In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Silico Molecular Docking Study
2.2. In Vitro Studies
2.2.1. Antioxidant DPPH Free Radical Scavenging Assay
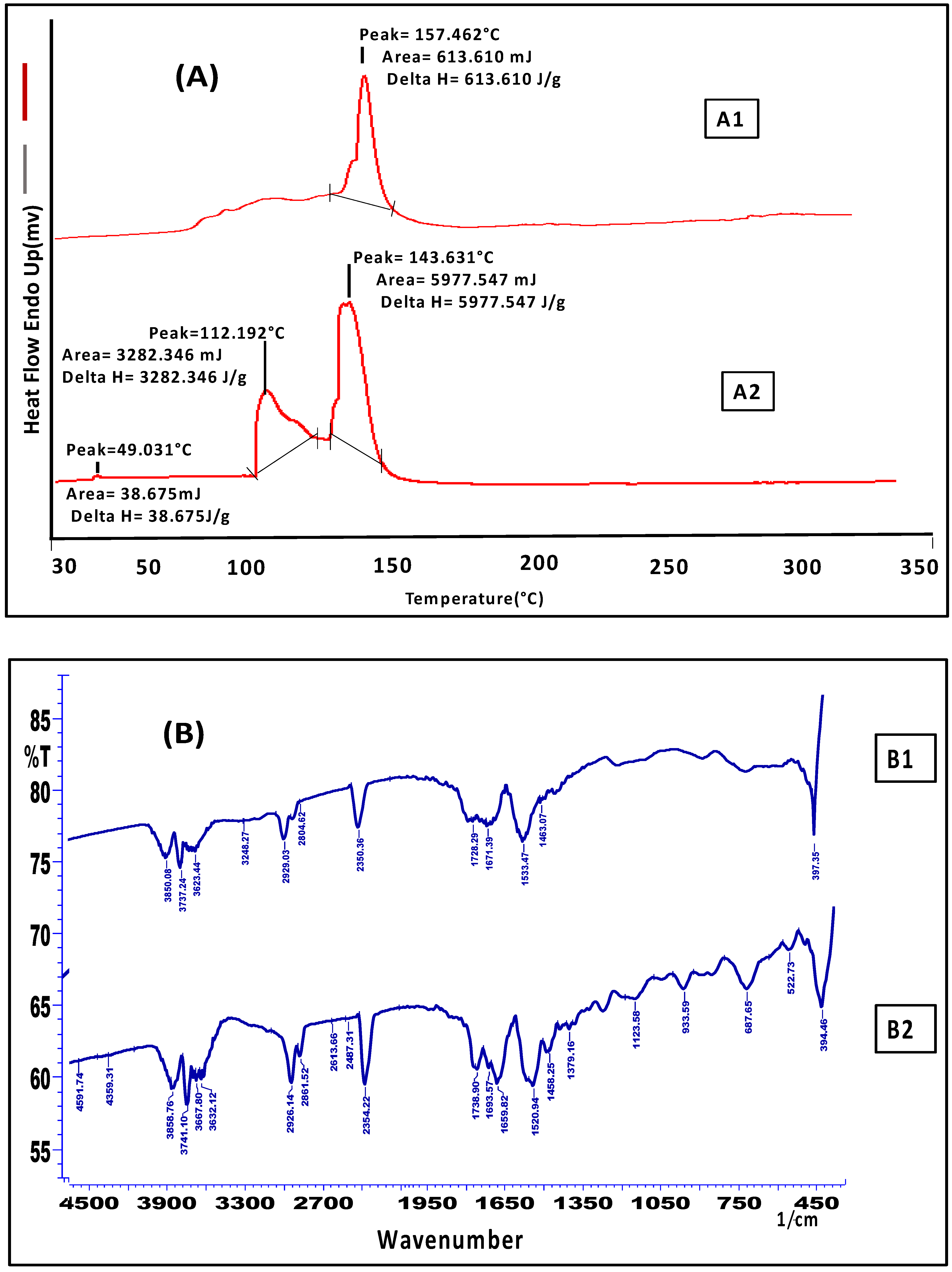
2.2.2. Skin Interaction Study of the Gel Formulation
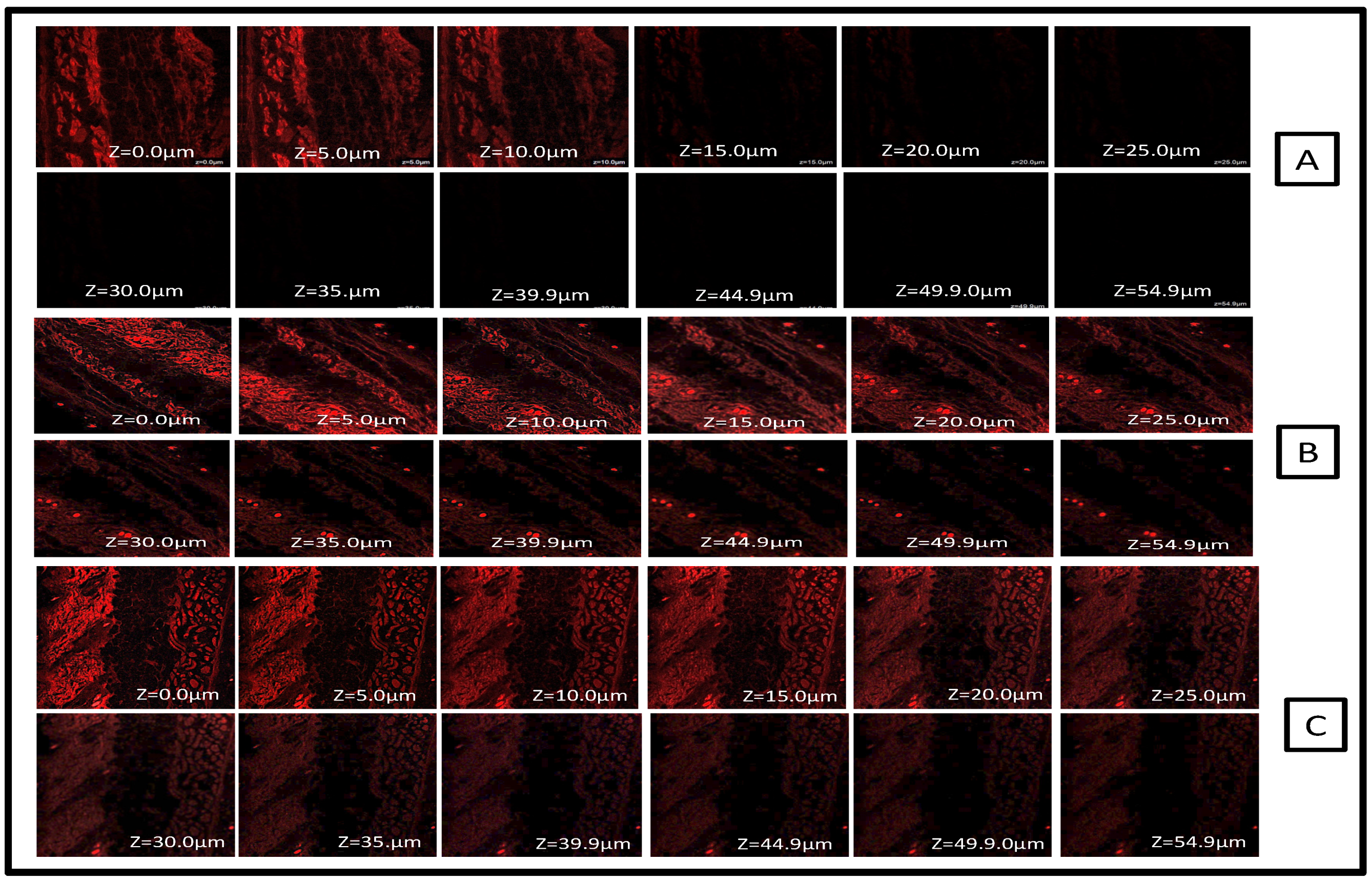
2.2.3. CLSM Study for Tissue Uptake
2.3. In Vivo Animal Study
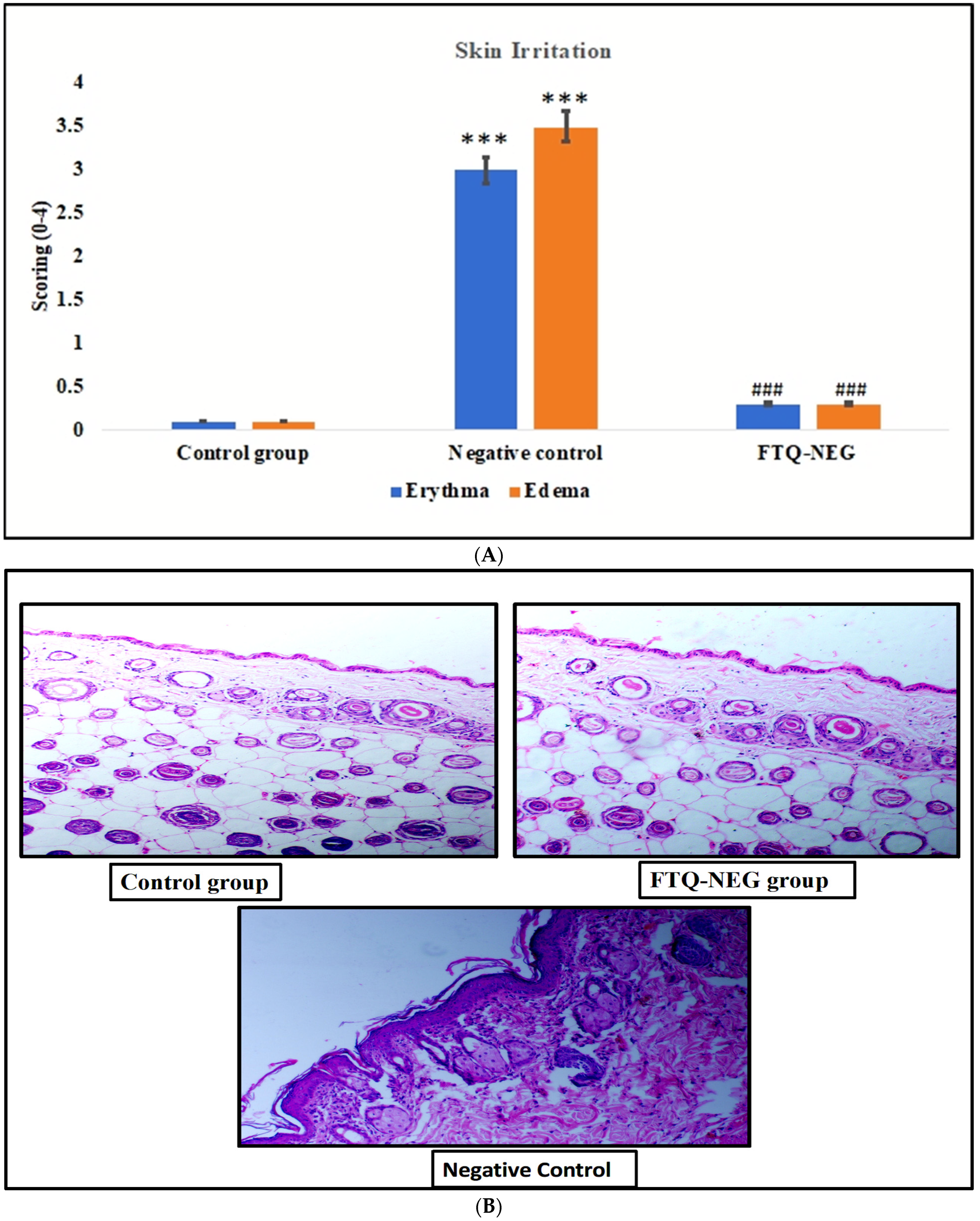
2.3.1. Skin Irritation Study
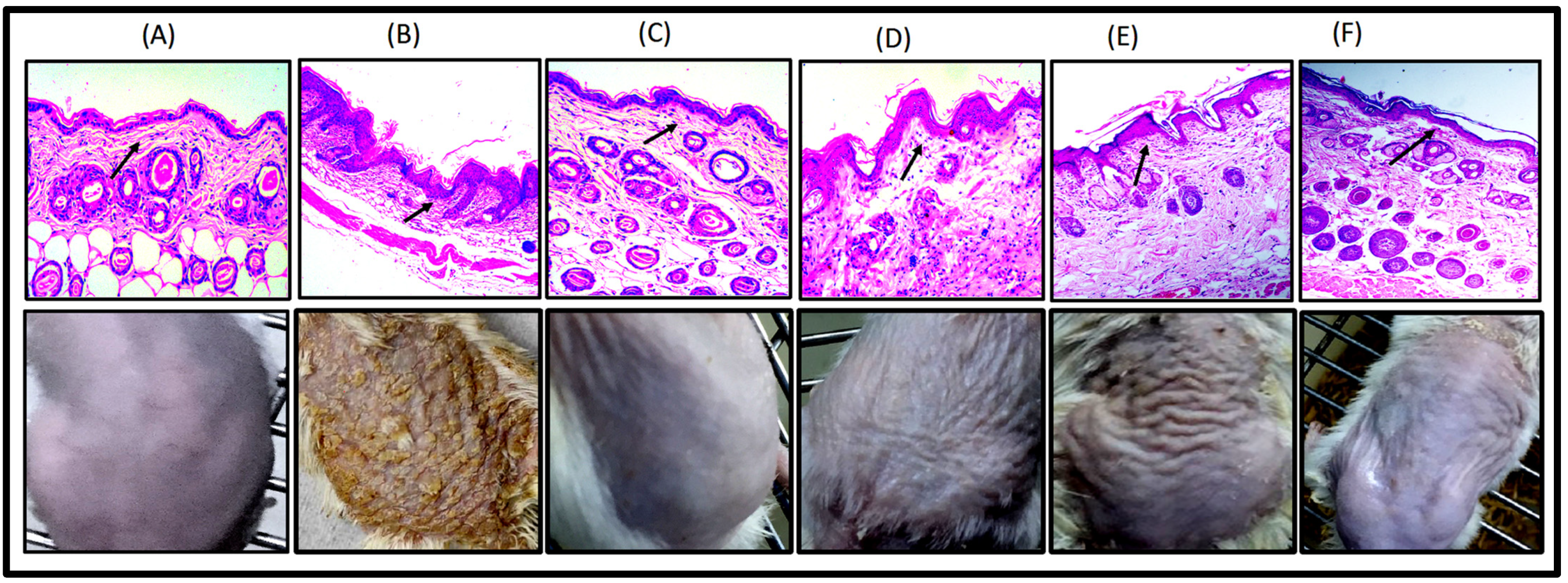
2.3.2. Imiquimod- (IMQ) Induced Psoriatic Plaque-Like Model
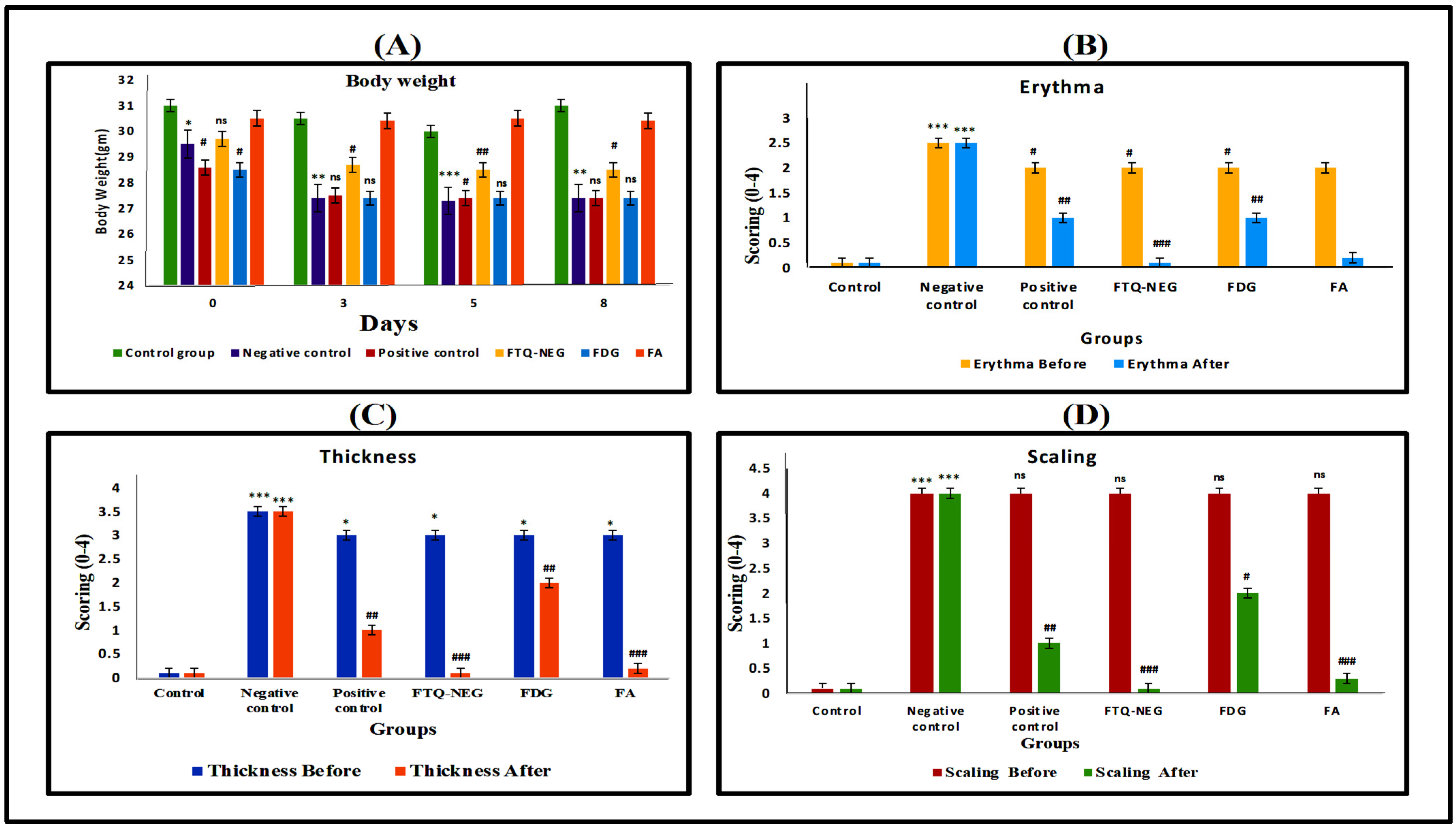
2.3.3. Ear Thickness and Inflammation Measurement via PASI (Psoriasis Area Severity Index) Scoring
Bodyweight and PASI Scoring
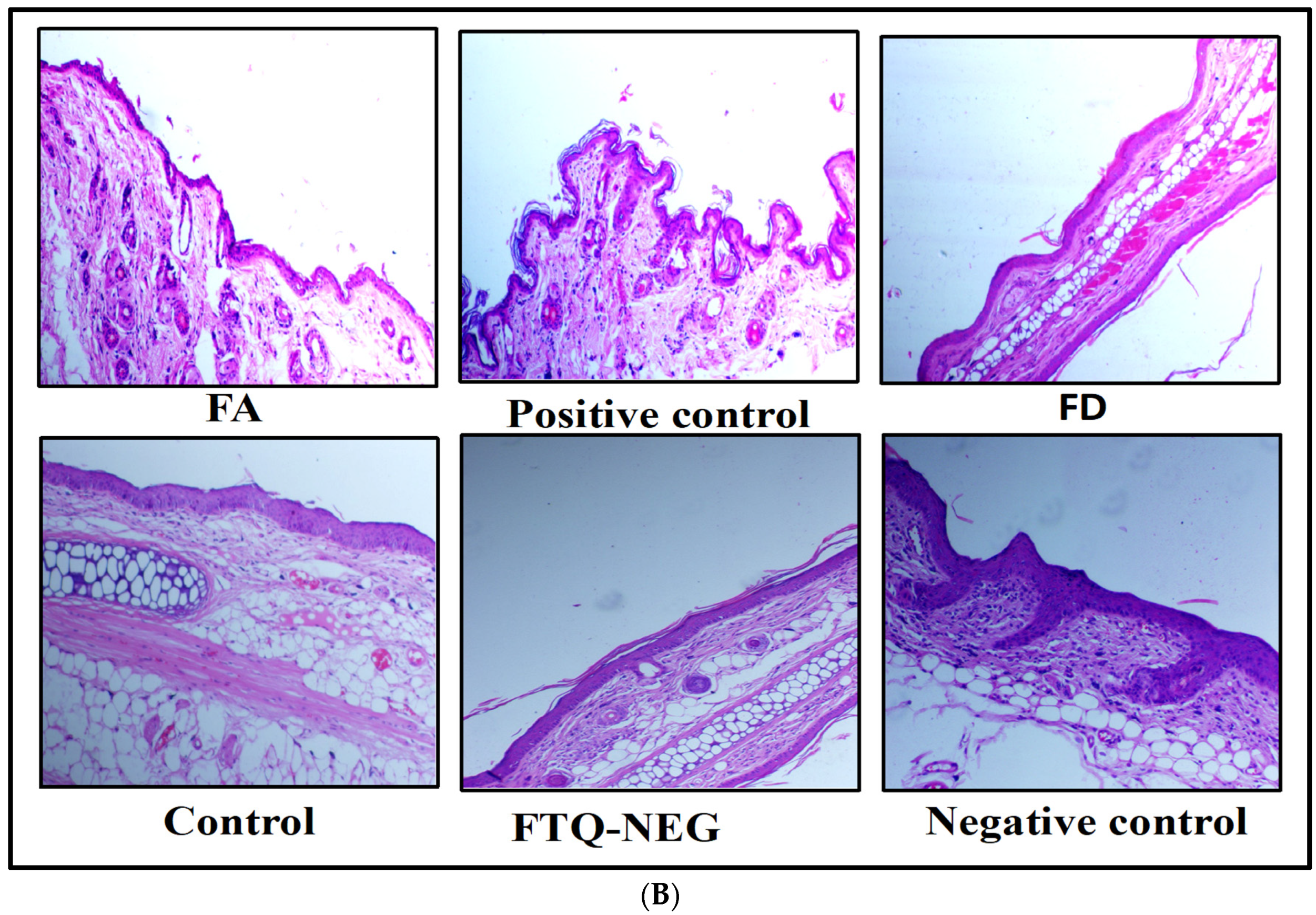
2.3.4. Spleen Weight and Histopathology
2.3.5. Immunohistochemistry Staining (IHC) of TNF-α and IL-6 in Skin and Spleen Tissue
Skin Tissue
Spleen Tissue
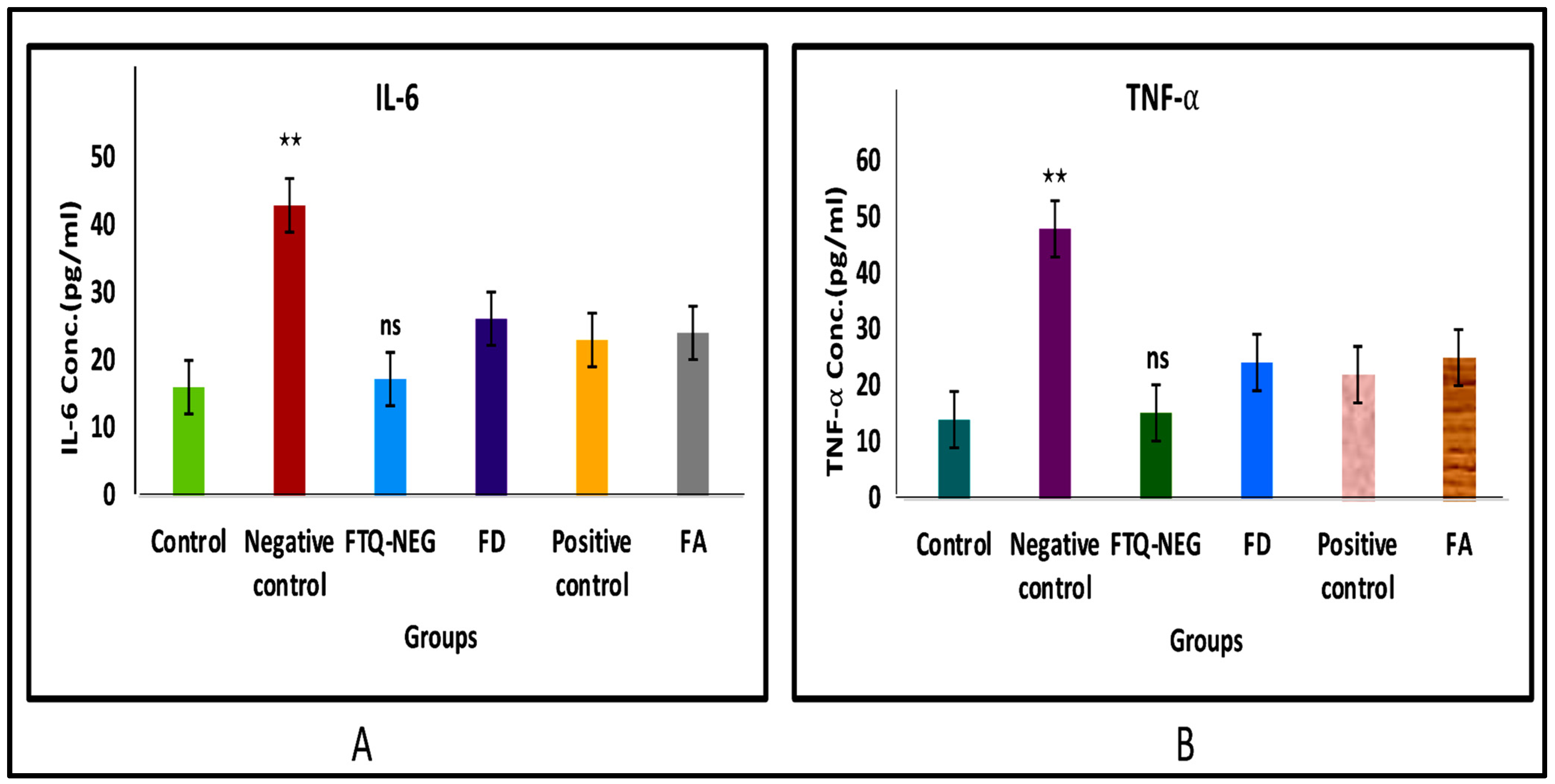
2.3.6. Analysis of Cytokine Levels in Skin Homogenate
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Methods
4.2.1. In-Silico Molecular Docking Study
4.2.2. In Vitro Studies
Antioxidant DPPH Free Radical Scavenging Assay
Skin Interaction Study of the Gel Formulation
CLSM Study for Tissue Uptake
4.2.3. In Vivo Studies
Skin Irritation Study
Imiquimod- (IMQ) Induced Psoriatic Plaque-Like Model
Ear Thickness, Body Weight, and Inflammation Measurements via PASI (Psoriasis Area Severity Index) Scoring
Spleen Weight and Histopathology
Immunohistochemistry Staining (IHC) of TNF-α and IL-6 in Skin and Spleen Tissue
Analysis of Cytokine Levels in Skin Homogenate
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffin, K.C.; Chandran, V.; Gladman, D.D.; Krueger, G.G.; Elder, J.T.; Rahman, P. Genetics of Psoriasis and Psoriatic Arthritis: Update and Future Direction. J. Rheumatol. 