The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review
Abstract
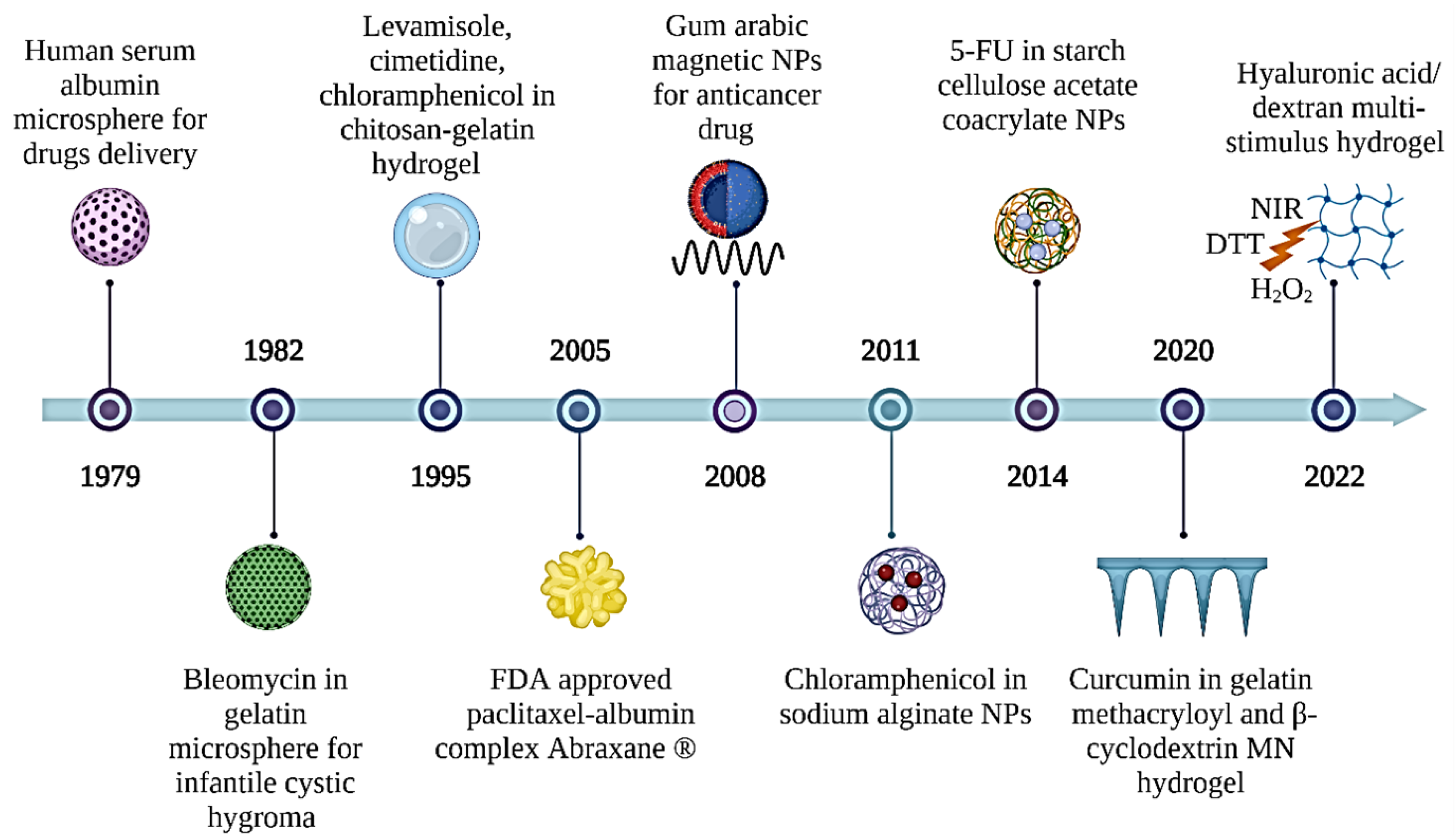
:1. Introduction
2. Applications of Natural Polymers in DDS
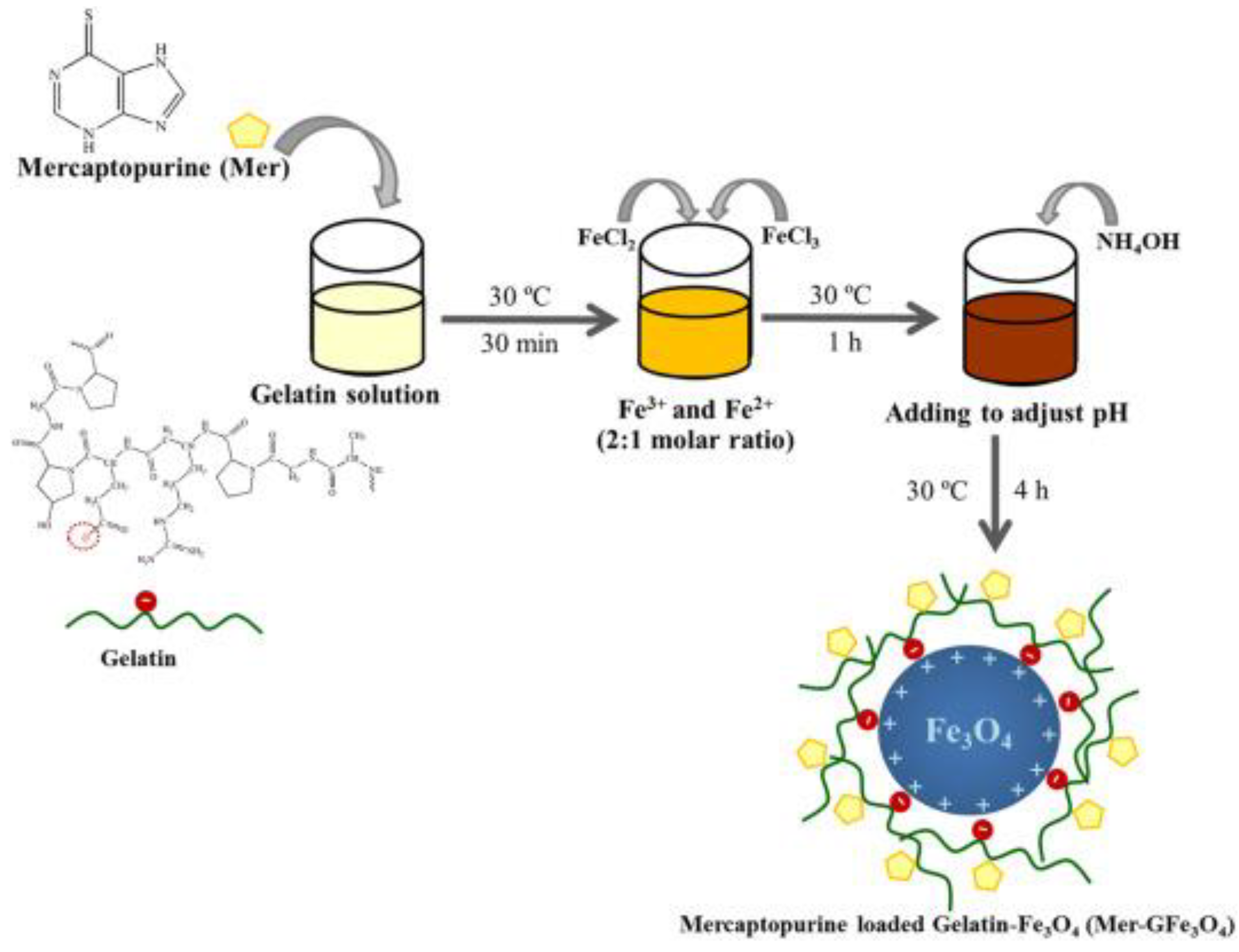
2.1. Gelatin
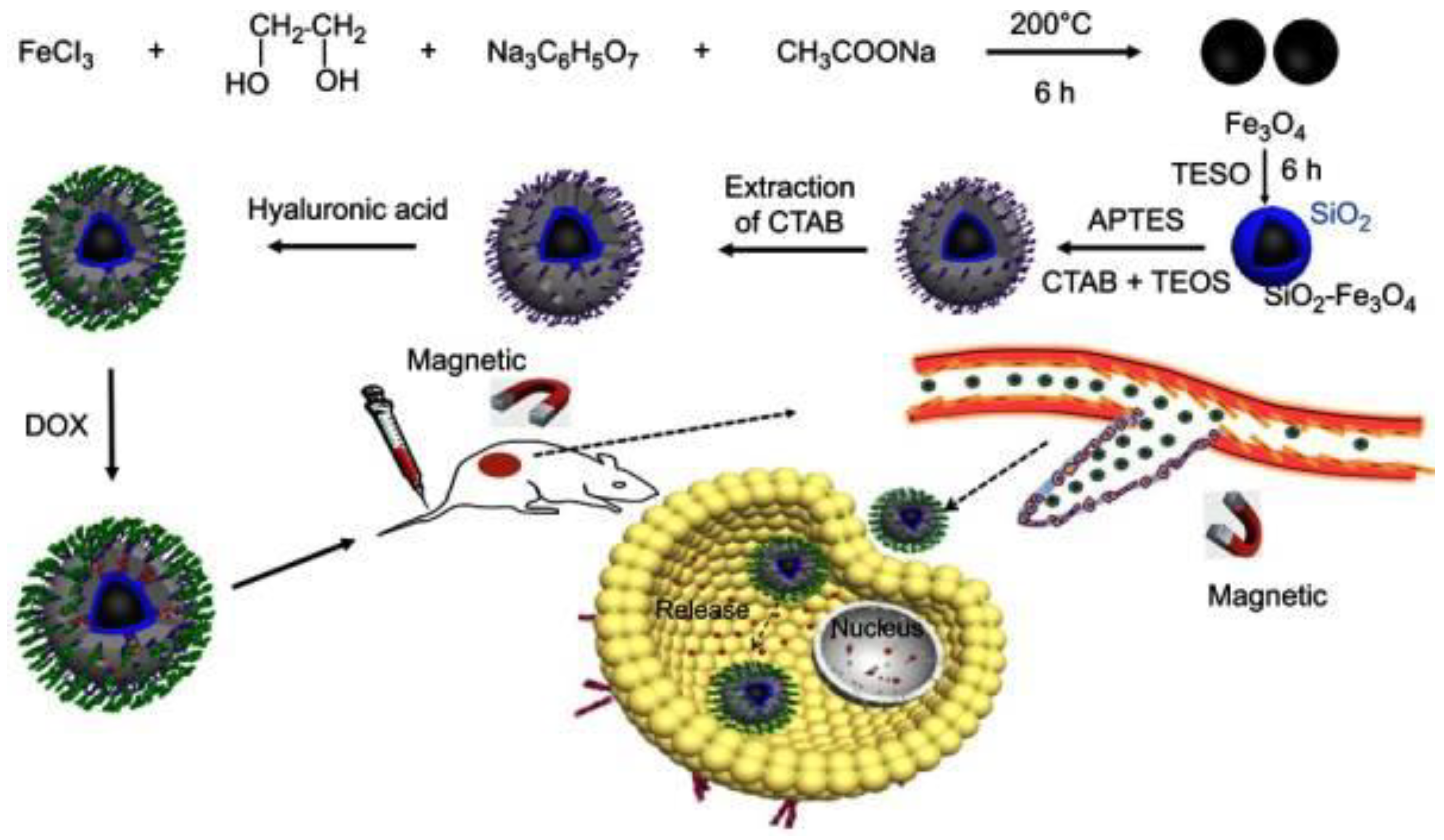
2.2. Hyaluronic Acid (HA)
2.3. Pectin
2.4. Starch
2.5. Xanthan Gum (XG)
2.6. Dextran
3. Applications of Natural Polymeric Fe3O4 MNPs Composites in DDSs
3.1. Effect of Fe3O4 MNPs Size in DDSs
3.2. Effect of Morphology or Anisotropy of Fe3O4 MNPs in DDSs
4. Applications of Polymeric Fe3O4 MNP Composites in DDSs
| Gelatin-Based Fe3O4 Composites | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Treatment | Loaded Drug | Observation | Ref. |
| DG/FA NPs | Nanoparticles | In vitro | Breast cancer | DOX | Decreased 48% cell viability. | [109] |
| Gel-MNPs | Nanocomposite | N/A | Lung and breast cancer | CUR | Loaded drugs are released in a pH-dependent manner, with a greater release rate in a moderately acidic environment. | [110] |
| Fe3O4/GQDs@GM | Microspheres | In vitro | Breast cancer | CUR | Exhibited high CUR loading capacity. | [111] |
| Alg-Gel/Fe3O4 | Hydrogel | In vitro and In vivo | Hela | DOX | Exhibited higher drug release value in acidic conditions. | [112] |
| HA-based Fe3O4 composites | ||||||
| CDHA–MGO | Nanocomposite | In vitro | Tumor | DOX | Targeting CD44 to accumulate inside tumor cells via HA conjugation. | [113] |
| Fe3O4-HA | Nanoparticles | In vitro and In vivo | Tumor | DOX | Enhanced antitumor and anti-metastasis effect. | [114] |
| Fe3O4@HA NPs | Nanoparticles | In vitro | Breast cancer | DOX | Enhanced DOX cancer-targeting capabilities. | [115] |
| MGO@CD-CA-HA | Nanocomposite | In vitro and In vivo | Liver cancer | CPT | Significantly reduced tumor growth (more than 90%). | [116] |
| PC/HA@DOX-Fe3O4 | Nanoparticles | In vivo | Xenograft tumor | DOX | Enhanced tumor growth suppression efficacy and significant DOX tumor-targeting capabilities. | [117] |
| Pectin-based Fe3O4 composites | ||||||
| Pectin/Fe3O4 | Nanoparticles | In vitro | Skin | 5-FU | Higher diffusion coefficients and shorter duration periods increased 5-FU release. | [96] |
| Fe3O4/Pectin | Nanoparticles | In vitro | Colorectal | BHT | Best antioxidant activities against DPPH. | [118] |
| AP-MA/PNIPAAm/Fe3O4 | Microgels | SGF and SIF | Colon cancer | CUR | Under the impact of EMF, a gradual and sustainable CUR release was made. | [100] |
| PEC-GO-Fe3O4 | Nanocomposite | In vitro | Aedes aegypti larvae | Permethrin | Enhanced drug loading and release performance up to 16 h. | [119] |
| Pec-gPolyDMAEMA@Fe3O4 | Nanoparticles | In vitro | Tumor | 5-FU | A 50 mT magnetic field dramatically (100%) boosted the 5-FU. | [120] |
| Starch-based Fe3O4 composites | ||||||
| CMC/PAA/St-Fe3O4 | Nanocomposite | In vitro | Colon cancer | 5-FU | 5-FU delivery to the intestinal fluid using an external magnetic source. | [101] |
| Fe3O4@CS-Starch/Cu | Nanocomposite | n.a. | Ovarian cancer | BHT | Ovarian cell viability decreased in a dose-dependent manner. | [121] |
| Fe3O4@Gr-IA/St-Alg | Hydrogel | In vitro | Wound healing | GFN | High efficiently loaded GFN and drug released in a controlled manner. | [122] |
| Dextran-based Fe3O4 composites | ||||||
| Dextran@Fe3O4 | Nanoparticles | In vitro | Prostate cancer | AUR | AUR release was considerably increased in an acidic medium. | [123] |
| Dextran-coated Fe3O4 | Nanoparticles | In vitro | Lung cancer | DOX | Enhanced the drug concentration in the tumor cell by applying EMF. | [124] |
| Dextran/Fe3O4 | Nanoparticles | In vitro and in vivo | Tumor | PBS | The dextran/Fe3O4 injection-induced tumors entirely vanished after 28 days. | [125] |
| Magnetic dextran | Microgel | In vitro | Tumor | DOX | DOX release profile that is both magnetic field and pH sensitive. | [126] |
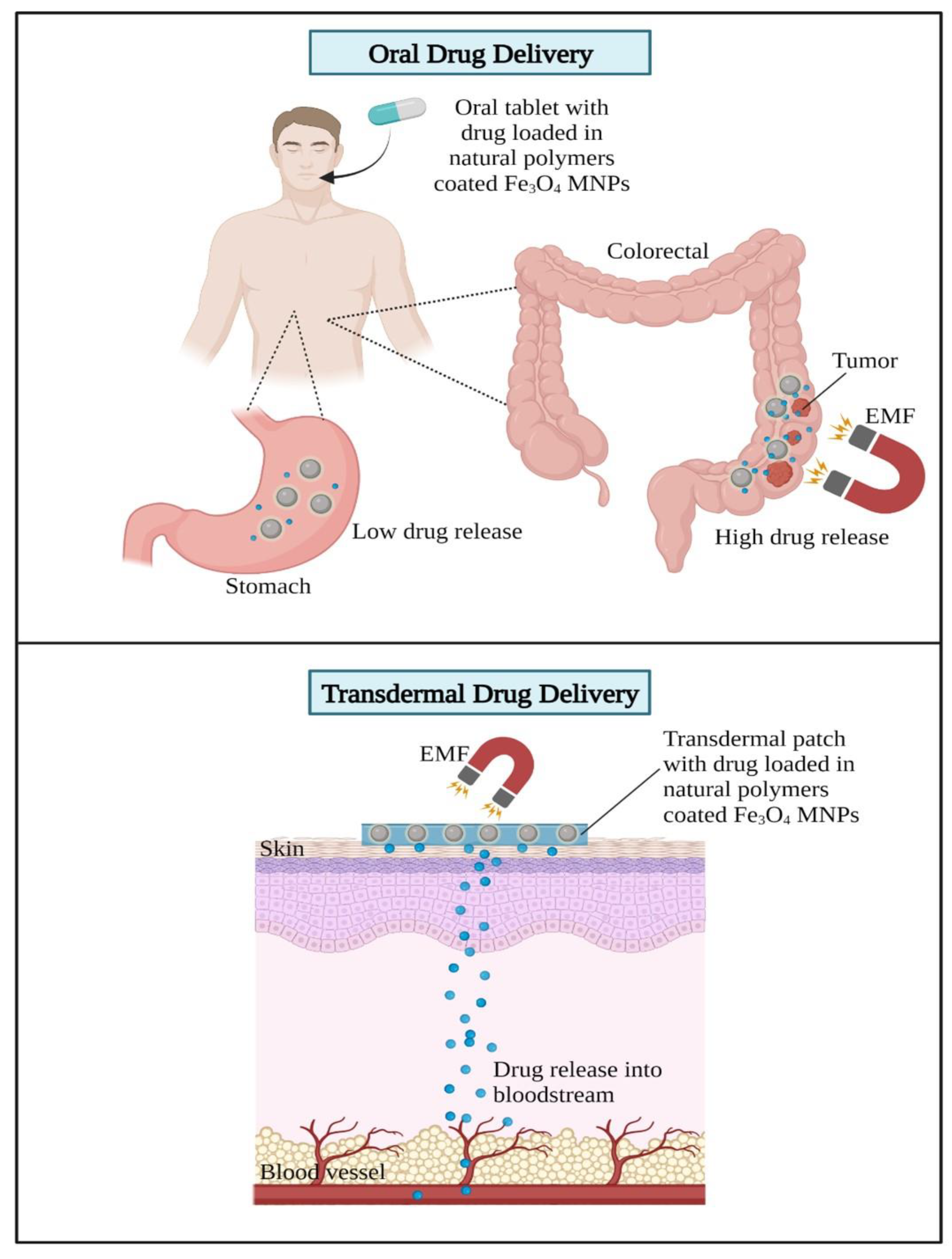
Biomedical Applications of Fe3O4 MNPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hrubý, M.; Filippov, S.K.; Štěpánek, P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur. Polym. J. 2015, 65, 82–97. [Google Scholar] [CrossRef]
