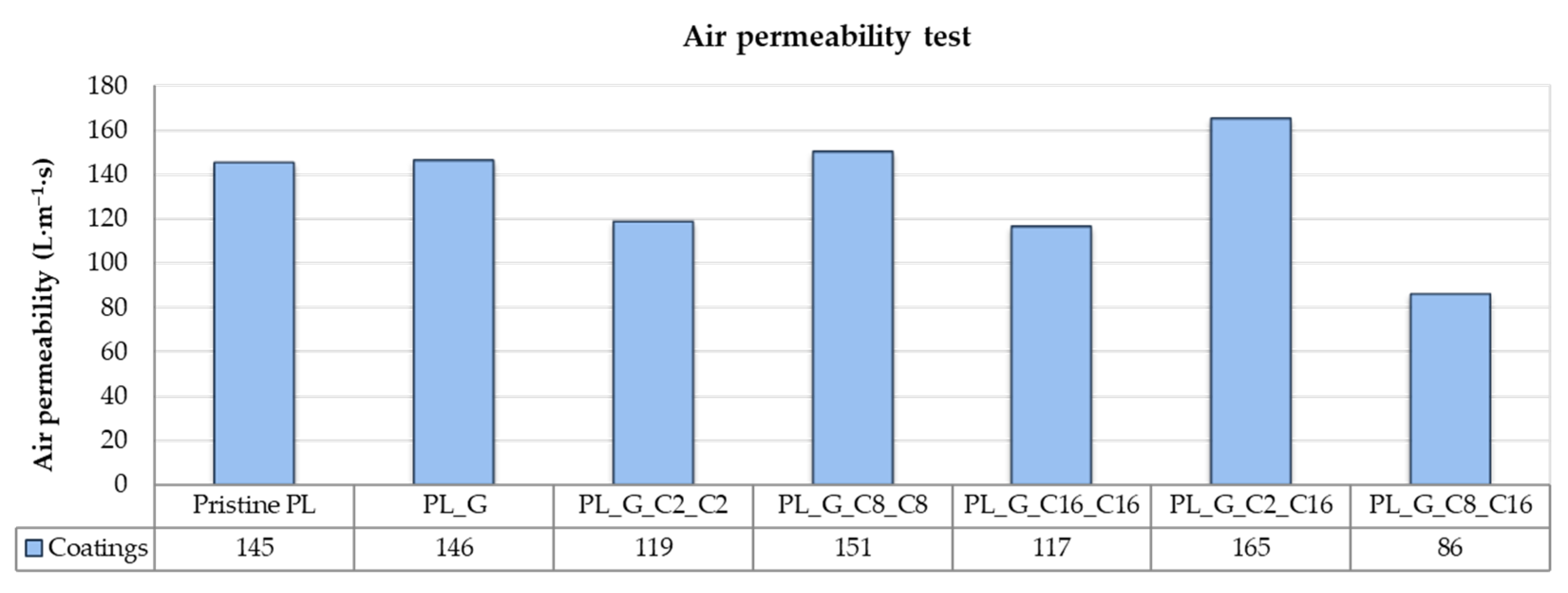
2.3.3. Air Permeability Test
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Nanohybrid Functional Sols Synthesis
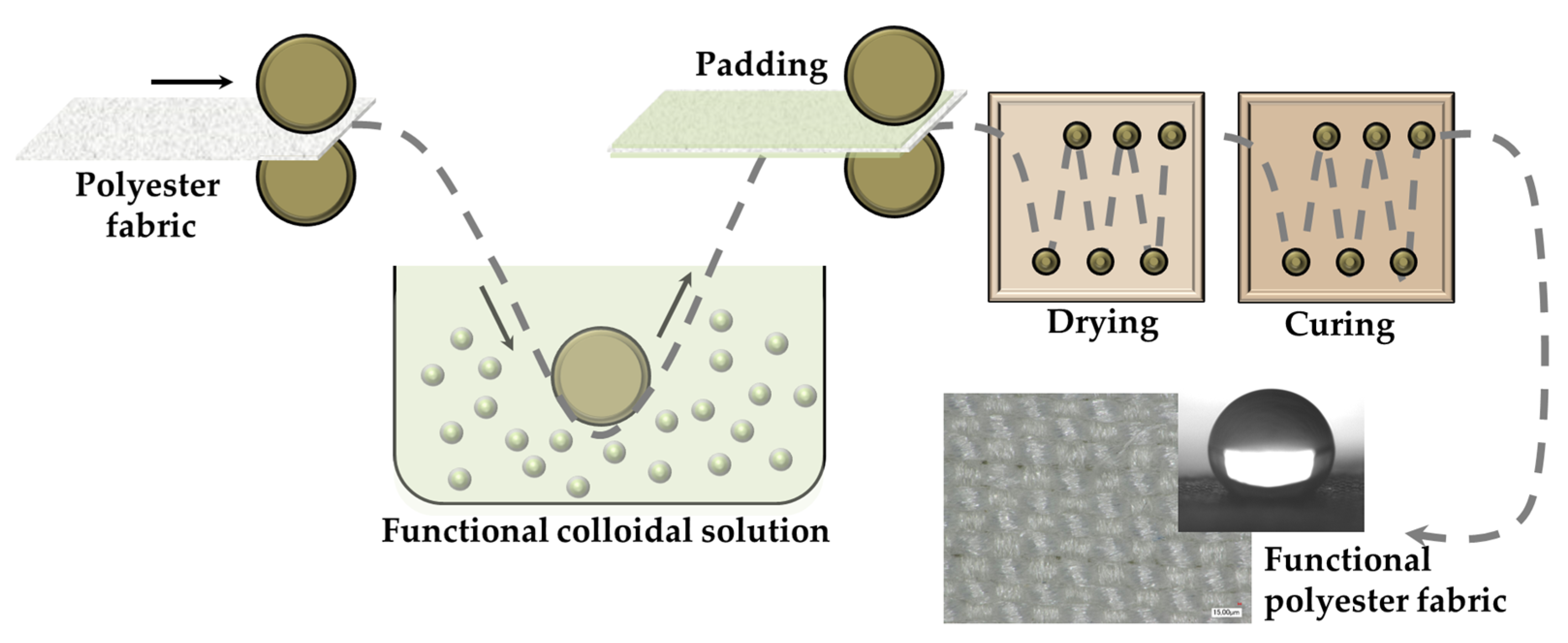
4.3. Fabrication of Superhydrophobic Fabric by Dip-Pad-Dry-Cure Method
4.4. Characterizations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Zhang, S.; Galluzzi, M.; Li, F.; Zhang, X.; Yang, X.; Liu, X.; Cai, X.; Zhu, X.; Du, B.; et al. Insight into multifunctional polyester fabrics finished by one-step eco-friendly strategy. Chem. Eng. J. 2019, 358, 634–642. [Google Scholar] [CrossRef]
- Chen, H.-L.; Burns, L.D. Environmental Analysis of Textile Products. Cloth. Text. Res. J. 2006, 24, 248–261. [Google Scholar] [CrossRef]
- Leonas, K.K. The Use of Recycled Fibers in Fashion and Home Products. In Textiles and Clothing Sustainability: Textile Science and Clothing Technology.; Muthu, S.S., Ed.; Springer: Singapore, 2017; pp. 55–77. ISBN 978-981-10-2146-6. [Google Scholar]
- Ke, W.H.; Hsia, C.F.; Chen, Y.J.; Huang, M.H. Synthesis of Ultrasmall Cu2O Nanocubes and Octahedra with Tunable Sizes for Facet-Dependent Optical Property Examination. Small 2016, 12, 3530–3534. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Athanassiou, A.; Bayer, I.S. Strain-responsive mercerized conductive cotton fabrics based on PEDOT:PSS/graphene. Mater. Des. 2017, 135, 213–222. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Chitnis, R.S.; Ramkrishnan, R. Waterproof breathable active sports wear fabrics. Man-Made Text. India 2004, 5, 166–171. [Google Scholar]
- Yang, L.; Liu, H.; Ding, S.; Wu, J.; Zhang, Y.; Wang, Z.; Wei, L.; Tian, M.; Tao, G. Superabsorbent Fibers for Comfortable Disposable Medical Protective Clothing. Adv. Fiber Mater. 2020, 2, 140–149. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef] [Green Version]
- Attar, R.M.S.; Alshareef, M.; Snari, R.M.; Alaysuy, O.; Aldawsari, A.M.; Abu-Melha, S.; El-Metwaly, N.M. Development of novel photoluminescent fibers from recycled polyester waste using plasma-assisted dyeing toward ultraviolet sensing and protective textiles. J. Mater. Res. Technol. 2022, 21, 1630–1642. [Google Scholar] [CrossRef]
- Adeakin, O.A.S.; Popoola, A.V.; Ajekwene, K.K. Effect of Solvent Pretreatment of Polyester Fiber on its Dye-Uptake Based on the Concept of Solubility Parameters. Fibers Polym. 2022, 23, 3118–3125. [Google Scholar] [CrossRef]
- Soto, D.; Ugur, A.; Farnham, T.A.; Gleason, K.K.; Varanasi, K.K. Short-Fluorinated iCVD Coatings for Nonwetting Fabrics. Adv. Funct. Mater. 2018, 28, 1707355. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, C.H. Superhydrophobic Textiles: Review of Theoretical Definitions, Fabrication and Functional Evaluation. J. Eng. Fiber. Fabr. 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Xu, L.; Xie, K.; Liu, Y.; Zhang, C. Stable super-hydrophobic and comfort PDMS-coated polyester fabric. e-Polymers 2021, 21, 654–661. [Google Scholar] [CrossRef]
