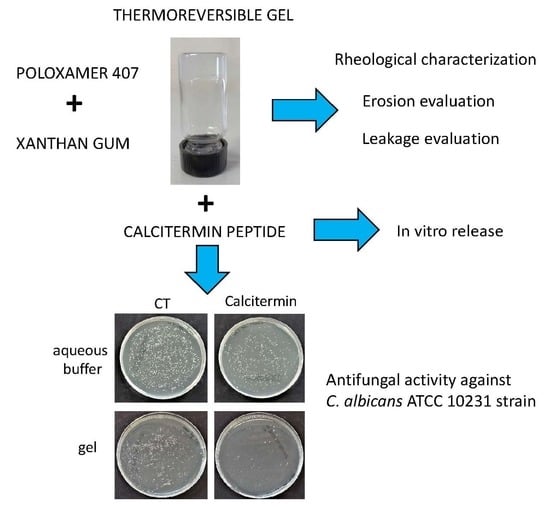
Calcitermin-Loaded Smart Gels Activity against Candida albicans: A Preliminary In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Gel Formulative Study
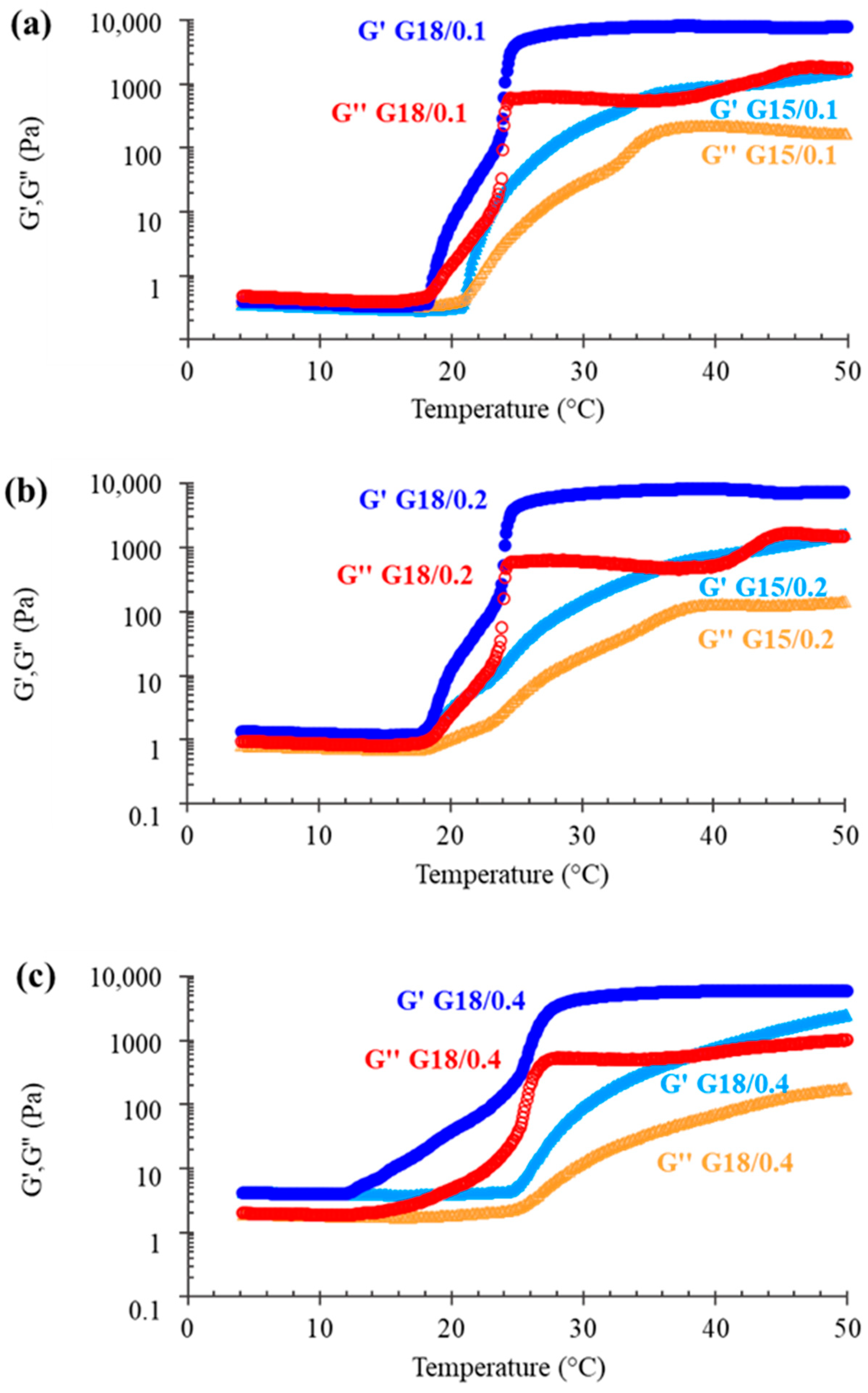
2.1.1. Rheological Characterization
2.1.2. Erosion Evaluation
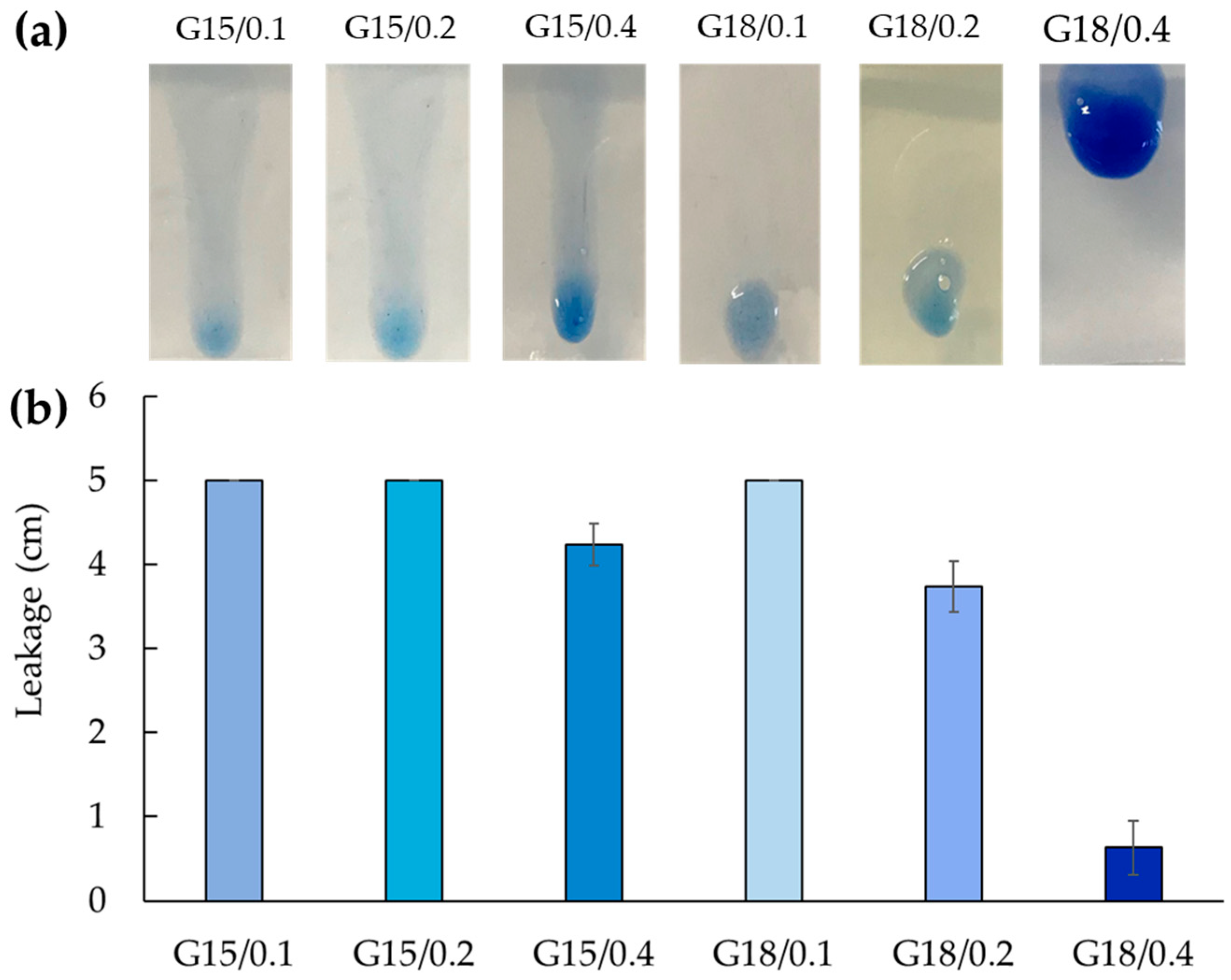
2.1.3. Leakage Evaluation
2.2. Cal-Loading in Gels
2.3. In Vitro Release Tests
2.4. Microbiological Evaluation
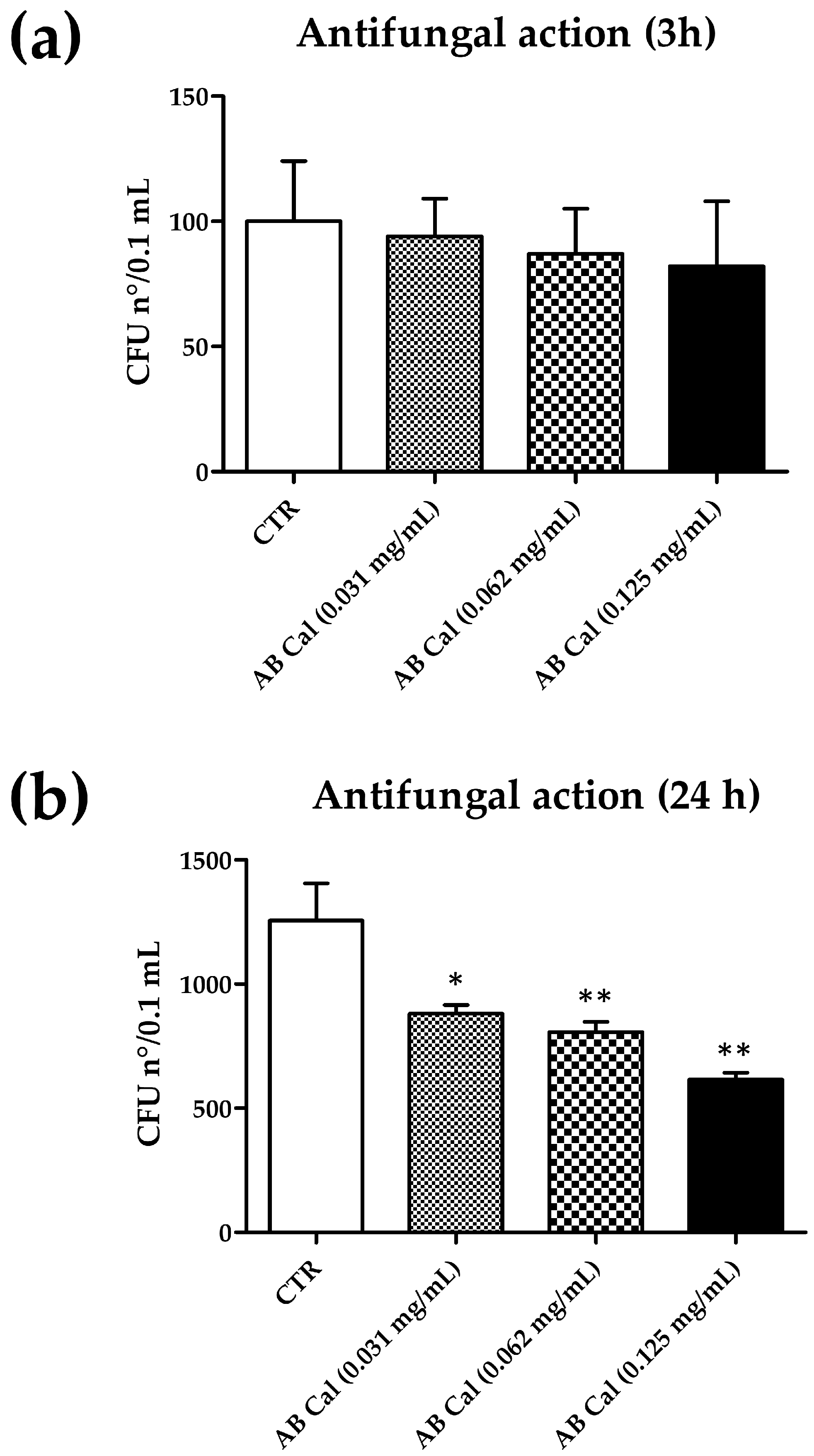
2.4.1. Antifungal Activity of Cal in Aqueous Buffer
2.4.2. Antifungal Activity of Cal in Gel
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Gel Preparation
5.3. Rheological Measurements
5.4. Erosion and Leakage Tests
5.5. Cal-Loaded Gels Preparation
Evaluation of Cal Content in Gels
5.6. In Vitro Release Test
5.7. HPLC Procedures
5.8. Antifungal Activity
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.E.; Bicanic, T. Drug Resistance and Novel Therapeutic Approaches in Invasive Candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.; Song, N.; Pang, Q.; Mei, H.; Zheng, H.; Li, D.; Cui, F.; Lv, G.; An, R.; Li, P.; et al. In Vitro Antifungal Activity of Azoles and Other Antifungal Agents Against Pathogenic Yeasts from Vulvovaginal Candidiasis in China. Mycopathologia 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Remelli, M. Lights and Shadows on the Therapeutic Use of Antimicrobial Peptides. Molecules 2022, 27, 4584. [Google Scholar] [CrossRef]
- Bastos, P.; Trindade, F.; da Costa, J.; Ferreira, R.; Vitorino, R. Human Antimicrobial Peptides in Bodily Fluids: Current Knowledge and Therapeutic Perspectives in the Postantibiotic Era. Med. Res. Rev. 2018, 38, 101–146. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, 1087–1093. [Google Scholar] [CrossRef]