2008, 35, 1449–1453. [Google Scholar] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and Classification of Psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.M.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of Care for the Management of Psoriasis and Psoriatic Arthritis. J. Am. Acad. Dermatol. 2009, 60, 4. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K. Depression and Suicidal Ideation in Dermatology Patients with Acne, Alopecia Areata, Atopic Dermatitis and Psoriasis. Br. J. Dermatol. 1998, 139, 846–850. [Google Scholar] [CrossRef]
- Palamara, F.; Meindl, S.; Holcmann, M.; Lührs, P.; Stingl, G.; Sibilia, M. Identification and Characterization of PDC-Like Cells in Normal Mouse Skin and Melanomas Treated with Imiquimod. J. Immunol. 2004, 173, 3051–3061. [Google Scholar] [CrossRef] [Green Version]
- Bailey, E.E.; Ference, E.H.; Alikhan, A.; Hession, M.T.; Armstrong, A.W. Combination Treatments for Psoriasis: A Systematic Review and Meta-Analysis. Arch. Dermatol. 2012, 148, 511–522. [Google Scholar]
- Basavaraj, K.H.; Navya, M.A.; Rashmi, R. Stress and Quality of Life in Psoriasis: An Update. Int. J. Dermatol. 2011, 50, 783–792. [Google Scholar] [CrossRef]
- Levy, A.R.; Davie, A.M.; Brazier, N.C.; Jivraj, F.; Albrecht, L.E.; Gratton, D.; Lynde, C.W. Economic Burden of Moderate to Severe Plaque Psoriasis in Canada. Int. J. Dermatol. 2012, 51, 1432–1440. [Google Scholar] [CrossRef]
- Nast, A.; Mrowietz, U.; Kragballe, K.; Puig, L.; Reich, K.; Warren, R.B.; Schmitt, J. National and Multinational Guidelines in Europe: Results from an Online Survey on Awareness of Different National and European Psoriasis Guidelines. Arch. Dermatol. Res. 2013, 305, 637–643. [Google Scholar] [CrossRef]