- Jain, K.K. Drug Delivery Systems—An Overview; Humana Press: Totowa, NJ, USA, 2008; pp. 1–50. [Google Scholar]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Amalraj, A.; Thomas, S. Effective drug delivery system of biopolymers based on nanomaterials and hydrogels—A review. Drug Des. 2016, 5, 2169-0138. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Wang, Y.; Powell, R.; Chan, P. Polymeric core-shell nanoparticles for therapeutics. Res. J. Pharm. Dos. Forms Technol. 2006, 33, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Bangar, B.; Shinde, N.; Deshmukh, S.; Kale, B. Natural polymers in drug delivery development. Res. J. Pharm. Dos. Forms Technol. 2014, 6, 54–57. [Google Scholar]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: A review of recent advances for biomedical applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Temperature/pH dual sensitive Hericium erinaceus residue carboxymethyl chitin/poly (N-isopropyl acrylamide) sequential IPN hydrogels. Cellulose 2020, 27, 825–838. [Google Scholar] [CrossRef]
- Omidi, S.; Pirhayati, M.; Kakanejadifard, A. Co-delivery of doxorubicin and curcumin by a pH-sensitive, injectable, and In Situ hydrogel composed of chitosan, graphene, and cellulose nanowhisker. Carbohydr. Polym. 2020, 231, 115745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, X.; Cao, H.; Tan, T. Preparation of injectable hydrogel with near-infrared light response and photo-controlled drug release. Bioresour. Bioprocess. 2020, 7, 1. [Google Scholar] [CrossRef]
- Oktay, S.; Alemdar, N. Electrically controlled release of 5-fluorouracil from conductive gelatin methacryloyl-based hydrogels. J. Appl. Polym. Sci. 2019, 136, 46914. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release. Cellulose 2019, 26, 6861–6877. [Google Scholar] [CrossRef]
- Hu, X.; Nian, G.; Liang, X.; Wu, L.; Yin, T.; Lu, H.; Qu, S.; Yang, W. Adhesive tough magnetic hydrogels with high Fe3O4 content. ACS Appl. Mater. Interfaces 2019, 11, 10292–10300. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Passive targeting of nanoparticles to cancer. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Cham, Switzerland, 2019; pp. 125–143. [Google Scholar] [CrossRef]
- Jain, A.K.; Das, M.; Swarnakar, N.K.; Jain, S. Engineered PLGA nanoparticles: An emerging delivery tool in cancer therapeutics. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 1–45. [Google Scholar] [CrossRef]
- Jain, K. Nanotechnology-based drug delivery for cancer. Technol. Cancer Res. Treat. 2005, 4, 407–416. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Cheng, H.-W.; Yen, W.-Y.; Tsai, J.-H.; Yeh, C.-Y.; Chen, C.-J.; Liu, J.T.; Chen, S.-Y.; Chang, S.-J. The Treatment of Keloid Scars via Modulating Heterogeneous Gelatin-Structured Composite Microneedles to Control Transdermal Dual-Drug Release. Polymers 2022, 14, 4436. [Google Scholar] [CrossRef]
- Yang, S.-W.; Chen, Y.-J.; Chen, C.-J.; Liu, J.-T.; Yang, C.-Y.; Tsai, J.-H.; Lu, H.-E.; Chen, S.-Y.; Chang, S.-J. High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair. Polymers 2022, 14, 809. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.J.; Wang, C.; Jiang, X.; Qu, M.; Zhu, J.; Ren, L.; Vajhadin, F.; et al. Biodegradable β-Cyclodextrin Conjugated Gelatin Methacryloyl Microneedle for Delivery of Water-Insoluble Drug. Adv. Healthc. Mater. 2020, 9, e2000527. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Gajipara, A.; Shelar, S.B.; Priyadarsini, K.I.; Hassan, P.A. Development of water-dispersible gelatin stabilized hydroxyapatite nanoformulation for curcumin delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102769. [Google Scholar] [CrossRef]
- Jaberifard, F.; Arsalani, N.; Ghorbani, M.; Mostafavi, H. Incorporating halloysite nanotube/carvedilol nanohybrids into gelatin microsphere as a novel oral pH-sensitive drug delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128122. [Google Scholar] [CrossRef]
- Pasomboon, P.; Chumnanpuen, P.; E-Kobon, T. Modified Genome-Scale Metabolic Model of Escherichia coli by Adding Hyaluronic Acid Biosynthesis-Related Enzymes (GLMU2 and HYAD) from Pasteurella multocida. Int. J. Biotechnol. Bioeng. 2021, 15, 54–58. [Google Scholar]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic acid: A review of the drug delivery capabilities of this naturally occurring polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Yaghobi, Z.; Movassaghpour, A.; Talebi, M.; Shadbad, M.A.; Hajiasgharzadeh, K.; Pourvahdani, S.; Baradaran, B. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 2021, 903, 174147. [Google Scholar] [CrossRef]
