Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM
Abstract
:1. Introduction
2. Results and Discussion
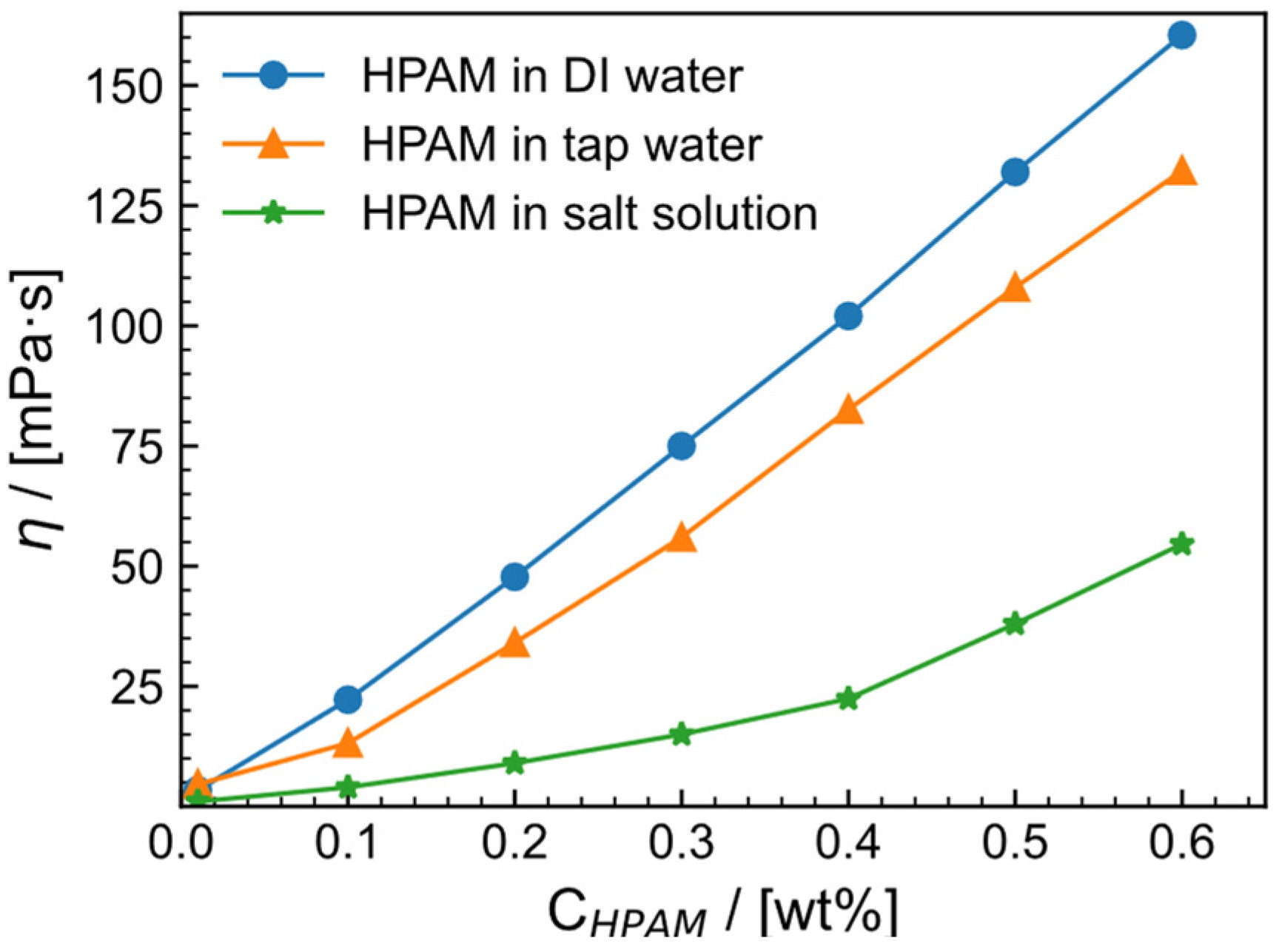
2.1. Apparent Viscosity of HPAM Solution
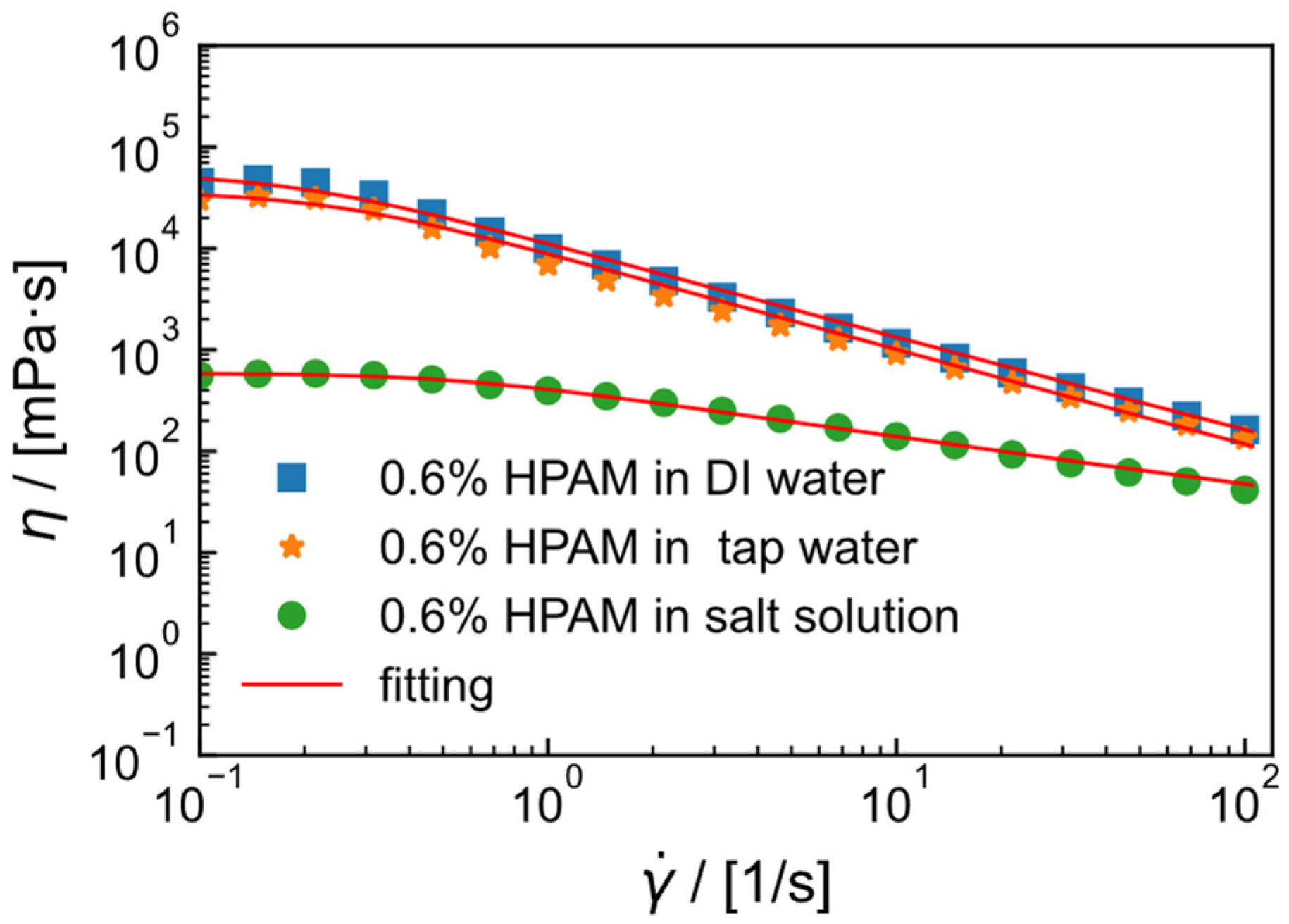
2.2. Flow Curve of HPAM Solution
2.3. Viscoelasticity of HPAM Solution
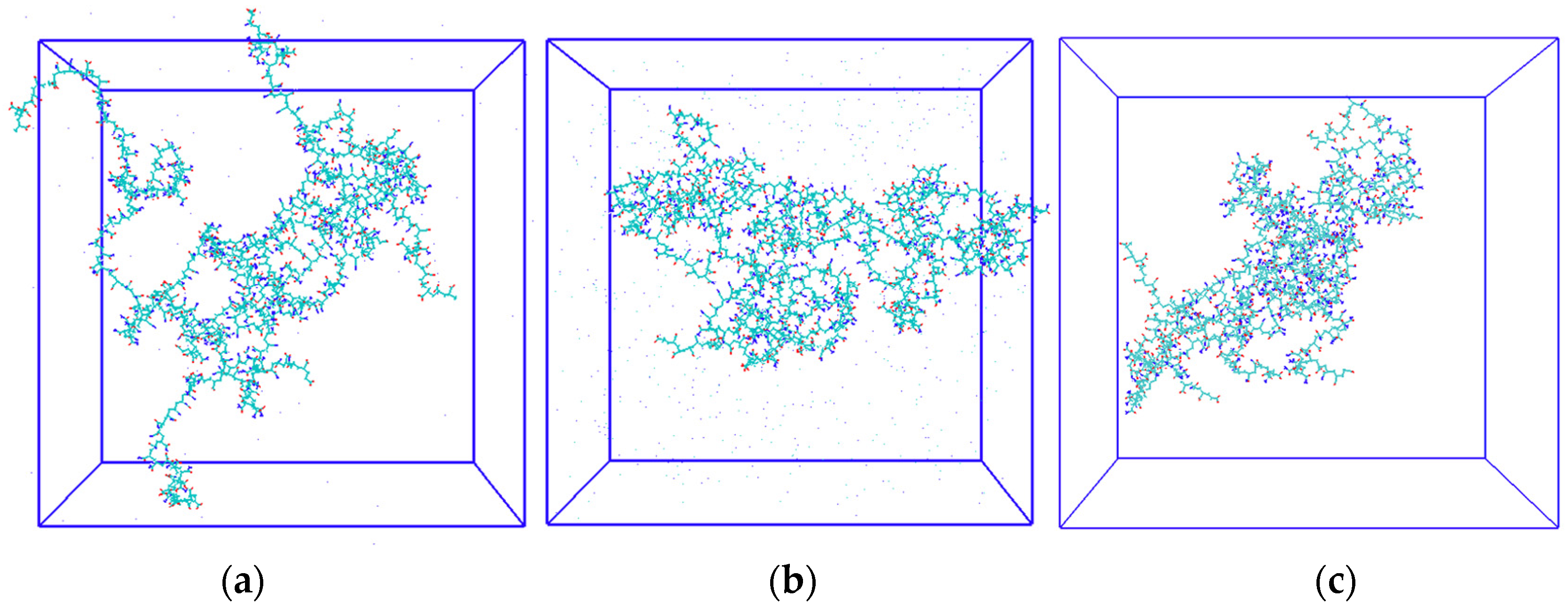
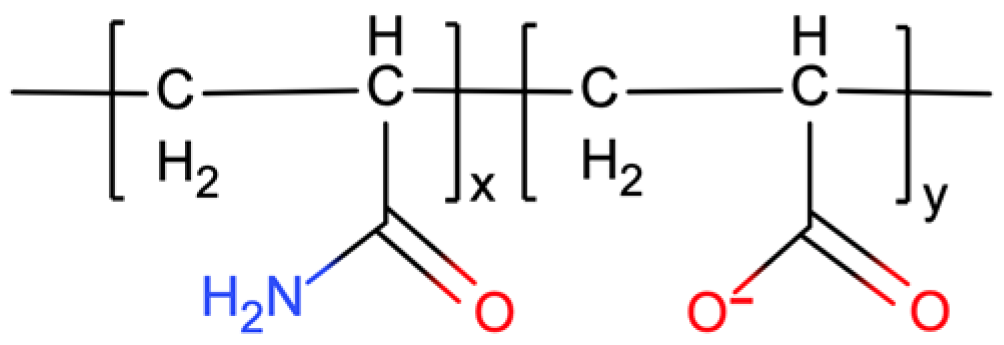
2.4. Conformation and Behavior of HPAM Solution
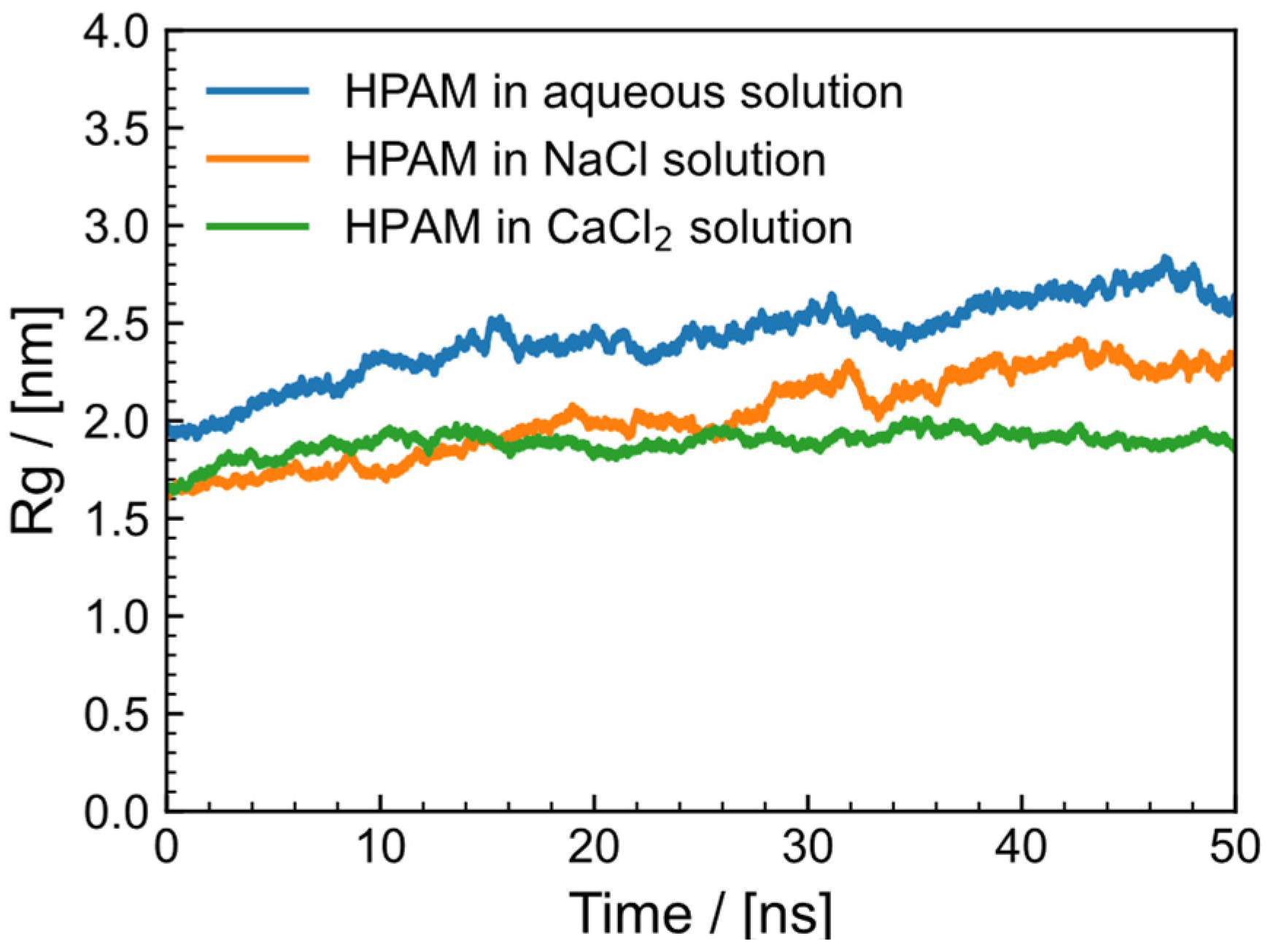
2.5. Gyration Radius of HPAM Solution
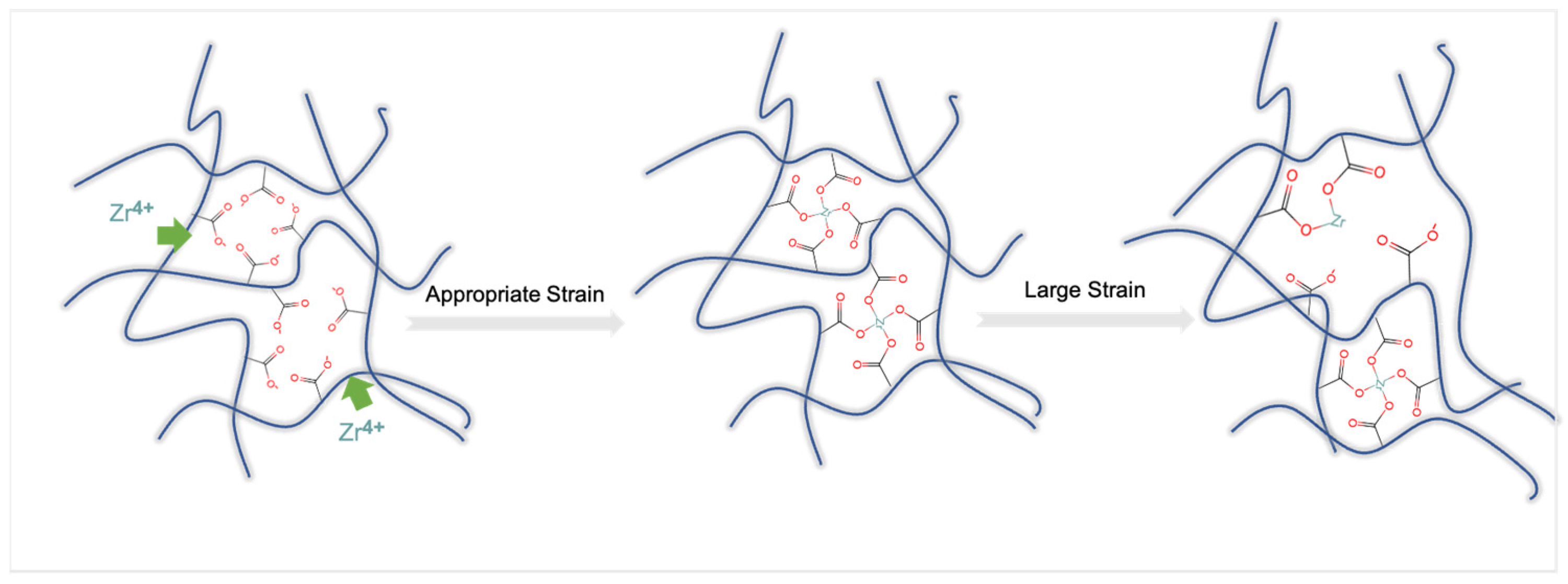
2.6. Ionic Bridge
2.7. Effect of Salt Solution on HPAM Fracturing Fluid
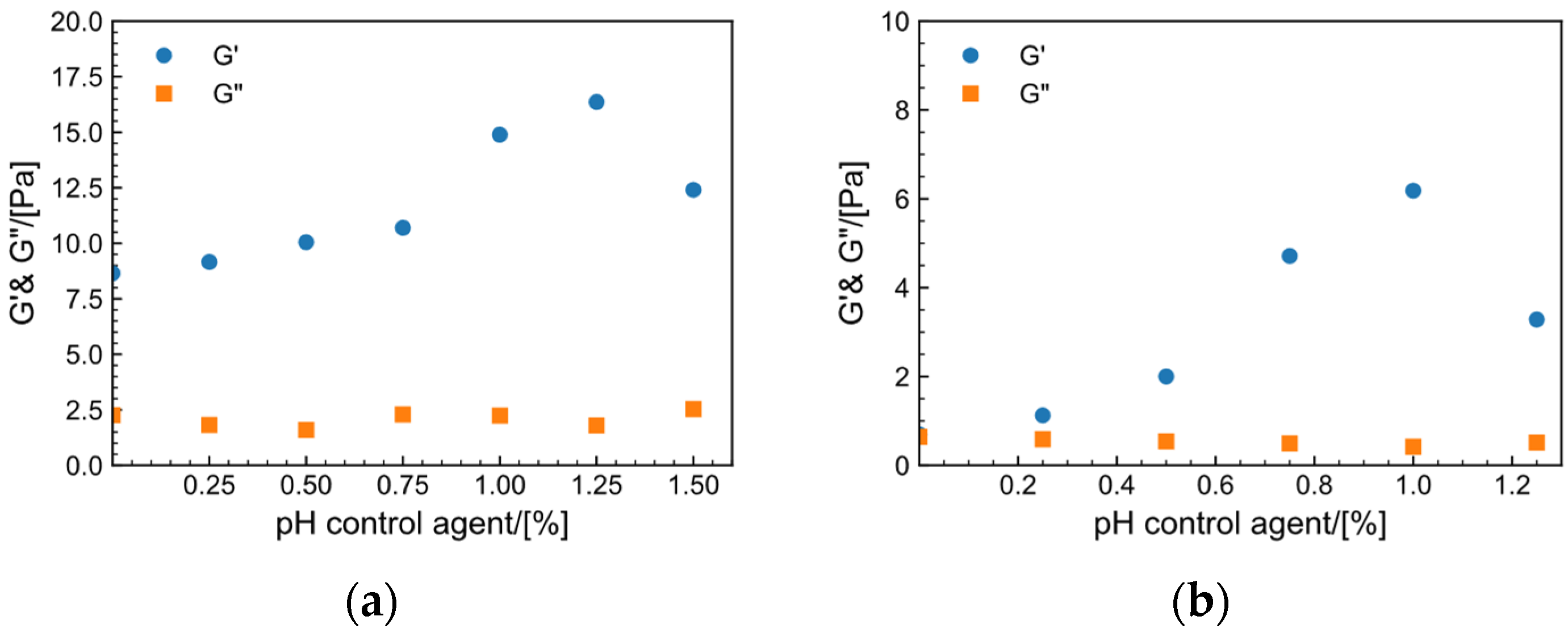
2.7.1. Effect of pH Control Agent
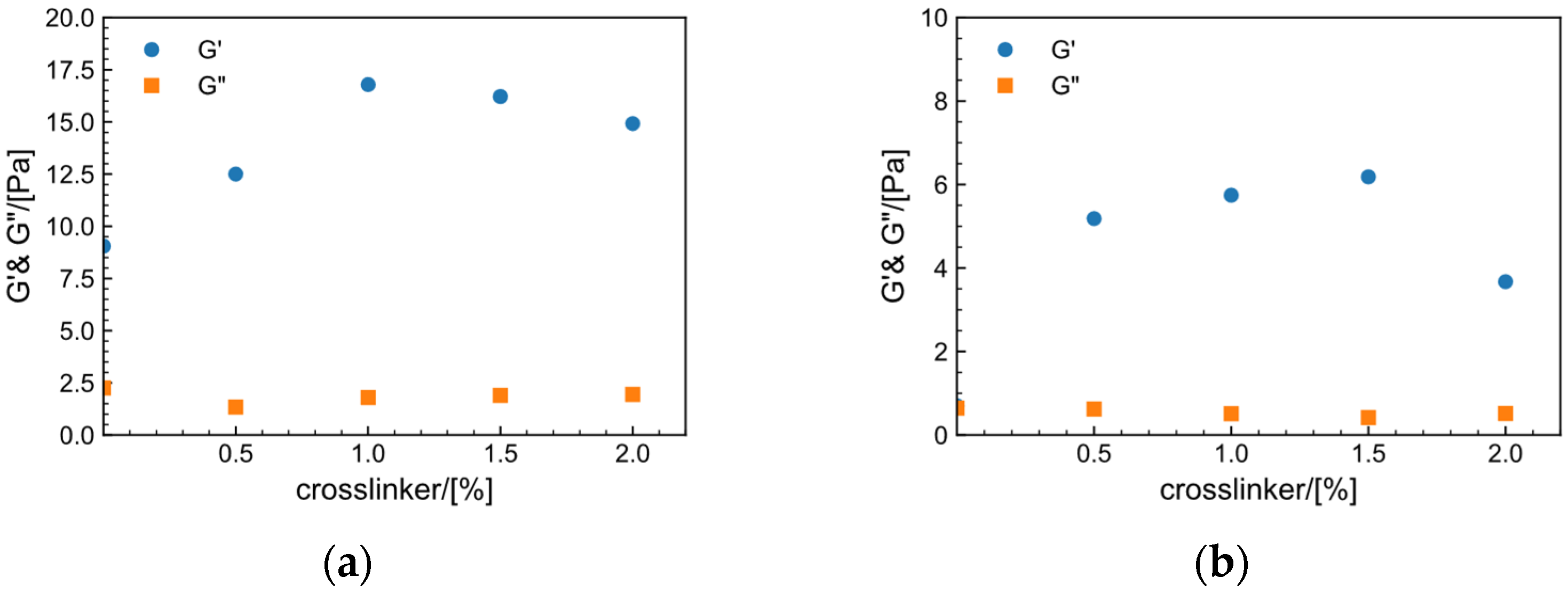
2.7.2. Effect of Crosslinker
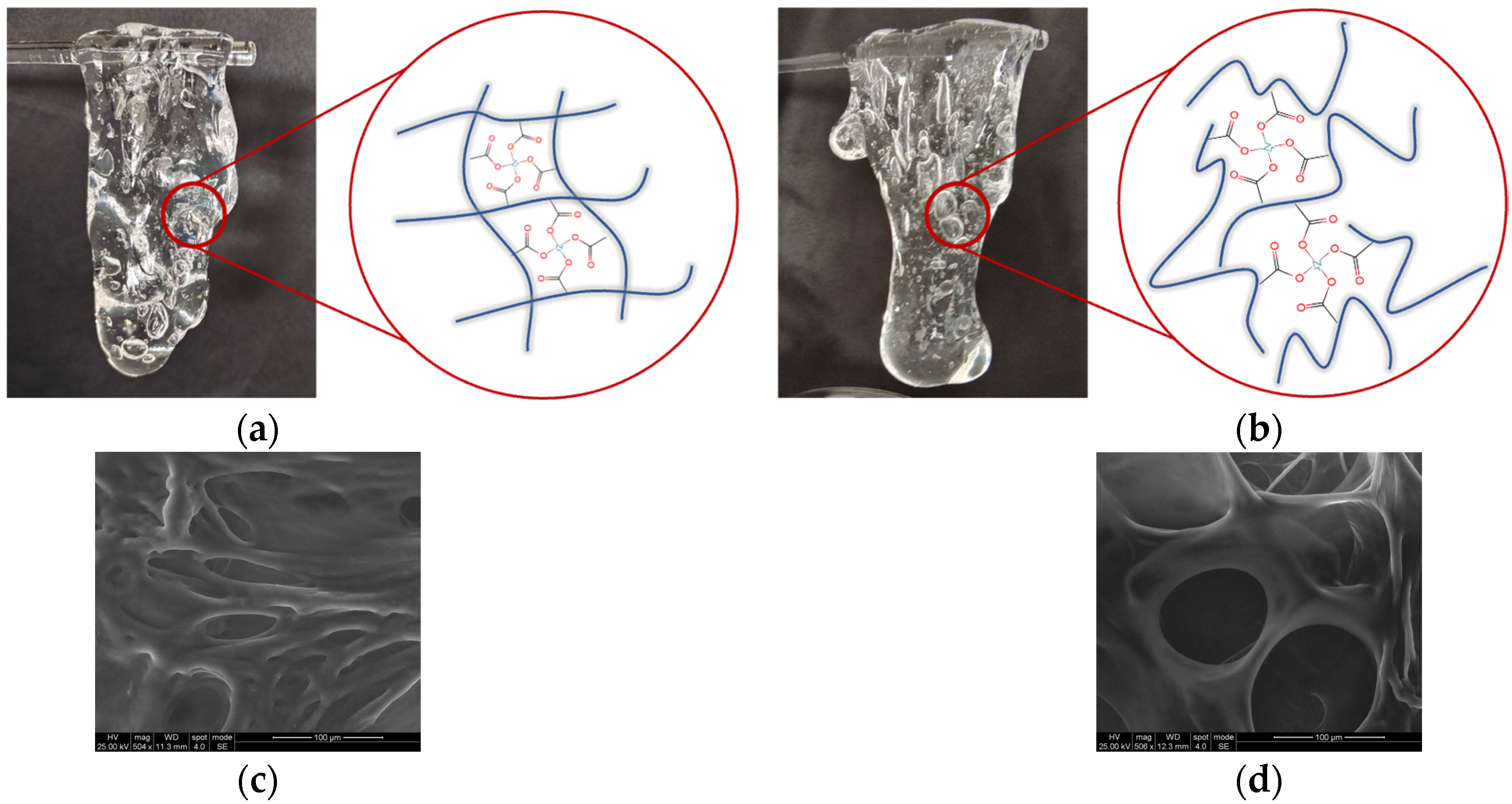
2.8. Microscopic Morphology and Picking Properties of Fracturing Fluid Gels
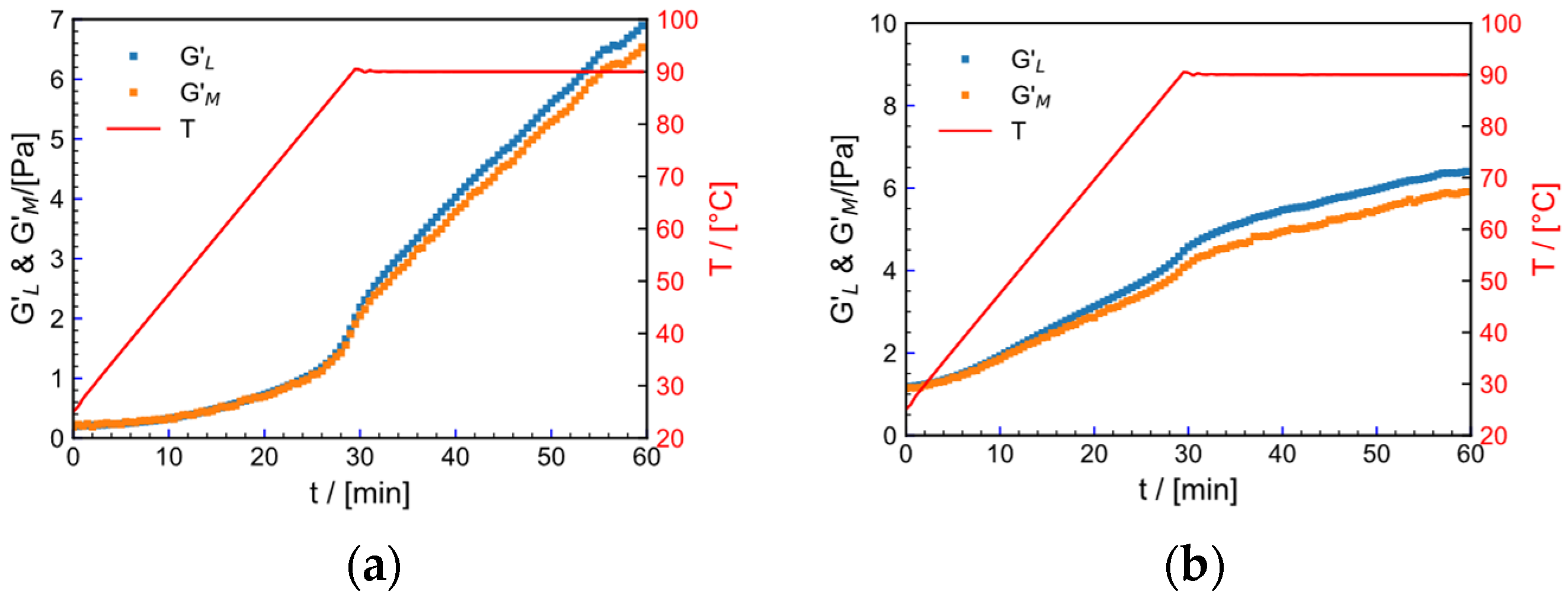
2.9. SAOS
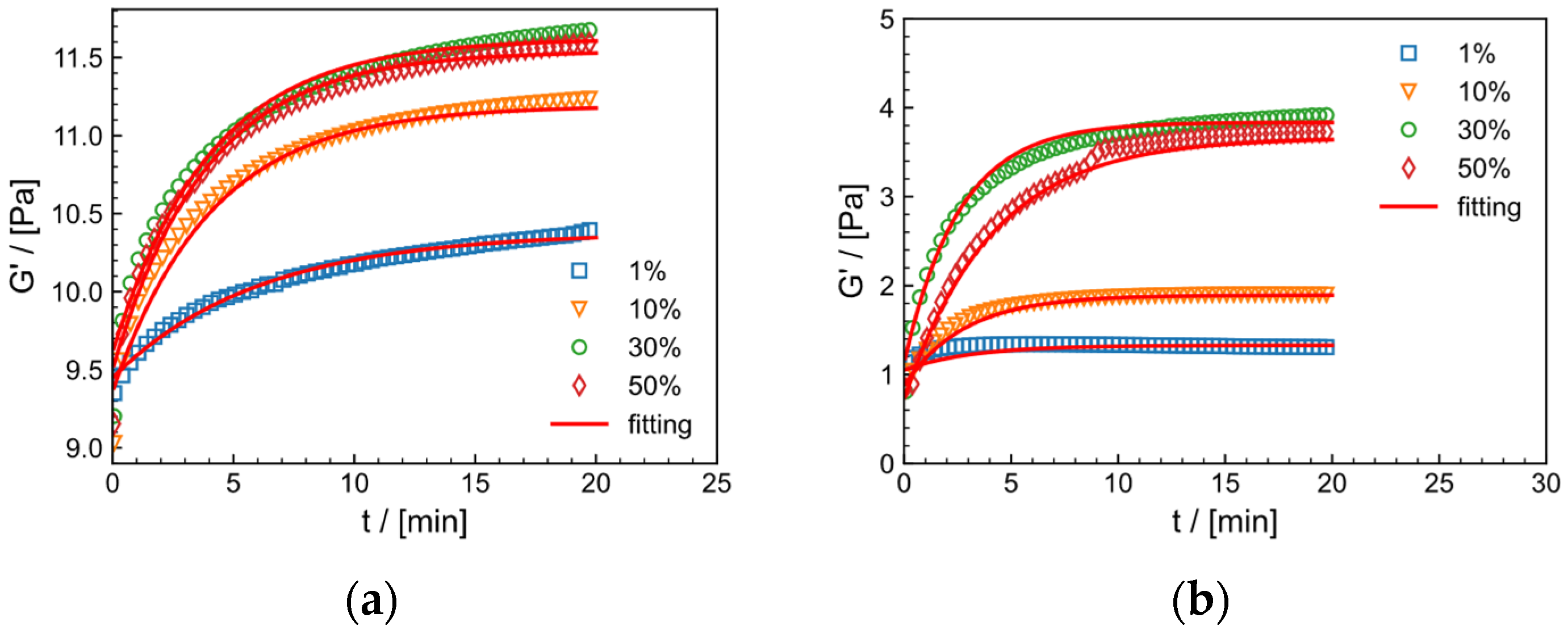
2.9.1. Effect of Strain on Crosslinking Process
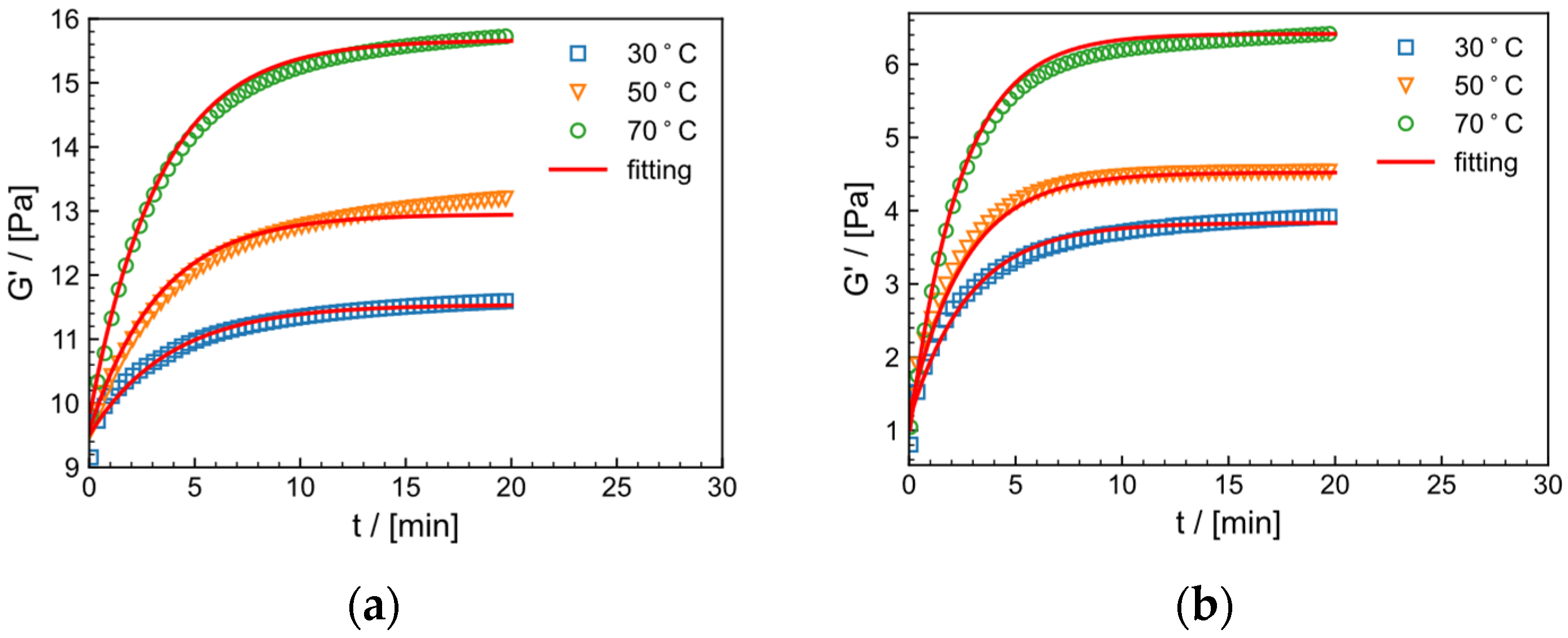
2.9.2. Effect of Temperature on Crosslinking Process
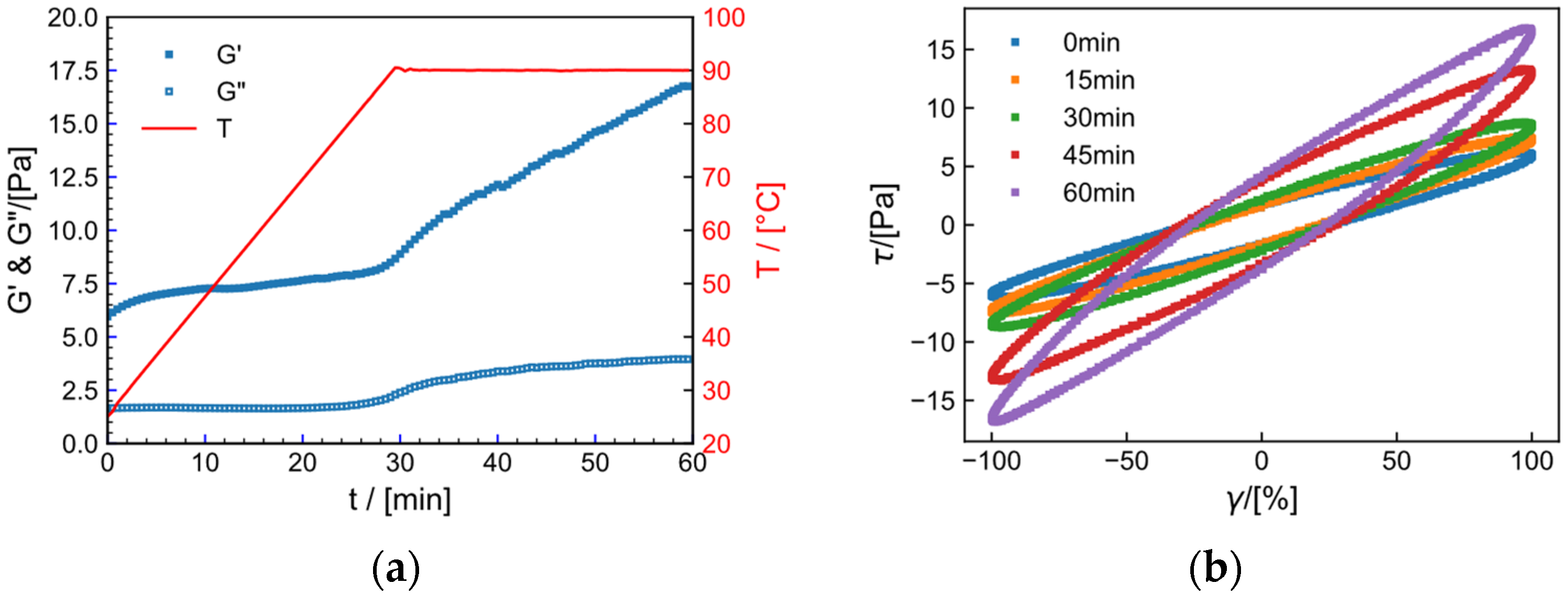
2.10. LAOS
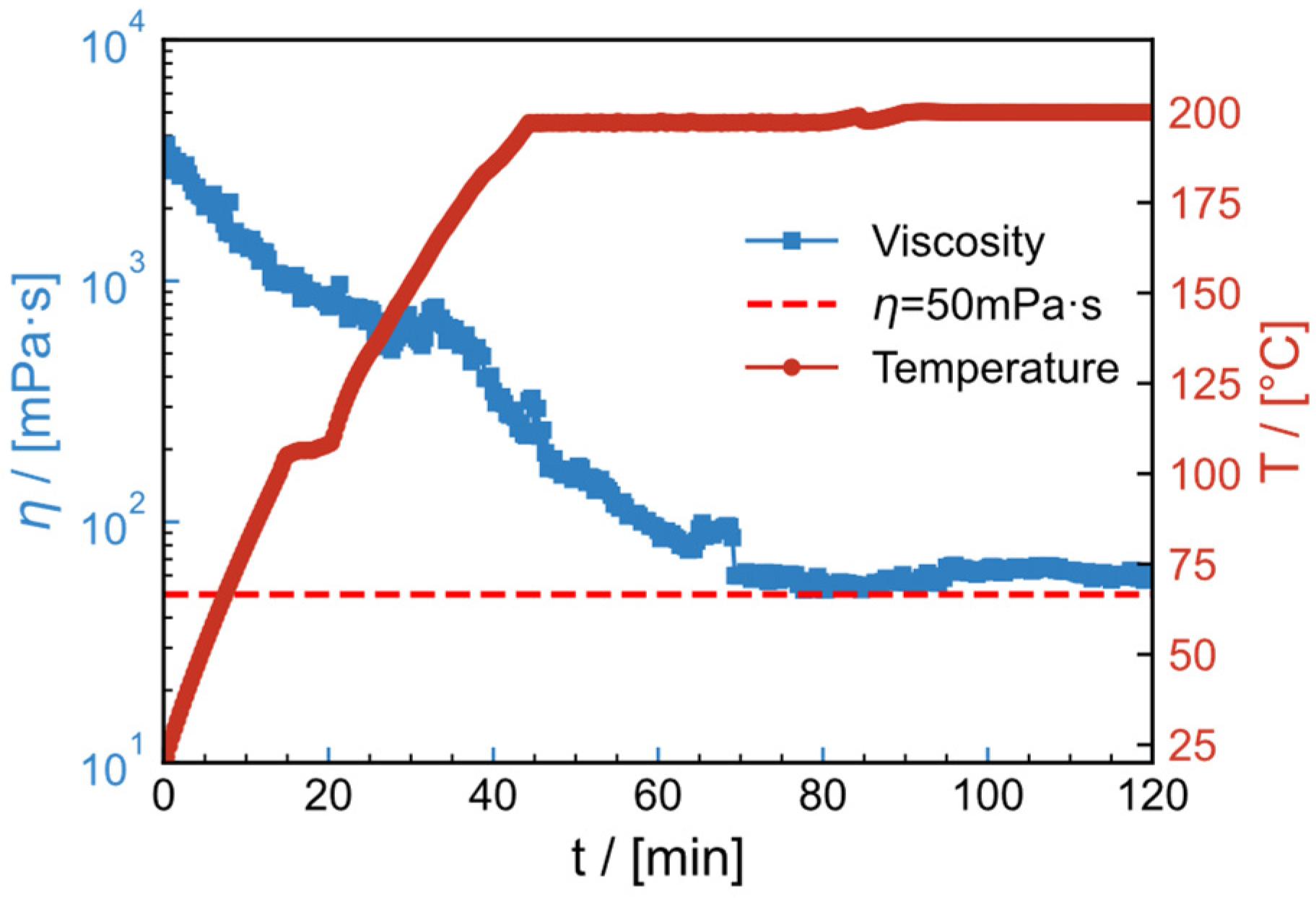
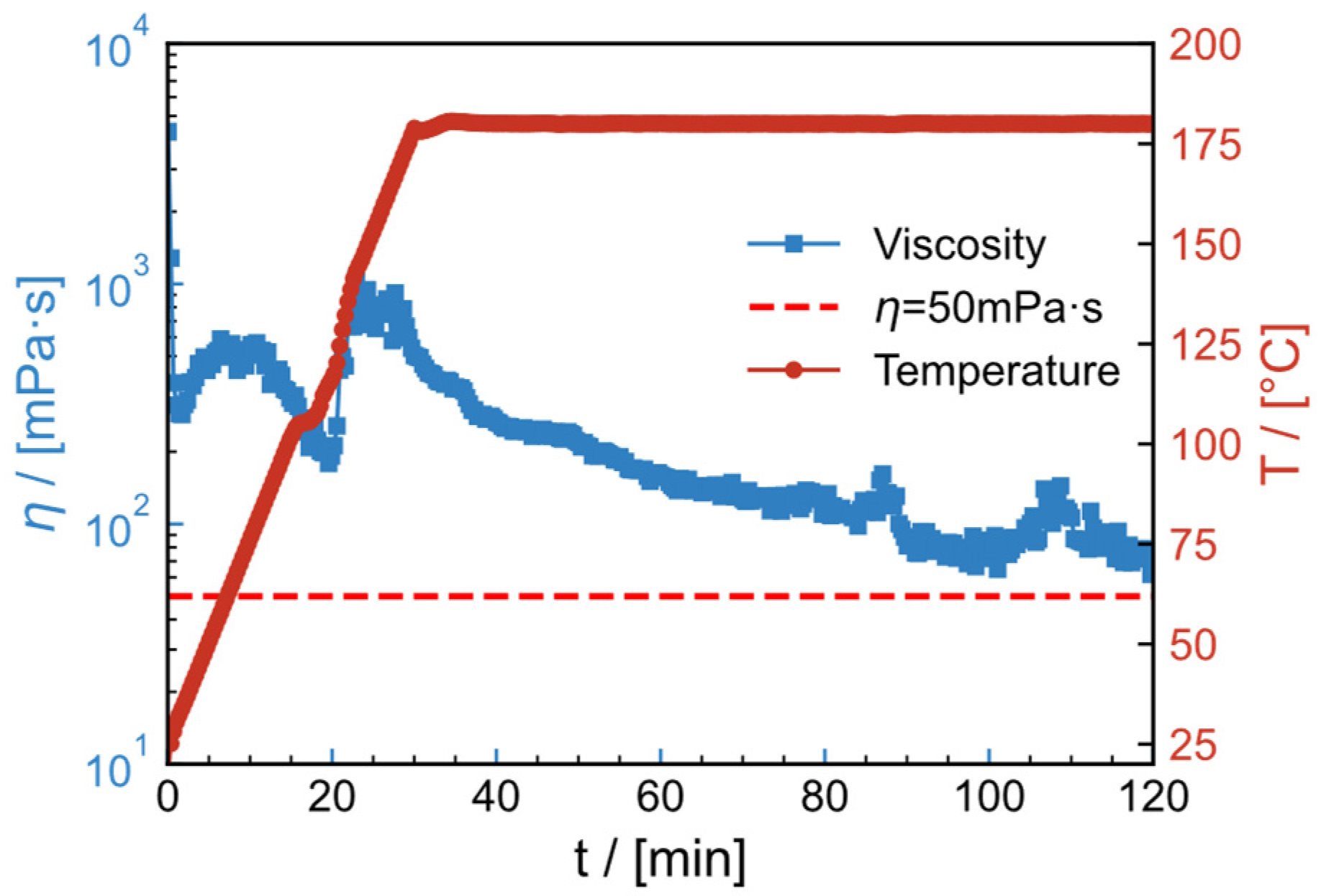
2.11. High-Temperature Resistance and Shear Resistance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Organic Zirconium Crosslinker
4.3. Preparation of Polymer Fracture Fluids Gel
4.4. Rheological Analysis
4.5. Thermal Stability Measurements
4.6. Morphological Analysis
4.7. Molecular Dynamics Simulation
4.7.1. Structural Models
4.7.2. Simulation Detail