- Nakamizo, Y. Topical Treatment of Psoriasis. Nishinihon J. Dermatol. 1977, 39, 855–859. [Google Scholar] [CrossRef]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by Design Based Risperidone Nano Soft Lipid Vesicle as a New Strategy for Enhanced Transdermal Drug Delivery: In-Vitro Characterization, and in-Vivo Appraisal. Mater. Sci. Eng. C 2017, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere Mediated Topical Delivery of Thymoquinone in the Treatment of Psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2251–2262. [Google Scholar] [CrossRef]
- Löbenberg, R.; Amidon, G.L. Modern Bioavailability, Bioequivalence and Biopharmaceutics Classification System. New Scientific Approaches to International Regulatory Standards. Eur. J. Pharm. Biopharm. 2000, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro Inhibitor, Isolated from the Edible Brown Algae Ecklonia Cava. Bioorganic Med. Chem. 2013, 21, 3730–3733. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous Solubility and Degradation Kinetics of the Phytochemical Anticancer Thymoquinone; Probing the Effects of Solvents, PH and Light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [Green Version]
- Gandy, J.J.; Snyman, J.R.; van Rensburg, C.E.J. Randomized, Parallel-Group, Double-Blind, Controlled Study to Evaluate the Efficacy and Safety of Carbohydrate-Derived Fulvic Acid in Topical Treatment of Eczema. Clin. Cosmet. Investig. Dermatol. 2011, 2011, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin Loaded Chitosan Polymer Based Nanoemulsion Gel: In Vitro, Ex Vivo Evaluation and in Vivo Wound Healing Studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Wilson, E.; Rajamanickam, G.V.; Dubey, G.P.; Klose, P.; Musial, F.; Saha, F.J.; Rampp, T.; Michalsen, A.; Dobos, G.J. Review on Shilajit Used in Traditional Indian Medicine. J. Ethnopharmacol. 2011, 136, 1–9. [Google Scholar] [CrossRef]
- Todd, W.J. Urban and Regional Land Use Change Detected by Using Landsat Data. J. Res. US Geol. Surv. 1977, 5, 529–534. [Google Scholar]