- Hu, X.; Hou, B.; Xu, Z.; Saeed, M.; Sun, F.; Gao, Z.; Lai, Y.; Zhu, T.; Zhang, F.; Zhang, W. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv. Sci. 2020, 7, 1903332. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Feng, Y.; Gao, K.; Shi, X.; Zhao, X. Fabrication of hyaluronic acid-based micelles with glutathione-responsiveness for targeted anticancer drug delivery. J. Colloid Interface Sci. 2022, 606, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Wu, F.; Li, Y.; Cai, X. Hyaluronic acid-functionalized halloysite nanotubes for targeted drug delivery to CD44-overexpressing cancer cells. Mater. Today Commun. 2021, 28, 102682. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Nicoletta, F.P.; Leggio, A.; Iemma, F. Dual-targeted hyaluronic acid/albumin micelle-like nanoparticles for the vectorization of doxorubicin. Pharmaceutics 2021, 13, 304. [Google Scholar] [CrossRef]
- Kim, M.J.; Seong, K.-Y.; Kim, D.S.; Jeong, J.S.; Kim, S.Y.; Lee, S.; Yang, S.Y.; An, B.-S. Minoxidil-loaded hyaluronic acid dissolving microneedles to alleviate hair loss in an alopecia animal model. Act. Biomater. 2022, 143, 189–202. [Google Scholar] [CrossRef]
- Noreen, S.; Pervaiz, F.; Ijaz, M.; Shoukat, H. Synthesis and characterization of pH-sensitive chemically crosslinked block copolymer [Hyaluronic acid/Poloxamer 407-co-poly (Methacrylic acid)] hydrogels for colon targeting. Polym. Plast. Technol. Mater. 2022, 61, 1071–1087. [Google Scholar]
- Jo, Y.-J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, X.; Peng, X.; Gou, D.; Fan, X.; Zheng, X.; Song, C.; Zhou, Y.; Cui, S. Dual crosslinking of folic acid-modified pectin nanoparticles for enhanced oral insulin delivery. Biomater. Adv. 2022, 135, 212746. [Google Scholar] [CrossRef]
- Shi, X.; Cui, S.; Song, X.; Rickel, A.P.; Sanyour, H.J.; Zheng, J.; Hu, J.; Hong, Z.; Zhou, Y.; Liu, Y. Gelatin-crosslinked pectin nanofiber mats allowing cell infiltration. Mater. Sci. Eng. C 2020, 112, 110941. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Arafat, M.; Tan, S.F.; Sarker, M.Z.I. Investigations of pectin nanostructures for enhanced percutaneous delivery of fusidic acid. J. Appl. Polym. Sci. 2022, 139, e52760. [Google Scholar] [CrossRef]
- Aroui, S.; Kenani, A. Cell-penetrating peptides: A challenge for drug delivery. In Cheminformatics and Its Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Falcão, L.d.S.; Coelho, D.B.; Veggi, P.C.; Campelo, P.H.; Albuquerque, P.M.; de Moraes, M.A. Starch as a Matrix for Incorporation and Release of Bioactive Compounds: Fundamentals and Applications. Polymers 2022, 14, 2361. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, E.; Namazi, H.; Pooresmaeil, M. Carboxymethyl starch encapsulated 5-FU and DOX co-loaded layered double hydroxide for evaluation of its In Vitro performance as a drug delivery agent. Int. J. Biol. Macromol. 2022, 201, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, S.; Chen, C.; Ming, Y.; Du, J.; Mu, J.; Luo, F.; Huang, D.; Wang, N.; Lin, Z.; et al. Colon-targeted oral nanoparticles based on ROS-scavenging hydroxyethyl starch-curcumin conjugates for efficient inflammatory bowel disease therapy. Int. J. Pharm. 2022, 623, 121884. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Bona, N.P.; Crizel, R.L.; Pedra, N.S.; Stefanello, F.M.; Lim, L.T.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.d.R. Electrospun Starch Nanofibers as a Delivery Carrier for Carvacrol as Anti-Glioma Agent. Starch Stärke 2022, 74, 2100115. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, P. Injectable shear-thinning xanthan gum hydrogel reinforced by mussel-inspired secondary crosslinking. RSC Adv. 2015, 5, 103292–103301. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maiti, S.; Moorthy, N.S.H.N. Repaglinide-laden hydrogel particles of xanthan gum derivatives for the management of diabetes. Carbohydr. Polym. 2022, 287, 119354. [Google Scholar] [CrossRef]
- Shukla, A.; Bhatt, N. Formulation and evaluation of glibenclamide loaded mouth dissolving films with xanthan gum as film forming polymer. Eur. J. Mol. Clin. Med. 2021, 8, 2021. [Google Scholar]
- Kumar, P.; Gupta, R.K. Formulation and Characterization of Mouth Dissolving Films of Amlodipine using Natural Polymer. Res. J. Pharm. Technol. 2022, 15, 3651–3655. [Google Scholar] [CrossRef]
- Alam, A.; Jawaid, T.; Alsanad, S.M.; Kamal, M.; Rawat, P.; Singh, V.; Alam, P.; Alam, P. Solubility Enhancement, Formulation Development, and Antibacterial Activity of Xanthan-Gum-Stabilized Colloidal Gold Nanogel of Hesperidin against Proteus vulgaris. Gels 2022, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers based on dextran. In Polysaccharides II; Springer: Berlin/Heidelberg, Germany, 2006; pp. 199–291. [Google Scholar]