- Som, A.; Yang, L.; Wong, G.C.L.; Tew, G.N. Divalent Metal Ion Triggered Activity of a Synthetic Antimicrobial in Cardiolipin Membranes. J. Am. Chem. Soc. 2009, 131, 15102–15103. [Google Scholar] [CrossRef]
- Dashper, S.G.; O’Brien-Simpson, N.M.; Cross, K.J.; Paolini, R.A.; Hoffmann, B.; Catmull, D.V.; Malkoski, M.; Reynolds, E.C. Divalent metal cations increase the activity of the antimicrobial Peptide kappacin. Antimicrob. Agents Chemother. 2005, 49, 2322–2328. [Google Scholar] [CrossRef]
- Melino, S.; Rufini, S.; Sette, M.; Morero, R.; Grottesi, A.; Paci, M.; Petruzzelli, R. Zn2+ Ions Selectively Induce Antimicrobial Salivary Peptide Histatin-5 To Fuse Negatively Charged Vesicles. Identification and Characterization of a Zinc-Binding Motif Present in the Functional Domain. Biochemistry 1999, 38, 9626–9633. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Thompson, Z.; Cowan, J.A. Antimicrobial Metallopeptides. ACS Chem. Biol. 2018, 13, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Łoboda, D.; Kozłowski, H.; Rowińska-Żyrek, M. Antimicrobial peptide–metal ion interactions—A potential way of activity enhancement. New J. Chem. 2018, 42, 7560–7568. [Google Scholar] [CrossRef]
- Libardo, M.D.J.; Angeles-Boza, A.M. Bioinorganic Chemistry of Antimicrobial and Host-Defense Peptides. Comments Inorg. Chem. 2014, 34, 42–58. [Google Scholar] [CrossRef]
- Cole, A.M.; Kim, Y.-H.; Tahk, S.; Hong, T.; Weis, P.; Waring, A.J.; Ganz, T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001, 504, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Toniolo, M.; Dudek, D.; Mikołajczyk, A.; Guerrini, R.; Matera-Witkiewicz, A.; Remelli, M.; Rowińska-Żyrek, M. Bioinorganic chemistry of calcitermin—The picklock of its antimicrobial activity. Dalton Trans. 2019, 48, 13740–13752. [Google Scholar] [CrossRef]