- Junek, R.; Morrow, R.; Schoenherr, J.I.; Schubert, R.; Kallmeyer, R.; Phull, S.; Klöcking, R. Bimodal Effect of Humic Acids on the LPS-Induced TNF-α Release from Differentiated U937 Cells. Phytomedicine 2009, 16, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Gnananath, K.; Nataraj, K.S.; Rao, B.G.; Kumar, K.P.; Mahnashi, M.H.; Anwer, M.K.; Umar, A.; Iqbal, Z.; Mirza, M.A. Exploration of Fulvic Acid as a Functional Excipient in Line with the Regulatory Requirement. Environ. Res. 2020, 187, 109642. [Google Scholar] [CrossRef] [PubMed]
- Sabi, R.; Very, P.; Van Rensburg, C.E.J. Carbohydrate-Derived Fulvic Acid (CHD-FA) Inhibits Carrageenan-Induced Inflammation and Enhances Wound Healing: Efficacy and Toxicity Study in Rats. Drug Dev. Res. 2012, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Khan, F.; Farooqui, A.; Ansari, I.A. Andrographolide Exhibits Anticancer Potential Against Human Colon Cancer Cells by Inducing Cell Cycle Arrest and Programmed Cell Death via Augmentation of Intracellular Reactive Oxygen Species Level. Nutr. Cancer 2018, 70, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel Colloidal Carriers for Psoriasis: Current Issues, Mechanistic Insight and Novel Delivery Approaches. J. Control. Release 2013, 170, 385–395. [Google Scholar] [CrossRef]
- Khandavilli, S.; Panchagnula, R. Nanoemulsions as Versatile Formulations for Paclitaxel Delivery: Peroral and Dermal Delivery Studies in Rats. J. Investig. Dermatol. 2007, 127, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.P.; Scarrone, A.L.; Santos, M.; Pohlmann, A.R.; Guterres, S.S. Human Skin Penetration and Distribution of Nimesulide from Hydrophilic Gels Containing Nanocarriers. Int. J. Pharm. 2007, 341, 215–220. [Google Scholar] [CrossRef]
- Karan, S.; Choudhury, H.; Chakra, B.K.; Chatterjee, T.K. Polymeric Microsphere Formulation for Colon Targeted Delivery of 5-Fluorouracil Using Biocompatible Natural Gum Katira. Asian Pac. J. Cancer Prev. 2019, 20, 2181–2194. [Google Scholar] [CrossRef]