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Zhao, L.; Liu, P.; Du, J.; Wang, Q.; An, Q.; Chang, B.; Luo, Z.; Zhang, N. Experimental Study and Field Verification of Fracturing Technique Using a Thermo-Responsive Diverting Agent. J. Nat. Gas Eng. 2021, 92, 103993. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Zhao, L.; Liu, P.; Chen, X.; Wang, Q.; Yu, M. Water-Soluble Polymers for High-Temperature Resistant Hydraulic Fracturing: A Review. J. Nat. Gas Eng. 2022, 104, 104673. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Z.; Zhao, M.; Gao, M.; Du, M.; Dai, C. Development and Evaluation of a Novel Seawater-Based Viscoelastic Fracturing Fluid System. J. Pet. Sci. Eng. 2019, 183, 106408. [Google Scholar] [CrossRef]
- Xu, K.; Lu, Y.; Chang, J.; Li, Y. Research Progress of High Temperature Resistant Fracturing Fluid System. J. Phys. Conf. Ser. 2021, 2076, 012039. [Google Scholar] [CrossRef]
- Gandossi, L.; Von Estorff, U. An Overview of Hydraulic Fracturing and Other Formation Stimulation Technologies for Shale Gas Production; Publications Office of the European Union: Luxembourg, 2013; pp. 10–13. [Google Scholar]
- Funkhouser, G.P.; Norman, L.R. Synthetic Polymer Fracturing Fluid for High-Temperature Applications. In International Symposium on Oilfield Chemistry; OnePetro: Richardson, TX, USA, 2003. [Google Scholar]
- Zhao, J.; Yang, B.; Mao, J.; Zhang, Y.; Yang, X.; Zhang, Z.; Shao, Y. A Novel Hydrophobic Associative Polymer by Raft-Madix Copolymerization for Fracturing Fluids with High Thermal Stability. Energy Fuels 2018, 32, 3039–3051. [Google Scholar] [CrossRef]
- Yang, B.; Mao, J.; Zhao, J.; Shao, Y.; Zhang, Y.; Zhang, Z.; Lu, Q. Improving the Thermal Stability of Hydrophobic Associative Polymer Aqueous Solution Using a “Triple-Protection” Strategy. Polymers 2019, 11, 949. [Google Scholar] [CrossRef]