- Massia, S.P.; Stark, J.; Letbetter, D.S. Surface-immobilized dextran limits cell adhesion and spreading. Biomaterials 2000, 21, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, S.G.; Shoichet, M.S. Synthesis of cell-adhesive dextran hydrogels and macroporous scaffolds. Biomaterials 2006, 27, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Saha, S.; Sarkar, S.; Kumar, P.; Gautam, A.K.; Sonkar, A.B. Chapter 12—Dextran-based nanomaterials in drug delivery applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 293–312. [Google Scholar]
- Entzian, K.; Aigner, A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Cornel, E.J.; Du, J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1323–1339. [Google Scholar] [CrossRef]
- Zamani, R.; Bizari, D.; Heiat, M. Synthesis and characterization of phase shift dextran stabilized nanodroplets for ultrasound-induced cancer therapy: A novel nanobiotechnology approach. J. Biotechnol. 2022, 350, 17–23. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, S.; Hua, Z.; Yang, D.; Hu, M.; Zhu, Y.-T.; Zhong, H. Near infrared (NIR) light therapy of eye diseases: A review. Int. J. Med. Sci. 2021, 18, 109. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Z.; Yang, F.; Wu, T.; Wen, S.; Zhang, J.; Huang, L.; Deng, S.; Tan, S. Fabrication of Laponite-Reinforced Dextran-Based Hydrogels for NIR-Responsive Controlled Drug Release. ACS Biomater. Sci. Eng. 2022, 8, 1554–1565. [Google Scholar] [CrossRef]
- Yu, C.; Liu, H.; Guo, C.; Chen, Q.; Su, Y.; Guo, H.; Hou, X.; Zhao, F.; Fan, H.; Xu, H.; et al. Dextran sulfate-based MMP-2 enzyme-sensitive SR-A receptor targeting nanomicelles for the treatment of rheumatoid arthritis. Drug Deliv. 2022, 29, 454–465. [Google Scholar] [CrossRef]
- Liu, P.; Huang, P.; Kang, E.-T. pH-Sensitive Dextran-Based Micelles from Copper-Free Click Reaction for Antitumor Drug Delivery. Langmuir 2021, 37, 12990–12999. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Urban, M.W. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Miyamoto, R.; Hirai, T.J.O.L. Spiropyran-conjugated thermoresponsive copolymer as a colorimetric thermometer with linear and reversible color change. Org. Lett. 2009, 11, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Maran, A.; Yaszemski, M.J.; Bolander, M.E.; Sarkar, G. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J. Mater. Sci. Mater. Med. 2009, 20, 347–350. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Shapiro, B. Towards dynamic control of magnetic fields to focus magnetic carriers to targets deep inside the body. J. Magn. Magn. Mater. 2009, 321, 1594–1599. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Namdeo, M.; Saxena, S.; Tankhiwale, R.; Bajpai, M.; Mohan, Y.á.; Bajpai, S. Magnetic nanoparticles for drug delivery applications. J. Nanosci. Nanotechnol. 2008, 8, 3247–3271. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and disadvantages of using magnetic nanoparticles for the treatment of complicated ocular disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, X.; Liu, X.; Shi, S.; Wang, J.; Shi, C.; Qian, Z.; Zhou, S. A strategy in the design of micellar shape for cancer therapy. Adv. Healthc. Mater. 2012, 1, 214–224. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Synergistic targeting of cell membrane, cytoplasm, and nucleus of cancer cells using rod-shaped nanoparticles. ACS Nano 2013, 7, 9558–9570. [Google Scholar] [CrossRef]
- Chen, J.; Clay, N.E.; Park, N.-h.; Kong, H. Non-spherical particles for targeted drug delivery. Chem. Eng. Sci. 2015, 125, 20–24. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, C.; Ma, X.; Li, J.; Wang, S.; Yue, B.; Huang, M. Hierarchical Core–Shell Fe3O4@ mSiO2@ Chitosan Nanoparticles for pH-Responsive Drug Delivery. J. Nanosci. Nanotechnol. 2021, 21, 3020–3027. [Google Scholar] [CrossRef]