- Bellotti, D.; Miller, A.; Rowińska-Żyrek, M.; Remelli, M. Zn2+ and Cu2+ Binding to the Extramembrane Loop of Zrt2, a Zinc Transporter of Candida albicans. Biomolecules 2022, 12, 121. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Looking into Candida albicans infection, host response, and antifungal strategies. Virulence 2015, 6, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans–epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Del. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Del. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Contado, C.; Costenaro, A.; Drechsler, M.; Rossi, D.; Menegatti, E.; Grandini, A.; Cortesi, R. Clotrimazole nanoparticle gel for mucosal administration. Mater. Sci. Eng. C 2013, 33, 411–418. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B. Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Patel, V.P.; Damasiya, H.M.; Kapupara, P.; Ashara, K.C. Temperature-dependent in Situ Gel of Clotrimazole: An Experimental Study. Folia Med. 2019, 61, 266–276. [Google Scholar] [CrossRef]
- Arslan, A.; Kose Ozkan, C.; Sig, A.K.; Dogan, E.; Esim, O.; Cetinkaya, S.; Atalay, F.; Tas, C.; Savaser, A.; Ozkan, Y. Evaluation of a novel oxiconazole nitrate formulation: The thermosensitive gel. Saudi Pharm. J. 2018, 26, 665–672. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincón, M.; Bozal, N.; Domenech, O.; Rodríguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.U.; Rajput, A.P.; Belgamwar, V.S.; Chalikwar, S.S. Development and characterization of amphotericin B nanoemulsion-loaded mucoadhesive gel for treatment of vulvovaginal candidiasis. Heliyon 2022, 8, e11489. [Google Scholar] [CrossRef]