- Su, X.; Feng, Y. Thermoviscosifying Smart Polymers for Oil and Gas Production: State of the Art. ChemPhysChem 2018, 19, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Elsaeed, S.M.; Zaki, E.G.; Omar, W.A.E.; Ashraf Soliman, A.; Attia, A.M. Guar Gum-Based Hydrogels as Potent Green Polymers for Enhanced Oil Recovery in High-Salinity Reservoirs. ACS Omega 2021, 6, 23421–23431. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent Advances in Cellulose and Its Derivatives for Oilfield Applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Z.; Wang, B.; Lu, H. Surfactant-Free Aqueous Foams Stabilized with Synergy of Xanthan-Based Amphiphilic Biopolymer and Nanoparticle as Potential Hydraulic Fracturing Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125215. [Google Scholar] [CrossRef]
- Tan, X.; Chen, J.; Fang, B.; Liu, B.; Gao, H.; Li, K.; Yu, L.; Xu, K.; Lu, Y.; Qiu, X. Rheology on High Temperature Resistant Novel Trimeric Cationic Viscoelastic Surfactant with Kcl. J. Dispers. Sci. Technol. 2022, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Tan, X.; Fang, B.; Liu, B.; Gao, H.; Li, K.; Yu, L.; Xu, K.; Lu, Y.; Qiu, X. Rheological Behavior of a Novel Fracturing Fluid Formed from Amine Oxide Surfactants. J. Surfactants Deterg. 2022, 25, 601–612. [Google Scholar] [CrossRef]
- Ge, Y.; Zhao, Z.; Cheng, X.; Chen, T.; Liu, T.; Guo, X. Research of a Novel Double Cross-Linking Fracturing Fluid. J. Pet. Explor. Prod. Technol. 2021, 11, 2191–2197. [Google Scholar] [CrossRef]
- Vega-Cantu, Y.I.; Hauge, R.H.; Norman, L.R.; Powell, R.J.; Billups, W.E. Effect of Magnesium and Iron on the Hydration and Hydrolysis of Guar Gum. Biomacromolecules 2006, 7, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Y.; Xie, X.; Slaný, M.; Dong, S.; Wu, Y.; Chen, G. Research of a Novel Fracturing-Production Integral Fluid Based on Cationic Surfactant. J. Mol. Liq. 2023, 369, 120858. [Google Scholar] [CrossRef]