- Shahin, R.; Yousefi, M.; Ziyadi, H.; Bikhof, M.; Hekmati, M. pH-Responsive and magnetic Fe3O4@UiO-66-NH2@PEI nanocomposite as drug nanocarrier: Loading and release study of Imatinib. Inorg. Chem. Commun. 2023, 147, 110186. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shadi, M.; Mohammadian, R.; Shaabani, A.; Ghorbani, M.; Rabiee, G.; Amini, M.M. Preparation of Fe3O4@SiO2@Tannic acid double core-shell magnetic nanoparticles via the Ugi multicomponent reaction strategy as a pH-responsive co-delivery of doxorubicin and methotrexate. Mater. Chem. Phys. 2020, 247, 122857. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.e.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Satapathy, B.S.; Majumder, S.; Sarkar, R.; Mukherjee, B.; Tudu, B. Engineered polymeric iron oxide nanoparticles as potential drug carrier for targeted delivery of docetaxel to breast cancer cells. J. Magn. Magn. Mater. 2019, 485, 165–173. [Google Scholar] [CrossRef]
- Khan, S.; Shah, Z.H.; Riaz, S.; Ahmad, N.; Islam, S.; Raza, M.A.; Naseem, S. Antimicrobial activity of citric acid functionalized iron oxide nanoparticles –Superparamagnetic effect. Ceram. Int. 2020, 46, 10942–10951. [Google Scholar] [CrossRef]
- Sirivat, A.; Paradee, N. Facile synthesis of gelatin-coated Fe3O4 nanoparticle: Effect of pH in single-step co-precipitation for cancer drug loading. Mater. Des. 2019, 181, 107942. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745. [Google Scholar] [CrossRef]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic acid-decorated PLGA-PEG nanoparticles for targeted delivery of SN-38 to ovarian cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, X.; Xu, Z.; Du, F.; Wang, W.; Shi, R.; Gao, D. Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles for targeted drug delivery. Int. J. Nanomed. 2019, 14, 5785. [Google Scholar] [CrossRef]
- Viratchaiboott, N.; Sakunpongpitiporn, P.; Niamlang, S.; Sirivat, A. Release of 5-FU loaded pectin/Fe3O4 from porous PBSA matrix under magnetic and electric fields. J. Alloys Compd. 2022, 906, 164239. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Oliveira, R.S.; Mauricio, M.R.; Cellet, T.S.; Pereira, G.M.; Kunita, M.H.; Muniz, E.C.; Rubira, A.F. Albumin release from a brain-resembling superabsorbent magnetic hydrogel based on starch. Soft Matter 2012, 8, 6629–6637. [Google Scholar] [CrossRef]
- Bueno, P.V.; Hilamatu, K.C.; Carmona-Ribeiro, A.M.; Petri, D.F. Magnetically triggered release of amoxicillin from xanthan/Fe3O4/albumin patches. Int. J. Biol. Macromol. 2018, 115, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, A.; Sadhasivam, J.; Gawas, P.; Nutalapati, V.; Pandian, R.; Perumal, S.K. Curcumin conjugated dextran coated Fe3O4 Nanoparticles: Cytotoxic effect on lung cancer cell line A549. Mater. Sci. Eng. B 2022, 286, 116047. [Google Scholar] [CrossRef]
- Almeida, E.A.; Bellettini, I.C.; Garcia, F.P.; Farinácio, M.T.; Nakamura, C.V.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Curcumin-loaded dual pH-and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017, 171, 259–266. [Google Scholar] [CrossRef]
- Mohammadi, R.; Saboury, A.; Javanbakht, S.; Foroutan, R.; Shaabani, A. Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur. Polym. J. 2021, 153, 110500. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, D.; Feng, S.; Zhang, L.; Ji, X.; Wang, Z.; Lu, Q.; Xi, C.; Pi, L.; Zhang, X. Effects of 3.5–23.0 T static magnetic fields on mice: A safety study. Neuroimage 2019, 199, 273–280. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Novotna, B.; Churpita, O.; Trošan, P.; Holáň, V.; Sykova, E.; Dejneka, A.; Kubinová, Š. Down-regulation of adipogenesis of mesenchymal stem cells by oscillating high-gradient magnetic fields and mechanical vibration. Appl. Phys. Lett. 2014, 105, 103702. [Google Scholar] [CrossRef]
- Oliveira, F.T.P.; Diedrichsen, J.; Verstynen, T.; Duque, J.; Ivry, R.B. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc. Natl. Acad. Sci. USA 2010, 107, 17751–17756. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Parekh, K.; Jain, N. In Vitro hyperthermic effect of magnetic fluid on cervical and breast cancer cells. Sci. Rep. 2020, 10, 15249. [Google Scholar] [CrossRef] [PubMed]
- Tkáč, I.; Benneyworth, M.A.; Nichols-Meade, T.; Steuer, E.L.; Larson, S.N.; Metzger, G.J.; Uğurbil, K. Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn. Reson. Med. 2021, 86, 1544–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, Y.; Li, Z.; Ji, X.; Wang, Z.; Wang, H.; Tian, X.; Yu, F.; Yang, Z.; Pi, L. 27 T ultra-high static magnetic field changes orientation and morphology of mitotic spindles in human cells. eLife 2017, 6, e22911. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Chuang, T.-J.; Ke, C.-J.; Yao, C.-H. Doxorubicin–gelatin/Fe3O4–alginate dual-layer magnetic nanoparticles as targeted anticancer drug delivery vehicles. Polymers 2020, 12, 1747. [Google Scholar] [CrossRef]