- Hsin, Y.K.; Thangarajoo, T.; Choudhury, H.; Pandey, M.; Meng, L.W.; Gorain, B. Stimuli-Responsive in situ Spray Gel of Miconazole Nitrate for Vaginal Candidiasis. J. Pharm. Sci. 2023, 112, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Hilmioglu-Polat, S.; Metin, D.Y.; Zekioglu, O.; Guneri, T.; Jones, D.S. In-situ gel formulations of econazole nitrate: Preparation and in-vitro and in-vivo evaluation. J. Pharm. Pharmacol. 2011, 63, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.C.R.; Nguyen, S.; Nazzal, S.; Alayoubi, A.; Jung, R.; Nesamony, J. Thermoresponsive fluconazole gels for topical delivery: Rheological and mechanical properties, in vitro drug release and anti-fungal efficacy. Pharm. Dev. Technol. 2015, 20, 41–49. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Şenyiğit, Z.A.; Eraç, B.; Limoncu, M.H.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: Formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2017, 22, 551–561. [Google Scholar] [CrossRef]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Bhowmik, M.; Kumari, P.; Sarkar, G.; Bain, M.K.; Bhowmick, B.; Mollick, M.M.R.; Mondal, D.; Maity, D.; Rana, D.; Bhattacharjee, D.; et al. Effect of xanthan gum and guar gum on in situ gelling ophthalmic drug delivery system based on poloxamer-407. Int. J. Biol. Macromol. 2013, 62, 117–123. [Google Scholar] [CrossRef]
- Grela, K.P.; Bagińska, I.; Burak, J.; Marciniak, D.M.; Karolewicz, B. Natural Gums As Viscosity-Enhancers in Pluronic® F-127 Thermogelling Solutions. Pharmazie 2019, 74, 334–339. [Google Scholar] [PubMed]
- Singh, S.; Gajra, B.; Rawat, M.; Muthu, M.S. Enhanced transdermal delivery of ketoprofen from bioadhesive gels. Pak. J. Pharm. Sci. 2009, 22, 193–198. [Google Scholar] [PubMed]