- Amir, Z.; Said, I.M.; Jan, B.M. In Situ Organically Cross-Linked Polymer Gel for High-Temperature Reservoir Conformance Control: A Review. Polym. Adv. Technol. 2019, 30, 13–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Mao, J.; Chen, A.; Yang, X.; Lin, C.; Wei, Z.; Huang, X.; Song, L.; Tang, F. Towards Sustainable Oil/Gas Fracking by Reusing Its Process Water: A Review on Fundamentals, Challenges, and Opportunities. J. Pet. Sci. Eng. 2022, 213, 110422. [Google Scholar] [CrossRef]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A Review of Crosslinked Fracturing Fluids Prepared with Produced Water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Gupta, D.; Carman, P.; Venugopal, R. A Stable Fracturing Fluid for Produced Water Applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TA, USA, 8–10 October 2012. [Google Scholar]
- Almubarak, T.; AlKhaldi, M.; Ng, J.H.; Nasr-El-Din, H.A. Design and Application of High-Temperature Raw-Seawater-Based Fracturing Fluids. SPE J. 2019, 24, 1929–1946. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S.; Alkhowaildi, M. Rheological Study of Seawater-Based Fracturing Fluid Containing Polymer, Crosslinker, and Chelating Agent. ACS omega 2022, 7, 31318–31326. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Xu, T.; Zhang, Z.; Yang, B.; Mao, J.; Yang, X. Preparation of a Novel Fracturing Fluid with Good Heat and Shear Resistance. RSC Adv. 2019, 9, 1199–1207. [Google Scholar] [CrossRef]
- Holtsclaw, J.; Funkhouser, G.P. A Crosslinkable Synthetic Polymer System for High-Temperature Hydraulic Fracturing Applications. In Proceedings of the SPE Tight Gas Completions Conference, San Antonio, TA, USA, 16–17 June 2009. [Google Scholar]