- Dutta, B.; Shelar, S.B.; Rajan, V.; Checker, S.; Barick, K.; Pandey, B.; Kumar, S.; Hassan, P. Gelatin grafted Fe3O4 based curcumin nanoformulation for cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 67, 102974. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. pH-sensitive ternary Fe3O4/GQDs@ G hybrid microspheres; Synthesis, characterization and drug delivery application. J. Alloys Compd. 2020, 846, 156419. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef]
- Liang, W.; Huang, Y.; Lu, D.; Ma, X.; Gong, T.; Cui, X.; Yu, B.; Yang, C.; Dong, C.; Shuang, S. β-cyclodextrin–hyaluronic acid polymer functionalized magnetic graphene oxide nanocomposites for targeted photo-chemotherapy of tumor cells. Polymers 2019, 11, 133. [Google Scholar] [CrossRef]
- Gong, T.; Dong, Z.; Fu, Y.; Gong, T.; Deng, L.; Zhang, Z. Hyaluronic acid modified doxorubicin loaded Fe3O4 nanoparticles effectively inhibit breast cancer metastasis. J. Mater. Chem. B 2019, 7, 5861–5872. [Google Scholar] [CrossRef]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef]
- Wen, C.; Cheng, R.; Gong, T.; Huang, Y.; Li, D.; Zhao, X.; Yu, B.; Su, D.; Song, Z.; Liang, W. β-Cyclodextrin-cholic acid-hyaluronic acid polymer coated Fe3O4-graphene oxide nanohybrids as local chemo-photothermal synergistic agents for enhanced liver tumor therapy. Colloids Surf. B Biointerfaces 2021, 199, 111510. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, X.; Liu, D.; Cong, M.; Song, Y.; Bai, S. Lipid/hyaluronic acid–Coated Doxorubicin-Fe3O4 as a dual-targeting nanoparticle for enhanced cancer therapy. AAPS Pharm. Sci. Technol. 2020, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, G.; Karmakar, B.; AlSalem, H.S.; Shati, A.A.; El-kott, A.F.; Elsaid, F.G.; Bani-Fwaz, M.Z.; Alsayegh, A.A.; Alkhayyat, S.S. Pectin mediated green synthesis of Fe3O4/Pectin nanoparticles under ultrasound condition as an anti-human colorectal carcinoma bionanocomposite. Arab. J. Chem. 2022, 15, 103867. [Google Scholar] [CrossRef]
- Wong, S.T.S.; Kamari, A.; Yusoff, S.N.M.; Hussein, M.Z.; Othman, H.; Ali, N.M.; Phillip, E. Gellan gum and pectin-functionalised magnetic graphene oxide nanocomposites as nanocarriers for permethrin to control mosquito larvae. Polym. Bull. 2022, 1–27. [Google Scholar] [CrossRef]
- Işıklan, N.; Polat, S. Synthesis and characterization of thermo/pH-sensitive pectin-graft-poly (dimethylaminoethyl methacrylate) coated magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 164, 4499–4515. [Google Scholar] [CrossRef]
- Ji, N.; Dong, C.; Jiang, J. Evaluation of antioxidant, cytotoxicity, and anti-ovarian cancer properties of the Fe3O4@ CS-Starch/Cu bio-nanocomposite. Inorg. Chem. Commun. 2022, 140, 109452. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Forouzandehdel, S.; Rami, M.R. Synthesis of a novel magnetic starch-alginic acid-based biomaterial for drug delivery. Carbohydr. Res. 2020, 487, 107889. [Google Scholar] [CrossRef]
- Naseri, N.; Iranshahi, M.; Rakhshani, S.; Mohtashami, L. Enhanced cytotoxicity of auraptene to prostate cancer cells by dextran-coated Fe3O4 nanoparticles. Nanomed. J. 2022, 9, 77–86. [Google Scholar]
- Zhang, Q.; Liu, Q.; Du, M.; Vermorken, A.; Cui, Y.; Zhang, L.; Guo, L.; Ma, L.; Chen, M. Cetuximab and Doxorubicin loaded dextran-coated Fe3O4 magnetic nanoparticles as novel targeted nanocarriers for non-small cell lung cancer. J. Magn. Magn. Mater. 2019, 481, 122–128. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Li, W.; Bai, H.; Gao, Y.; Ma, J.; Liu, W.; Xi, G. Dextran coated Fe3O4 nanoparticles as a near-infrared laser-driven photothermal agent for efficient ablation of cancer cells In Vitro and In Vivo. Mater. Sci. Eng. C 2018, 90, 46–56. [Google Scholar] [CrossRef]
- He, L.; Zheng, R.; Min, J.; Lu, F.; Wu, C.; Zhi, Y.; Shan, S.; Su, H. Preparation of magnetic microgels based on dextran for stimuli-responsive release of doxorubicin. J. Magn. Magn. Mater. 2021, 517, 167394. [Google Scholar] [CrossRef]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar] [PubMed]
- Koda, J.; Venook, A.; Walser, E.; Goodwin, S. A multicenter, phase I/II trial of hepatic intra-arterial delivery of doxorubicin hydrochloride adsorbed to magnetic targeted carriers in patients with hepatocellular carcinoma. Eur. J. Cancer 2002, 38, S18. [Google Scholar]