- Park, J.B.; Prodduturi, S.; Morott, J.; Kulkarni, V.I.; Jacob, M.R.; Khan, S.I.; Stodghill, S.P.; Repka, M.A. Development of an Antifungal Denture Adhesive Film for Oral Candidiasis Utilizing Hot Melt Extrusion Technology. Expert Opin. Drug Deliv. 2015, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Mirza, M.A.; Aqil, M.; Hassan, N.; Zakir, F.; Ansari, M.J.; Iqbal, Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels 2022, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bast, F. In Silico Molecular Docking Study of Natural Compounds on Wild and Mutated Epidermal Growth Factor Receptor. Med. Chem. Res. 2014, 23, 5047–5085. [Google Scholar] [CrossRef]
- Kchaou, W.; Abbès, F.; Attia, H.; Besbes, S. In Vitro Antioxidant Activities of Three Selected Dates from Tunisia (Phoenix dactylifera L.). J. Chem. 2014, 2014, 367681. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Ahmed, S.; Nikita, N.; Moon, G.; Aqil, M.; Sultana, Y. Chemical Engineering of a Lipid Nano-Scaffold for the Solubility Enhancement of an Antihyperlipidaemic Drug, Simvastatin; Preparation, Optimization, Physicochemical Characterization and Pharmacodynamic Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1908–1919. [Google Scholar] [CrossRef] [Green Version]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of Transethosomes Formulation for Dermal Fisetin Delivery: Box–Behnken Design, Optimization, in Vitro Skin Penetration, Vesicles–Skin Interaction and Dermatokinetic Studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Weil, C.S.; Scala, R.A. Study of Intra- and Interlaboratory Variability in the Results of Rabbit Eye and Skin Irritation Tests. Toxicol. Appl. Pharmacol. 1971, 19, 276–360. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.I.; Khan, D.; Rehman, A.U.; Elaissari, A.; Ahmed, N. Development and in Vitro/in Vivo Evaluation of Ph-Sensitive Polymeric Nanoparticles Loaded Hydrogel for the Management of Psoriasis. Nanomaterials 2021, 11, 3433. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Naseem, F.; Farid, A.; Rather, I.A.; Hakeem, K.R. Olive Oil Based Methotrexate Loaded Topical Nanoemulsion Gel for the Treatment of Imiquimod Induced Psoriasis-like Skin Inflammation in an Animal Model. Biology 2021, 10, 1121. [Google Scholar] [CrossRef]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and Curcumin Co-Loaded Liposphere Gel: Synergistic Combination towards Management of Psoriasis. J. Control. Release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Sahu, S.; Katiyar, S.S.; Kushwah, V.; Jain, S. Active Natural Oil-Based Nanoemulsion Containing Tacrolimus for Synergistic Antipsoriatic Efficacy. Nanomedicine 2018, 13, 1985–1998. [Google Scholar] [CrossRef]
- Reis, M.M.; Higuchi, M.D.L.; Benvenuti, L.A.; Aiello, V.D.; Gutierrez, P.S.; Bellotti, G.; Pileggi, F. An in Situ Quantitative Immunohistochemical Study of Cytokines and IL-2R+ in Chronic Human Chagasic Myocarditis: Correlation with the Presence of Myocardial Trypanosoma Cruzi Antigens. Clin. Immunol. Immunopathol. 1997, 83, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion Loaded Gel for Topical Co-Delivery of Clobitasol Propionate and Calcipotriol in Psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1473–1482. [Google Scholar] [CrossRef]
- Flint, M.S.; Dearman, R.J.; Kimber, I.; Hotchkiss, S.A.M. Production and in Situ Localization of Cutaneous Tumour Necrosis Factor α (TNF-α) and Interleukin 6 (IL-6) Following Skin Sensitization. Cytokine 1998, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Singh, M.; Sulaiman, S.; Jain, P.; Sharma, K.; Nandy, S.; Dudeja, M.; Ali, A.; Iqbal, Z. Molecular Docking-Guided Ungual Drug-Delivery Design for Amelioration of Onychomycosis. ACS Omega 2019, 4, 9583–9592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, C.; Upadhyay, N.K.; Sharma, A.; Lal, U.R.; Raza, K.; Negi, P. Phospholipid Nanoformulation of Thymoquinone with Enhanced Bioavailability: Development, Characterization and Anti-Inflammatory Activity. J. Drug Deliv. Sci. Technol. 