- Xu, K.; Lu, Y.; Chang, J.; Lu, X.; Wang, P. Research and Performance Analysis of 245 °C Ultra-High Temperature Fracturing Liquid. In Proceedings of the International Field Exploration and Development Conference, Singapore, 27–29 September 2022. [Google Scholar]
- Shao, Y.; Mao, J.; Yang, B.; Zhao, J.; Yang, X. High Performance Hydrophobic Associated Polymer for Fracturing Fluids with Low-Dosage. Pet. Chem. 2020, 60, 219–225. [Google Scholar] [CrossRef]
- Yan, S.; Tang, J.; Yan, S.; Wang, Y.; Han, J.; Shi, S. Preparation and Performance of Novel Temperature-Resistant Thickening Agent. Polym. Adv. Technol. 2018, 29, 1022–1029. [Google Scholar] [CrossRef]
- Li, N.; Yu, J.; Wang, D.; Wang, C.; Kang, J.; Liu, P.; Huang, C.; Xiong, Y. Development Status of Crosslinking Agent in High-Temperature and Pressure Fracturing Fluid: A Review. J. Nat. Gas Eng. 2022, 107, 104369. [Google Scholar] [CrossRef]
- Carl, M. Fracturing Fluid Components. In Effective and Sustainable Hydraulic Fracturing; Andrew, P.B., John, M., Rob, J., Eds.; IntechOpen: Rijeka, Croatia, 2013; Volume 2, pp. 42–43. [Google Scholar]
- Zhou, M.; Zhang, J.; Zuo, Z.; Liao, M.; Peng, P. Preparation and Property Evaluation of a Temperature-Resistant Zr-Crosslinked Fracturing Fluid. J. Ind. Eng. Chem. 2021, 96, 121–129. [Google Scholar] [CrossRef]
- Almubarak, T.; Li, L.; Ng, J.H.; Nasr-El-Din, H.; AlKhaldi, M. New Insights into Hydraulic Fracturing Fluids Used for High-Temperature Wells. Petroleum 2021, 7, 70–79. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.C.; Nasr-El-Din, H.A.; Almubarak, M.; AlKhaldi, M. Influence of Zirconium Crosslinker Chemical Structure and Polymer Choice on the Performance of Crosslinked Fracturing Fluids. Can. J. Chem. Eng. 2022, 100, 1141–1157. [Google Scholar] [CrossRef]