- Wilson, M.W.; Kerlan Jr, R.K.; Fidelman, N.A.; Venook, A.P.; LaBerge, J.M.; Koda, J.; Gordon, R.L. Hepatocellular carcinoma: Regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/conventional angiography suite—Initial experience with four patients. Radiology 2004, 230, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bartolozzi, C.; Lencioni, R.; Donati, F.; Cioni, D. Abdominal MR: Liver and pancreas. Eur. Radiol. 1999, 9, 1496–1512. [Google Scholar] [CrossRef]
- Senéterre, E.; Taourel, P.; Bouvier, Y.; Pradel, J.; Van Beers, B.; Daures, J.-P.; Pringot, J.; Mathieu, D.; Bruel, J.-M. Detection of hepatic metastases: Ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology 1996, 200, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Ros, P.R.; Freeny, P.C.; Harms, S.E.; Seltzer, S.E.; Davis, P.L.; Chan, T.W.; Stillman, A.E.; Muroff, L.R.; Runge, V.M.; Nissenbaum, M.A. Hepatic MR imaging with ferumoxides: A multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology 1995, 196, 481–488. [Google Scholar] [CrossRef]
- McLachlan, S.J.; Morris, M.R.; Lucas, M.A.; Fisco, R.A.; Eakins, M.N.; Fowler, D.R.; Scheetz, R.B.; Olukotun, A.Y. Phase I clinical evaluation of a new iron oxide MR contrast agent. J. Magn. Reson. Imaging JMRI 1994, 4, 301–307. [Google Scholar] [CrossRef]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakamura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef]
- Taruno, K.; Kuwahata, A.; Sekino, M.; Nakagawa, T.; Kurita, T.; Enokido, K.; Nakamura, S.; Takei, H.; Kusakabe, M. Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation. Cancers 2022, 14, 1409. [Google Scholar] [CrossRef]
- Wang, L.; Balasubramanian, P.; Chen, A.P.; Kummar, S.; Evrard, Y.A.; Kinders, R.J. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin. Oncol. 2016, 43, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, H.; Buchanan, J.K.; Kandile, N.G.; Plieger, P.G. Iron Oxide Nanoparticles: Physicochemical Characteristics and Historical Developments to Commercialization for Potential Technological Applications. ACS Biomater. Sci. Eng. 2021, 7, 5432–5450. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Thomas, J.; Panwar, V.; Ali, H.; Chopra, V.; Sharma, A.; Tomar, R.; Ghosh, D. In Situ biosynthesized superparamagnetic iron oxide nanoparticles (SPIONS) induce efficient hyperthermia in cancer cells. ACS Appl. Bio Mater. 2020, 3, 779–788. [Google Scholar] [CrossRef] [PubMed]




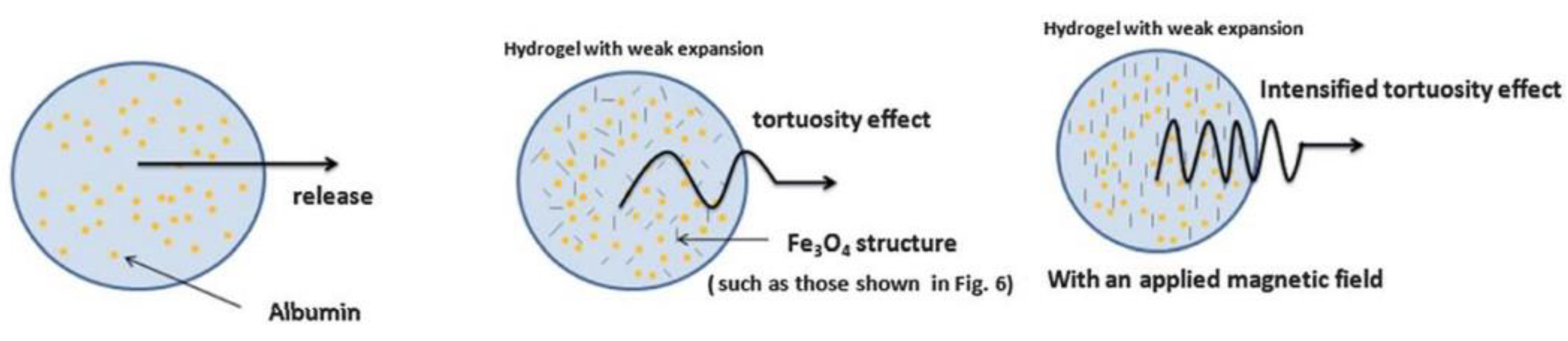
| Gelatin-Based DDS | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effects | Ref. |
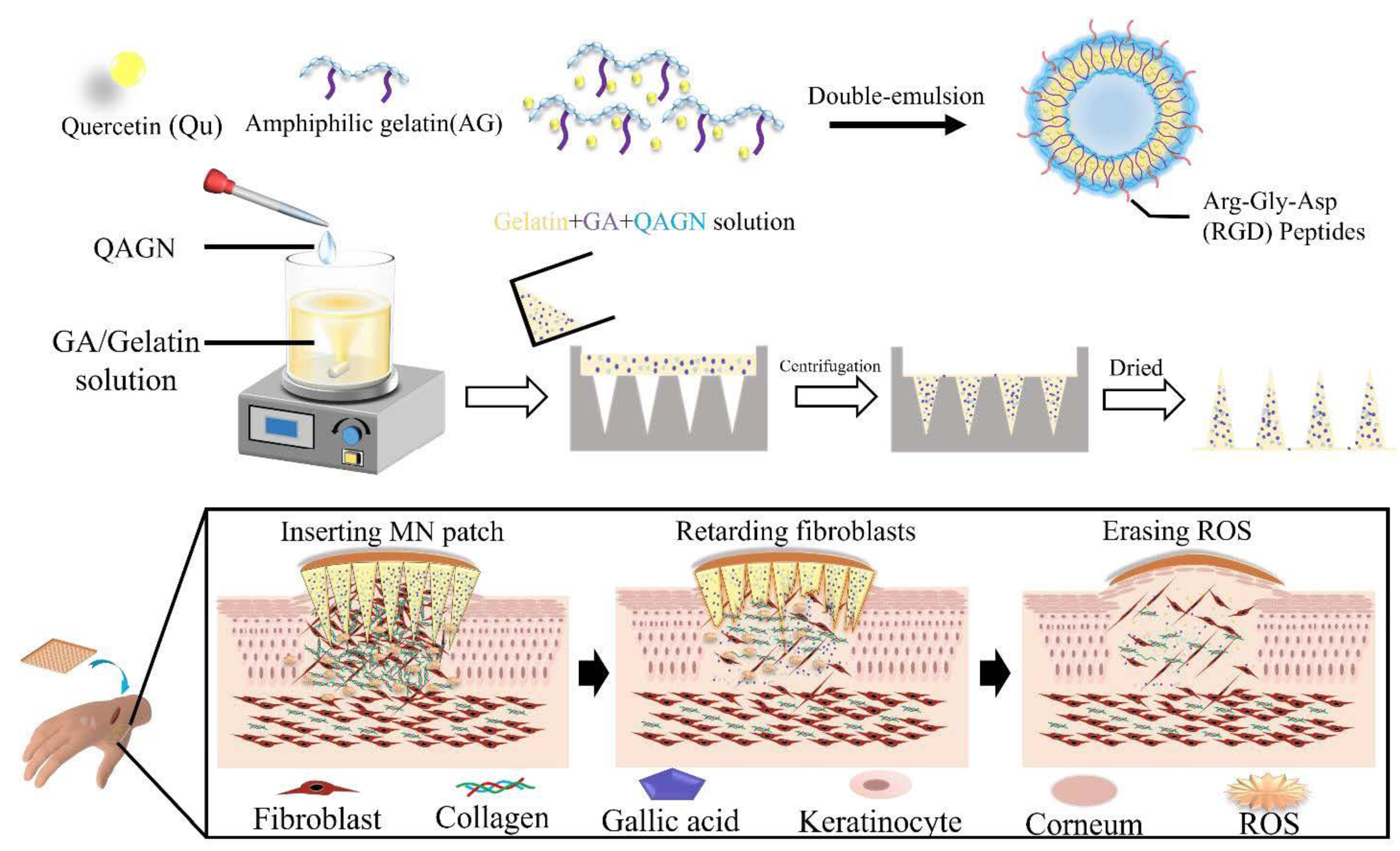
| Gelatin | Hydrogel-based MN | In vitro | Keloid scarring | Gallic acid QAGN | Produced a controlled drug release of QAGN. Downregulated the gene expression of fibroblasts. | [22] |
| Gelatin methacryloyl and β-cyclodextrin | Hydrogel-based MN | In vitro and in vivo | Melanoma cancer | Curcumin | Higher therapeutic efficacy compared to non-transdermal patches. Verified biocompatibility and degradability. | [25] |
| Gelatin and hydroxyapatite | NPs | In vitro | Lung cancer | Curcumin | Sustained release of curcumin. Higher increase in cellular internalization and toxicity towards A549 cells than free curcumin. | [26] |
| pH-sensitive gelatin | Microsphere | In vitro | Respiratory disease | Carvedilol | Rapid drug release under acidic state (pH = 1.2) and non-toxicity against Caco-2 cells | [27] |
| Hyaluronic Acid (HA)-Based DDS | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effects | Ref. |
| HA-tetraphenyl ethylene | Micelles | In vitro | Cancer | DOX | Great efficacy in unloading DOX through fast glutathione-triggered dissociation. | [33] |
| HA-modified | Halloysite nanotube | In vitro | Cancer | DOX | Enhanced the therapeutic efficacy of DOX. High antitumor efficacy in CD44-positive Hela cells. | [34] |
| HA–human serum albumin | Micelle-like NPs | In vitro and in vivo | Breast cancer | DOX | Greater cytotoxicity of MDA-MB231 cells. CD44-mediated internalization of nanoparticles. | [35] |
| HA | MN | In vivo | Alopecia | MDX | Enhancement of HDP cells. Reduced hair loss in alopecia. | [36] |
| HA | Hydrogel | In vitro and in vivo | Colorectal cancer | 5-Fu | 5-FU is retained in a coordinated manner for a more extended period. Toxicity assessment on rabbits also showed compatibility. | [37] |
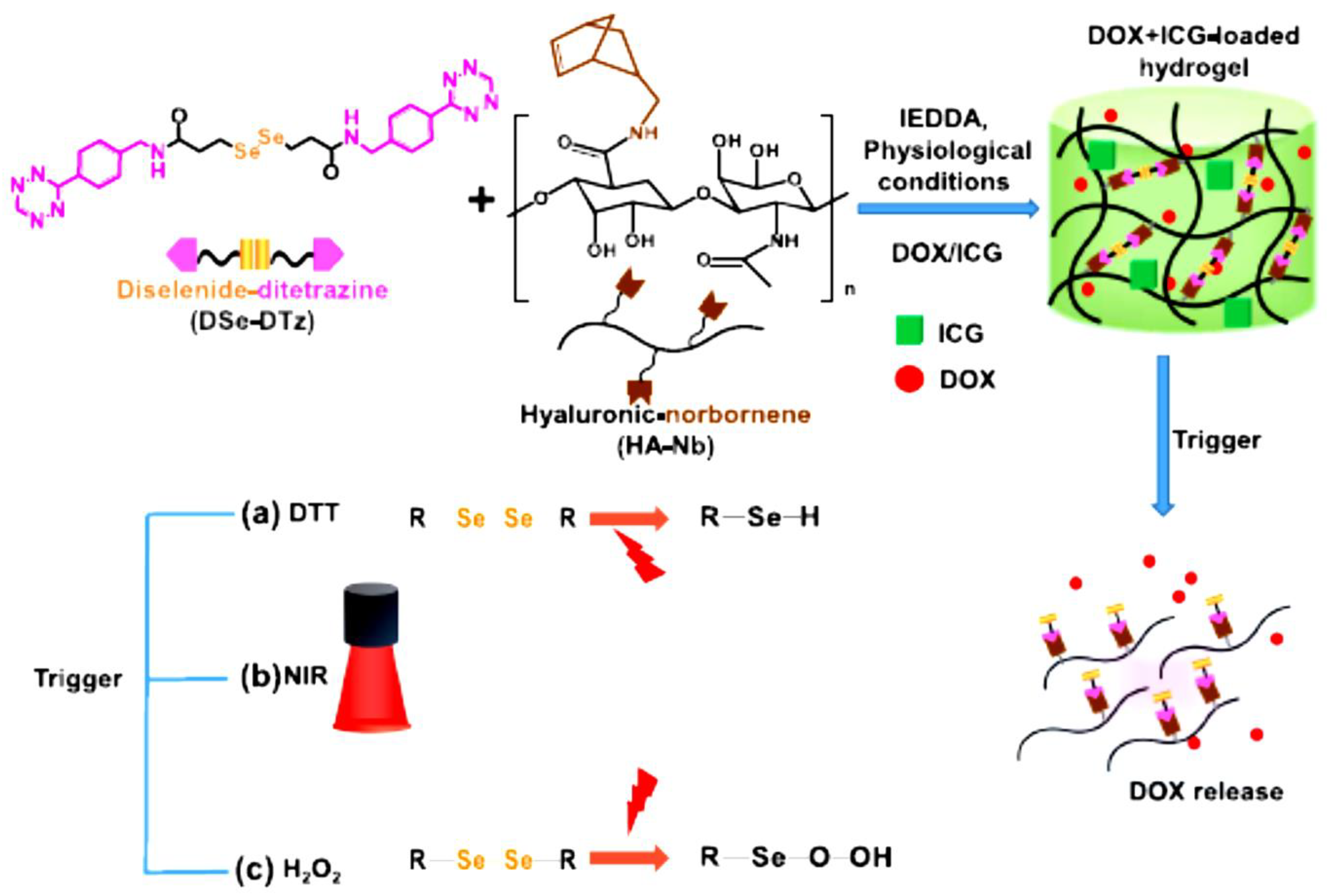
| HA | Multi-stimuli responsive hydrogel | In vitro | Cancer | DOX | Antitumor effect in breast cancer cells (BT-29) | [38] |