2019, 52, 316–324. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin Loaded Binary Ethosomes for Management of Skin Cancer by Dermal Application on UV Exposed Mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Khatoon, K.; Ali, A.; Ahmad, F.J.; Hafeez, Z.; Rizvi, M.M.A.; Akhter, S.; Beg, S. Novel Nanoemulsion Gel Containing Triple Natural Bio-Actives Combination of Curcumin, Thymoquinone, and Resveratrol Improves Psoriasis Therapy: In Vitro and in Vivo Studies. Drug Deliv. Transl. Res. 2021, 11, 1245–1260. [Google Scholar] [CrossRef]
- Ali, A.; Ali, S.; Aqil, M.; Imam, S.S.; Ahad, A.; Qadir, A. Thymoquinone Loaded Dermal Lipid Nano Particles: Box Behnken Design Optimization to Preclinical Psoriasis Assessment. J. Drug Deliv. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Boakye, C.H.A.; Patel, K.; Doddapaneni, R.; Bagde, A.; Marepally, S.; Singh, M. Novel Amphiphilic Lipid Augments the Co-Delivery of Erlotinib and IL36 SiRNA into the Skin for Psoriasis Treatment. J. Control. Release 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safak, A.A.; Simsek, E.; Bahcebasi, T. Sonographic Assessment of the Normal Limits and Percentile Curves of Liver, Spleen, and Kidney Dimensions in Healthy School-Aged Children. J. Ultrasound Med. 2005. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. In Methods in Molecular Biology; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]





| Protein (PDB:ID) | Compound Name (PubChem ID) | Docking Score (kcal/mol.) | Hydrogen Bonding Interactions | Distance of Hydrogen Bond (Å) | Hydrophobic Interactions |
|---|---|---|---|---|---|
| IL-6 (4CNI) | TQ(10281) | −4.4 | Leu11 N…O2 | 3.12 | Pro154 (alkyl), Pro156 and Pro209 (pi–alkyl) |