- Li, G.; Fang, B.; Lu, Y.; Li, K.; Ma, M.; Yang, M.; Qiu, X.; Wang, L.; Liu, Y. Rheological Properties and Crosslinking Rheo-Kinetics of Cmhec/Ctab Synergistic Systems. J. Dispers. Sci. Technol. 2016, 37, 1826–1831. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Cao, Z.; Hao, T.; Liu, Y.; Wu, W. High-Temperature Performance and Cross-Linking Mechanism of Different Types of Gel Systems in Saline Environment. J. Appl. Polym. Sci. 2022, 139, 51452. [Google Scholar] [CrossRef]
- Al-Hamairi, A.; AlAmeri, W. Development of a Novel Model to Predict Hpam Viscosity with the Effects of Concentration, Salinity and Divalent Content. J. Pet. Explor. Prod. Technol. 2020, 10, 1949–1963. [Google Scholar] [CrossRef] [Green Version]
- Kawale, D.; Marques, E.; Zitha, P.L.; Kreutzer, M.T.; Rossen, W.R.; Boukany, P.E. Elastic Instabilities During the Flow of Hydrolyzed Polyacrylamide Solution in Porous Media: Effect of Pore-Shape and Salt. Soft Matter 2017, 13, 765–775. [Google Scholar] [CrossRef]
- Mitishita, R.S.; Elfring, G.J.; Frigaard, I.A. Statistics and Spectral Analysis of Turbulent Duct Flows with Flexible and Rigid Polymer Solutions. J. Non-Newtonian Fluid Mech. 2022, 311, 104952. [Google Scholar] [CrossRef]
- Chen, P.; Yao, L.; Liu, Y.; Luo, J.; Zhou, G.; Jiang, B. Experimental and Theoretical Study of Dilute Polyacrylamide Solutions: Effect of Salt Concentration. J. Mol. Model. 2012, 18, 3153–3160. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhu, Y.; Ji, Y.; Xu, H.; Zhang, H.; Yuan, S. Effect of Salt-Resistant Monomers on Viscosity of Modified Polymers Based on the Hydrolyzed Poly-Acrylamide (Hpam): A Molecular Dynamics Study. J. Mol. Liq. 2021, 325, 115161. [Google Scholar] [CrossRef]