| Pectin-Based DDS | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effects | Ref. |
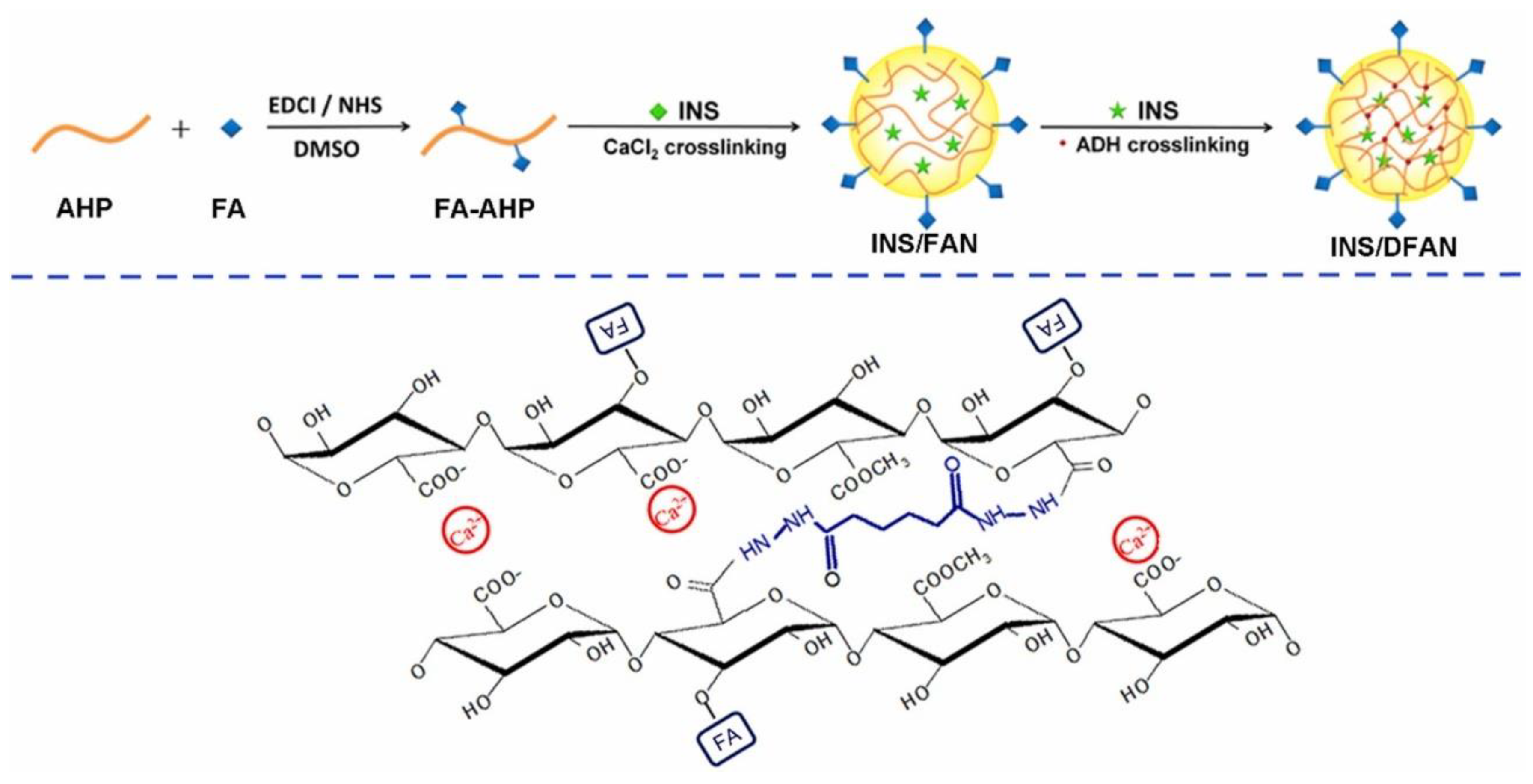
| Folic acid-modified pectin | NPs | In vitro and in vivo | Type 1 diabetes | Insulin | Prevent the premature release of insulin. High encapsulation efficiency. Excellent stability. Enhanced insulin delivery. Improved hypoglycaemic effects on type 1 diabetes rats. | [39] |
| Amphipathically modified pectin | Spherical nano-structures | In vitro | Skin-related disease | Fusidic acid | Fusidic acid was released in a more controlled manner. HaCaT cells showed a non-cytotoxicity profile. Two-fold greater penetration rate. | [41] |
| Starch-Based DDS | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effects | Ref. |
| Hydroxyethyl starch | NPs | In vitro and in vivo | Ulcerative colitis | Curcumin and DEX | Drugs released in an α-amylase-responsive manner. Effective internalization and cytocompatibility with macrophages. Greater in efficacy compared to free DEX. | [45] |
| Starch | Nanofiber | In vitro and in vivo | Cancer | Carvacrol | The system is resisting in vitro digestion. 50% reduction in cancer cells of rat C6 glioma cells. | [46] |
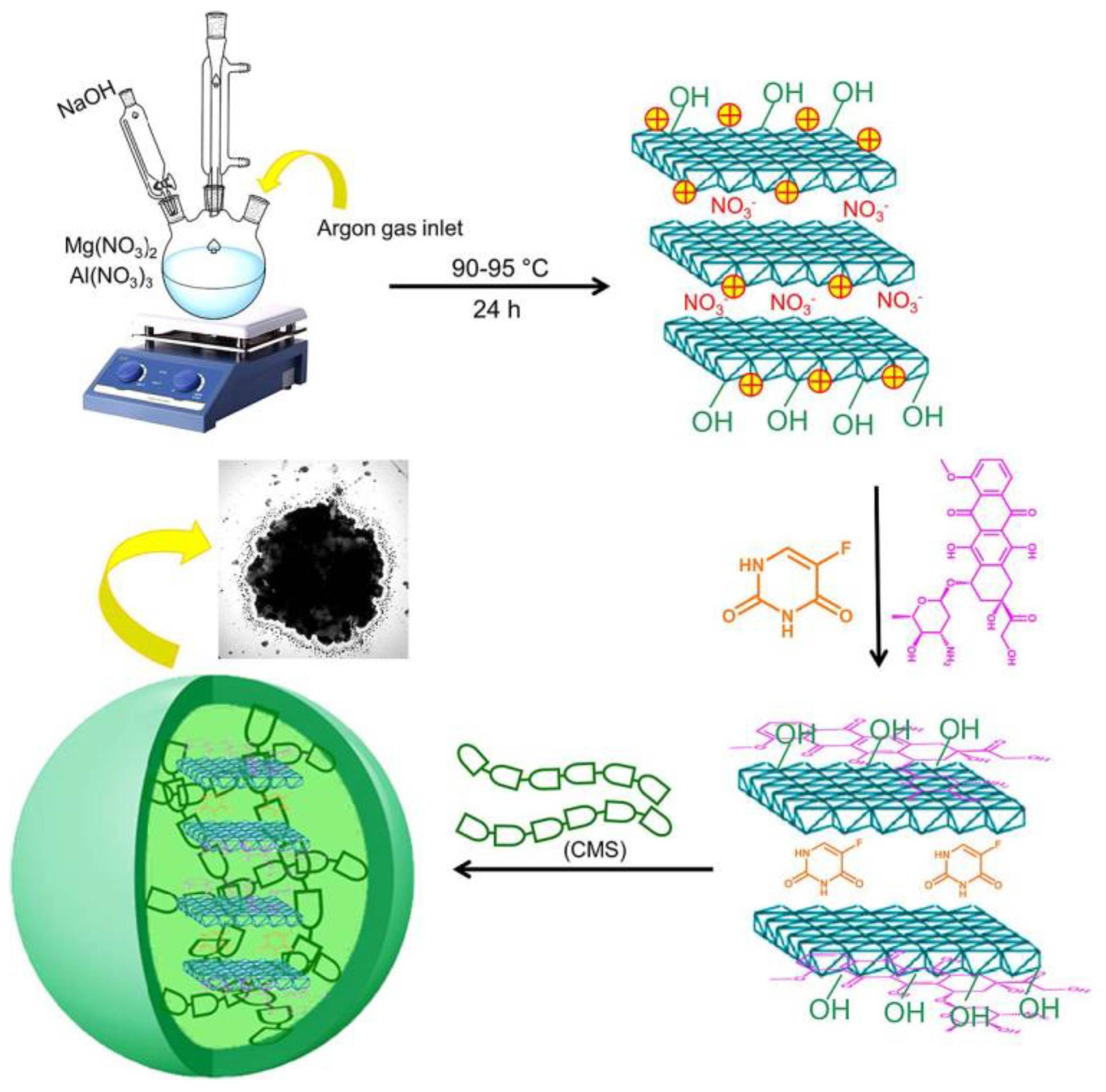
| Carboxymethyl starch -CMS@LDH(MgAl)@DOX,5-Fu | Microspheres | In vitro | Colon cancer | 5-Fu and DOX | Sustained drug release pattern and controlled release profile of DOX and 5-Fu. | [44] |
| Xanthan Gum (XG)-Based DDSs | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effects | Ref. |
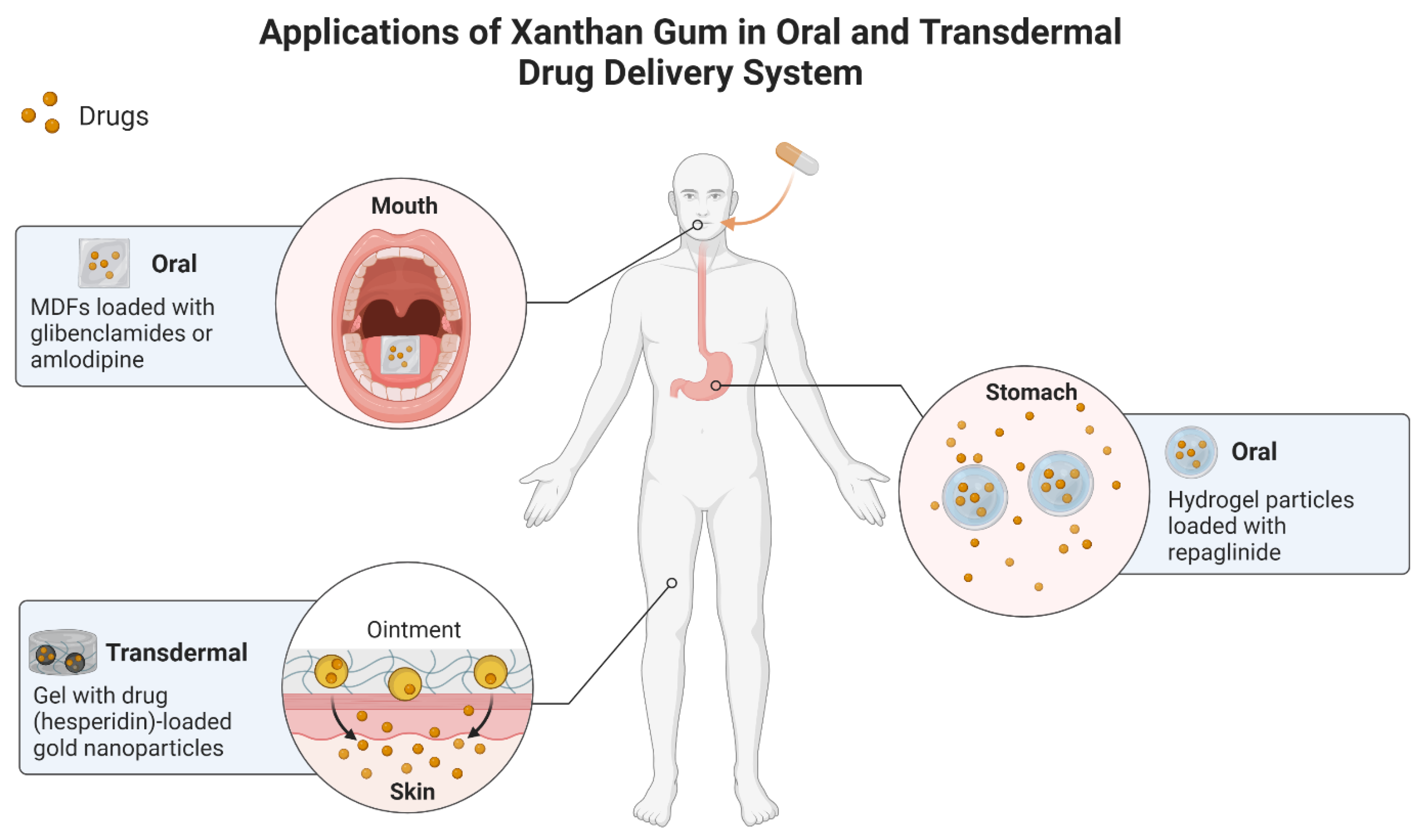
| Carboxyethyl XG-carboxymethyl XG | Hydrogel particle | In vitro and in vivo | Type 2 diabetes | Repaglinide | The system released 97% drug in 4 h. Prolonged drug release for 8 h. Reduction in blood glucose levels in diabetic rats. | [51] |
| XG | MDFs | In vitro | Type 2 diabetes | Glibenclamide | Instant release of drug. Drug rapid dissolution.Optimum mechanical strength. | [52] |
| XG | MDFs | In vitro | Hypertension | Amlodipine | Complete drug release within 10 min. | [53] |
| XG | Gel-AuNPs | In vitro | P. vulgaris infection | Hesperidin | Gel showed antimicrobial activity against P. vulgaris. | [54] |
| Dextran-Based DDSs | ||||||
|---|---|---|---|---|---|---|
| Formulation | Drug Delivery Vehicle | Platform | Disease | Loaded Drug | Observed Effect | Ref. |
| Dextran-stabilized perfluorohexane | Ultrasound-responsive nanodroplets | In vitro | Cancer | DOX | Particle size and encapsulation efficiency. Biphasic drug release system of DOX. | [62] |
| Vinyl-functionalized dextran, vinyl-modified graphene oxide-Laponite | NIR light-responsive hydrogel | Ex vivo | Microbial infection | Ciproflo-xacin | Drug dispersion in NIR-controllable. Antibacterial effect. Good compatibility with blood. | [64] |
| Modified dextran (dextran-sulfate-PVGLIG) | Nanomicelles | In vitro and in vivo | Rheumatoid arthritis | Cel | High entrapment of drug in nanomicelles. Effectively delivered the drug to the inflammatory joint. Greater anti-RA effects. Lower systemic toxicity in comparison to free Cel. | [65] |
| Dextran-graft-poly(2-(diisopropylamino) ethyl methacrylate-co-2-(2′,3′,5′-triiodobenzoyl) ethyl methacrylate) | pH-sensitive micelle | In vitro and in vivo | Breast cancer | DOX | Optimally release DOX into MCF-7 cells. Excellent anticancer efficacy. Effectively reduce the growth of the tumor. | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W.; et al. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels 2023, 9, 121. https://doi.org/10.3390/gels9020121
Nordin AH, Ahmad Z, Husna SMN, Ilyas RA, Azemi AK, Ismail N, Nordin ML, Ngadi N, Siti NH, Nabgan W, et al. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels. 2023; 9(2):121. https://doi.org/10.3390/gels9020121
Chicago/Turabian StyleNordin, Abu Hassan, Zuliahani Ahmad, Siti Muhamad Nur Husna, Rushdan Ahmad Ilyas, Ahmad Khusairi Azemi, Noraznawati Ismail, Muhammad Luqman Nordin, Norzita Ngadi, Nordin Hawa Siti, Walid Nabgan, and et al. 2023. "The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review" Gels 9, no. 2: 121. https://doi.org/10.3390/gels9020121
APA StyleNordin, A. H., Ahmad, Z., Husna, S. M. N., Ilyas, R. A., Azemi, A. K., Ismail, N., Nordin, M. L., Ngadi, N., Siti, N. H., Nabgan, W., Norfarhana, A. S., & Azami, M. S. M. (2023). The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels, 9(2), 121. https://doi.org/10.3390/gels9020121