- Wang, D.M. Environmental protection in clothing industry. In Sustainable Development; World Scientific: Singapore, 2015; pp. 729–735. ISBN 978-981-4749-90-9. [Google Scholar]
- Rahmatinejad, J.; Khoddami, A.; Mazrouei-Sebdani, Z.; Avinc, O. Polyester hydrophobicity enhancement via UV-Ozone irradiation, chemical pre-treatment and fluorocarbon finishing combination. Prog. Org. Coatings 2016, 101, 51–58. [Google Scholar] [CrossRef]
- Ramesh, S.; Khan, S.; Park, Y.; Ford, E.; Menegatti, S.; Genzer, J. Self-healing and repair of fabrics: A comprehensive review of the application toolkit. Mater. Today 2022, 54, 90–109. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.-C.; Zhang, G.; Xu, L.; Sun, W.-J.; Zhong, G.-J.; Lei, J.; Yan, D.-X.; Li, Z.-M. Robustly Superhydrophobic Conductive Textile for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2019, 11, 1680–1688. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M.; Shahsavan, S. A new method to stabilize nanoparticles on textile surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 345, 202–210. [Google Scholar] [CrossRef]
- Chruściel, J.J. Modifications of Textile Materials with Functional Silanes, Liquid Silicone Softeners, and Silicone Rubbers—A Review. Polymers 2022, 14, 4382. [Google Scholar] [CrossRef]
- Liu, X.; Zou, X.; Ge, Z.; Zhang, W.; Luo, Y. Novel waterborne polyurethanes containing long-chain alkanes: Their synthesis and application to water repellency. RSC Adv. 2019, 9, 31357–31369. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Shi, K.; Ning, J.; Zheng, Z.; Yu, H.; Yang, Z.; Liu, J. Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric. Polymers 2021, 13, 2980. [Google Scholar] [CrossRef]
- Sfameni, S.; Hadhri, M.; Rando, G.; Drommi, D.; Rosace, G.; Trovato, V.; Plutino, M.R. Inorganic Finishing for Textile Fabrics: Recent Advances in Wear-Resistant, UV Protection and Antimicrobial Treatments. Inorganics 2023, 11, 19. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured surface finishing and coatings: Functional properties and applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Trovato, V.; Sfameni, S.; Rando, G.; Rosace, G.; Libertino, S.; Ferri, A.; Plutino, M.R. A Review of Stimuli-Responsive Smart Materials for Wearable Technology in Healthcare: Retrospective, Perspective, and Prospective. Molecules 2022, 27, 5709. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Marchetta, A.; Scolaro, C.; Cappello, S.; Urzì, C.; Visco, A.; Plutino, M.R. Development of Eco-Friendly Hydrophobic and Fouling-Release Coatings for Blue-Growth Environmental Applications: Synthesis, Mechanical Characterization and Biological Activity. Gels 2022, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Galletta, M.; Ielo, I.; Brucale, M.; De Leo, F.; Cardiano, P.; Cappello, S.; Visco, A.; Trovato, V.; et al. Design and Development of Fluorinated and Biocide-Free Sol–Gel Based Hybrid Functional Coatings for Anti-Biofouling/Foul-Release Activity. Gels 2022, 8, 538. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef]