- Abdel-Azeim, S.; Kanj, M.Y. Dynamics, Aggregation, and Interfacial Properties of the Partially Hydrolyzed Polyacrylamide Polymer for Enhanced Oil Recovery Applications: Insights from Molecular Dynamics Simulations. Energy Fuels 2018, 32, 3335–3343. [Google Scholar] [CrossRef]
- Quezada, G.R.; Saavedra, J.H.; Rozas, R.E.; Toledo, P.G. Molecular Dynamics Simulations of the Conformation and Diffusion of Partially Hydrolyzed Polyacrylamide in Highly Saline Solutions. Chem. Eng. Sci. 2020, 214, 115366. [Google Scholar] [CrossRef]
- Belyadi, H.; Fathi, E.; Belyadi, F. Chapter Eight—Hydraulic Fracturing Chemical Selection and Design. In Hydraulic Fracturing in Unconventional Reservoirs, 2nd ed.; Belyadi, H., Fathi, E., Belyadi, F., Eds.; Gulf Professional Publishing: Oxford, UK, 2019; pp. 107–120. [Google Scholar]
- Wang, Z.; Lin, M.; Xiang, Y.; Zeng, T.; Dong, Z.; Zhang, J.; Yang, Z. Zr-Induced Thermostable Polymeric Nanospheres with Double-Cross-Linked Architectures for Oil Recovery. Energy Fuels 2019, 33, 10356–10364. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Xu, Z.; Wang, K.; Gao, M.; Lv, W.; Dai, C. Dynamic Cross-Linking Mechanism of Acid Gel Fracturing Fluid. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125471. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A Review of Nonlinear Oscillatory Shear Tests: Analysis and Application of Large Amplitude Oscillatory Shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Ewoldt, R.H.; Hosoi, A.; McKinley, G.H. New Measures for Characterizing Nonlinear Viscoelasticity in Large Amplitude Oscillatory Shear. J. Rheol. 2008, 52, 1427–1458. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. Gromacs: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber Ff99sb Protein Force Field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling through Velocity Rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]