| FA(5359407) | −6.0 | Val118 O…H24 Thr93 OG1…O23 Ala175 O…H26 Tyr183 OH…O2 | 2.93 2.91 2.12 3.08 | Pro41 and Leu177 (pi–alkyl) | |
| TNF-α (6RMJ) | TQ(10281) | −4.8 | Ala145 NH…O2 Phe144 NH…O2 | 2.67 2.22 | Phe144 (alkyl, pi–pi, T-shaped), Leu142 (pi–alkyl) |
| FA(5359407) | −6.4 | Asp143 OD1…H20 Asp143 HA…O19 Phe144 NH…O19 Asp140 HA…O25 | 2.41 2.84 2.14 3.05 | Phe144 (pi–pi, T-shaped) |
| S. No | Groups | Category | No. of Mice | Duration/Days |
|---|---|---|---|---|
| 1 | Group-1 | Control group (No treatment) | 5 | 8 |
| 2 | Group-2 | Negative control (Disease induced) | 5 | 8 |
| 3 | Group-3 | Positive control (Tenovate M cream; 40 mg/cm2 marketed formulation) | 5 | 8 |
| 4 | Group-4 | Free drug (FD) TQ in gel (50 mg/cm2) | 5 | 8 |
| 5 | Group-5 | FTQ-NEG (50 mg/cm2) | 5 | 8 |
| 6 | Group-6 | FA in gel (100 mg/cm2) | 5 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Mirza, M.A.; Aqil, M.; Alex, T.S.; Raj, N.; Manzoor, N.; Naseef, P.P.; Saheer Kuruniyan, M.; Iqbal, Z. In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis. Gels 2023, 9, 112. https://doi.org/10.3390/gels9020112
Khan R, Mirza MA, Aqil M, Alex TS, Raj N, Manzoor N, Naseef PP, Saheer Kuruniyan M, Iqbal Z. In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis. Gels. 2023; 9(2):112. https://doi.org/10.3390/gels9020112
Chicago/Turabian StyleKhan, Rahmuddin, Mohd. Aamir Mirza, Mohd Aqil, Thomson Santosh Alex, Nafis Raj, Nikhat Manzoor, Punnoth Poonkuzhi Naseef, Mohamed Saheer Kuruniyan, and Zeenat Iqbal. 2023. "In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis" Gels 9, no. 2: 112. https://doi.org/10.3390/gels9020112
APA StyleKhan, R., Mirza, M. A., Aqil, M., Alex, T. S., Raj, N., Manzoor, N., Naseef, P. P., Saheer Kuruniyan, M., & Iqbal, Z. (2023). In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis. Gels, 9(2), 112. https://doi.org/10.3390/gels9020112