- Sfameni, S.; Del Tedesco, A.; Rando, G.; Truant, F.; Visco, A.; Plutino, M.R. Waterborne Eco-Sustainable Sol–Gel Coatings Based on Phytic Acid Intercalated Graphene Oxide for Corrosion Protection of Metallic Surfaces. Int. J. Mol. Sci. 2022, 23, 12021. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol-gel treatment of textiles for the entrapping of an antioxidant/anti-inflammatory molecule: Functional coating morphological characterization and drug release evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 020016. [Google Scholar] [CrossRef]
- Youn, S.; Hee Park, C. Development of breathable Janus superhydrophobic polyester fabrics using alkaline hydrolysis and blade coating. Text. Res. J. 2018, 89, 959–974. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, F.; Periolatto, M. Modification of surface energy and wetting of textile fibers. In Wetting Wettability; IntechOpen: London, UK, 2015; pp. 139–168. [Google Scholar]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Regenerated cationic dyeable polyester deriving from poly(ethylene terephthalate) waste. Polym. Degrad. Stab. 2020, 179, 109261. [Google Scholar] [CrossRef]
- Kamel, M.M.; El Zawahry, M.M.; Helmy, H.; Eid, M.A. Improvements in the dyeability of polyester fabrics by atmospheric pressure oxygen plasma treatment. J. Text. Inst. 2011, 102, 220–231. [Google Scholar] [CrossRef]
- Trovato, V.; Mezzi, A.; Brucale, M.; Abdeh, H.; Drommi, D.; Rosace, G.; Plutino, M.R. Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors. Polymers 2022, 14, 2788. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sensors Actuators B 2017, 238, 281–291. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Ranjan, R.; Brittain, W.J. Surface initiated polymerizations from silica nanoparticles. Soft Matter 2006, 2, 386–396. [Google Scholar] [CrossRef]
- Koo, K.; Park, Y. Characteristics of double-layer coated fabrics with and without phase change materials and nano-particles. Fibers Polym. 2014, 15, 1641–1647. [Google Scholar] [CrossRef]
- Pan, H.; Wang, W.; Shen, Q.; Pan, Y.; Song, L.; Hu, Y.; Lu, Y. Fabrication of flame retardant coating on cotton fabric by alternate assembly of exfoliated layered double hydroxides and alginate. RSC Adv. 2016, 6, 111950–111958. [Google Scholar] [CrossRef]
- Kramar, A.D.; Obradović, B.M.; Vesel, A.; Kuraica, M.M.; Kostić, M.M. Preparation of Hydrophobic Viscose Fabric Using Nitrogen DBD and Copper Ions Sorption. Plasma Process. Polym. 2015, 12, 1095–1103. [Google Scholar] [CrossRef]