| CHPAM = 0.6 wt% | Model Parameters | R | |||
|---|---|---|---|---|---|
| /s | |||||
| In DI water | 54,310 ) | 7.80 () | 4.73 () | 0.99 | |
| In tap water | 36,440 | 7.27 ) | 4.29 () | 0.04 ( | 0.99 |
| In salt solution | 586.9 | 6.32 ( | 1.82 () | 0.49 () | 0.99 |
| Model Parameters | ||||
|---|---|---|---|---|
| Tap Water System | ||||
| /s | ||||
| 1 | 10.38 | 9.45 | 0.17 | 0.98 |
| 10 | 11.19 | 9.36 | 0.24 | 0.98 |
| 30 | 11.54 | 9.50 | 0.26 | 0.99 |
| 50 | 11.62 | 9.61 | 0.25 | 0.99 |
| salt solution system | ||||
| 1 | 1.33 | 1.05 | 0.32 | 0.97 |
| 10 | 1.89 | 0.87 | 0.35 | 0.98 |
| 30 | 3.83 | 1.13 | 0.36 | 0.98 |
| 50 | 3.66 | 0.73 | 0.24 | 0.99 |
| /°C | Model Parameters | |||
|---|---|---|---|---|
| Tap Water System | ||||
| /s | ||||
| 30 | 11.54 | 9.50 | 0.26 | 0.99 |
| 50 | 12.95 | 9.57 | 0.29 | 0.97 |
| 70 | 15. 67 | 9.78 | 0.30 | 0.98 |
| salt solution system | ||||
| 30 | 3.83 | 1.13 | 0.36 | 0.98 |
| 50 | 4.52 | 1.22 | 0.38 | 0.98 |
| 70 | 6.41 | 0.91 | 0.42 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.; Fang, B.; Yu, L.; Lu, Y.; Xu, K.; Li, K. Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM. Gels 2023, 9, 151. https://doi.org/10.3390/gels9020151
Xin H, Fang B, Yu L, Lu Y, Xu K, Li K. Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM. Gels. 2023; 9(2):151. https://doi.org/10.3390/gels9020151
Chicago/Turabian StyleXin, Hui, Bo Fang, Luyao Yu, Yongjun Lu, Ke Xu, and Kejing Li. 2023. "Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM" Gels 9, no. 2: 151. https://doi.org/10.3390/gels9020151
APA StyleXin, H., Fang, B., Yu, L., Lu, Y., Xu, K., & Li, K. (2023). Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM. Gels, 9(2), 151. https://doi.org/10.3390/gels9020151