- Liu, Y.; Fuentes, C.A.; Willem Van Vuure, A.; Zhang, D.; Seveno, D. Wettability of a hybrid PTFE/Kevlar fabrics at micro-, meso-, and macroscales. Appl. Surf. Sci. 2022, 604, 154613. [Google Scholar] [CrossRef]
- Wang, Z.; Zuilhof, H. Antifouling Properties of Fluoropolymer Brushes toward Organic Polymers: The Influence of Composition, Thickness, Brush Architecture, and Annealing. Langmuir 2016, 32, 6571–6581. [Google Scholar] [CrossRef] [Green Version]
- Achagri, G.; Essamlali, Y.; Amadine, O.; Majdoub, M.; Chakir, A.; Zahouily, M. Surface modification of highly hydrophobic polyester fabric coated with octadecylamine-functionalized graphene nanosheets. RSC Adv. 2020, 10, 24941–24950. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.R.; Yashomala, M.A.D.H.; Elkaduwe, R.K.W.H.M.K.; Karunaratne, D.G.G.P.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G.; Manipura, A.; Mantilaka, M.M.M.G.P.G. Fabrication of water-repellent polyester textile via dip-coating of in-situ surface-modified superhydrophobic calcium carbonate from dolomite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127397. [Google Scholar] [CrossRef]
- Mousaa, I.M.; Elhady, M.A.; El-Sayyad, G.S.; Attia, R.M. Development of a highly hydrophobic and antimicrobial surface for polyester fabrics treated with (vinyl acetate versatic ester/paraffin wax) blend containing sodium chloride using electron beam irradiation. Prog. Org. Coatings 2023, 174, 107230. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, W.; Ma, T.; Zhang, C.; Kuang, J.; Wang, R. Anti-UV and hydrophobic dual-functional coating fabrication for flame retardant polyester fabrics by surface-initiated PET RAFT technique. Eur. Polym. J. 2022, 173, 111275. [Google Scholar] [CrossRef]
- Narakaew, S.; Au-Pree, S.; Baison, W.; Thungprasert, S.; Wattalo, I.; Jaipor, P.; Promanan, T.; Sukdee, S.; Santakij, P.; Chanogkun, C.; et al. The finished polyester fabric with hot NH4OH pretreatment and mixed ZnO-Zn(OH)2 nanoparticles for hydrophobic property. J. Met. Mater. Miner. 2022, 32, 109–117. [Google Scholar] [CrossRef]
- Katiyar, P.; Mishra, S.; Srivastava, A.; Prasad, N.E. Preparation of TiO2–SiO2 Hybrid Nanosols Coated Flame-Retardant Polyester Fabric Possessing Dual Contradictory Characteristics of Superhydrophobicity and Self Cleaning Ability. J. Nanosci. Nanotechnol. 2020, 20, 1780–1789. [Google Scholar] [CrossRef]
- Yun, C.; Islam, M.I.; LeHew, M.; Kim, J. Assessment of environmental and economic impacts made by the reduced laundering of self-cleaning fabrics. Fibers Polym. 2016, 17, 1296–1304. [Google Scholar] [CrossRef]
- Ren, G.; Song, Y.; Li, X.; Wang, B.; Zhou, Y.; Wang, Y.; Ge, B.; Zhu, X. A simple way to an ultra-robust superhydrophobic fabric with mechanical stability, UV durability, and UV shielding property. J. Colloid Interface Sci. 2018, 522, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, M.; Zong, Y.; Li, Z. Modification of fabric via co-grafted with fluorine-free carbene polymer and its hydrophobicity. Polymer 2022, 247, 124802. [Google Scholar] [CrossRef]
- Guo, F.; Fang, K.; Song, Y.; Fu, R.; Li, H.; Zhang, C. Optimizing a fabricating program for wearable super-hydrophobic cotton by clean production technology of plasma and reducing chemical consumption. J. Clean. Prod. 2020, 276, 124233. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Fluorocarbon nano-coating of polyester fabrics by atmospheric air plasma with aerosol. Appl. Surf. Sci. 2008, 254, 3902–3908. [Google Scholar] [CrossRef]
- Brzeziński, S.; Kowalczyk, D.; Borak, B.; Jasiorski, M.; Tracz, A. Applying the sol-gel method to the deposition of nanocoats on textiles to improve their abrasion resistance. J. Appl. Polym. Sci. 2012, 125, 3058–3067. [Google Scholar] [CrossRef]
- Dhanapal, V.K.; Dhanakodi, R. Influence of Moisture Management Properties on Socks Made from Recycled Polyester, Virgin Cotton and its Blends. Fibres Text. East. Eur. 2020, 4, 76–81. [Google Scholar]
- Belliveau, R.G.; DeJong, S.A.; Boltin, N.D.; Lu, Z.; Cassidy, B.M.; Morgan, S.L.; Myrick, M.L. Mid-infrared emissivity of nylon, cotton, acrylic, and polyester fabrics as a function of moisture content. Text. Res. J. 2019, 90, 1431–1445. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Boskhomodgiev, A.P.; Iordanskii, A.L.; Bonartseva, G.A.; Rebrov, A.V.; Makhina, T.K.; Myshkina, V.L.; Yakovlev, S.A.; Filatova, E.A.; Ivanov, E.A.; et al. Hydrolytic Degradation of Poly(3-hydroxybutyrate), Polylactide and their Derivatives: Kinetics, Crystallinity, and Surface Morphology. Mol. Cryst. Liq. Cryst. 2012, 556, 288–300. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions. J. Appl. Polym. Sci. 2015, 132, 42189. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef]
- Zhu, F.L.; Feng, Q.Q. Recent advances in textile materials for personal radiative thermal management in indoor and outdoor environments. Int. J. Therm. Sci. 2021, 165, 106899. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 53001. [Google Scholar] [CrossRef]
- Ullah, H.M.K.; Lejeune, J.; Cayla, A.; Monceaux, M.; Campagne, C.; Devaux, É. A review of noteworthy/major innovations in wearable clothing for thermal and moisture management from material to fabric structure. Text. Res. J. 2021, 92, 3351–3386. [Google Scholar] [CrossRef]



| Sample Code | Aqueous Solution Repellency Grade Number | Composition (by Volume) | Surface Tensions (Dynes/cm at 25 °C) |
|---|---|---|---|
| PL_G | 2 | 95:5/Water:isopropyl alcohol | 50.0 |
| PL_G_C2_ C2 | 3 | 90:10/Water:isopropyl alcohol | 42.0 |
| PL_G_C8_ C8 | 3 | 90:10/Water:isopropyl alcohol | 42.0 |
| PL_G_C16_ C16 | 4 | 80:20/Water:isopropyl alcohol | 33.0 |
| PL_G_C2_ C16 | 3 | 90:10/Water:isopropyl alcohol | 42.0 |
| PL_G_C8_ C16 | 3 | 90:10/Water:isopropyl alcohol | 42.0 |
| Coating System | Coated Fabric | Deposition Method | WCA/° | Ref. |
|---|---|---|---|---|
| Organophilic graphene nanosheets | Knit polyester | Dip-coating | 148 | [47] |
| Superhydrophobic precipitated calcium carbonate | 89% polyester: 11% elasthane | Dip-coating | 150.2 | [48] |
| Vinyl acetate versatic ester/paraffin wax and sodium chloride | Polyester | Electron beam irradiation | 111 | [49] |
| Poly(glycidyl methacrylate) and benzotriazole | PET-co-CEPPA | Surface-initiated PET RAFT 1 | 132 | [50] |
| Ammonium hydroxide and ZnO-Zn(OH)2 nanoparticles | Polyester | Dip-coating | 138 | [51] |
| TTIP, TEOS and HDTMS | Polyester | Padding | 145 | [52] |
| GPTMS, C2, C8 and C16 | Polyester | Dip-pad-dry-cure | 150.2 | [This work] |
| Sample Name | Wettability Level | Water Features |
|---|---|---|
| PL_G | 50 (ISO 1) | Complete wetting of the entire specimen face |
| PL_G_C2_C2 | 50 (ISO 1) | Complete wetting of the entire specimen face |
| PL_G_C8_C8 | 100 (ISO 5) | No wetting of the specimen face |
| PL_G_C16_C16 | 100 (ISO 5) | No wetting of the specimen face |
| PL_G_C2_C16 | 100 (ISO 5) | No wetting of the specimen face |
| PL_G_C8_C16 | 100 (ISO 5) | No wetting of the specimen face |
| Sample Code | Weight (g) | Drying Temperature (°C) | Drying Time (s) 1 | Humidity (%) |
|---|---|---|---|---|
| PL | 1.189 | 130 | 54 | 1.26 |
| PL_G | 1.571 | 130 | 51 | 0.76 |
| PL_G_C2_C2 | 1.645 | 130 | 52 | 1.03 |
| PL_G_C8_C8 | 1.609 | 130 | 51 | 1.06 |
| PL_G_C16_C16 | 1.693 | 130 | 51 | 1.00 |
| PL_G_C2_C16 | 1.564 | 130 | 50 | 1.02 |
| PL_G_C8_C16 | 1.545 | 130 | 48 | 0.97 |
| Sample Code | First Deposition | Second Deposition | Total Add on wt. % (A) | Sample Appearance |
|---|---|---|---|---|
| PL_G | G | – | 0.9 |  |
| PL_G_C2_C2 | G and C2 | C2 | 2.16 |  |
| PL_G_C8_C8 | G and C8 | C8 | 5.7 |  |
| PL_G_C16_C16 | G and C16 | C16 | 11.8 |  |
| PL_G_C2_C16 | G and C2 | C16 | 2.6 |  |
| PL_G_C8_C16 | G and C8 | C16 | 4.4 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings. Gels 2023, 9, 109. https://doi.org/10.3390/gels9020109
Sfameni S, Lawnick T, Rando G, Visco A, Textor T, Plutino MR. Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings. Gels. 2023; 9(2):109. https://doi.org/10.3390/gels9020109
Chicago/Turabian StyleSfameni, Silvia, Tim Lawnick, Giulia Rando, Annamaria Visco, Torsten Textor, and Maria Rosaria Plutino. 2023. "Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings" Gels 9, no. 2: 109. https://doi.org/10.3390/gels9020109
APA StyleSfameni, S., Lawnick, T., Rando, G., Visco, A., Textor, T., & Plutino, M. R. (2023). Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings. Gels, 9(2), 109. https://doi.org/10.3390/gels9020109