- Abu-Huwaij, R.; Obaidat, R.M.; Sweidan, K.; Al-Hiari, Y. Formulation and In Vitro Evaluation of Xanthan Gum or Carbopol 934-Based Mucoadhesive Patches, Loaded with Nicotine. AAPS PharmSciTech 2011, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Faccendini, A.; Puccio, A.; Caramella, C. Comparison of poloxamer- and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug Dev. Ind. Pharm. 2014, 40, 352–360. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Dessì, M.; Borzacchiello, A.; Mohamed, T.H.A.; Abdel-Fattah, W.I.; Ambrosio, L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 2984–2993. [Google Scholar] [CrossRef]
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Methylcellulose Based Thermally Reversible Hydrogel System for Tissue Engineering Applications. Cells 2013, 2, 460–475. [Google Scholar] [CrossRef]
- FDA Guidance for Industry: SUPAC-SS: Nonsterile Semisolid Dosage Forms; Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls; In-Vitro Release Testing and In Vivo Bioequivalence Documentation—ECA Academy. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ss-nonsterile-semisolid-dosage-forms-scale-and-post-approval-changes-chemistry-manufacturing (accessed on 1 February 2023).
- Sicurella, M.; Sguizzato, M.; Cortesi, R.; Huang, N.; Simelière, F.; Montesi, L.; Marconi, P.; Esposito, E. Mangiferin-Loaded Smart Gels for HSV-1 Treatment. Pharmaceutics 2021, 13, 1323. [Google Scholar] [CrossRef]
- Shah, V.P.; Simona Miron, D.; Ștefan Rădulescu, F.; Cardot, J.-M.; Maibach, H.I. In vitro release test (IVRT): Principles and applications. Int. J. Pharm. 2022, 626, 122159. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Snigdha, S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Polymer–Water Interactions in Hydrogels. In Nano Hydrogels: Physico-Chemical Properties and Recent Advances in Structural Designing; Jose, J., Thomas, S., Thakur, V.K., Eds.; Springer: Singapore, 2021; pp. 127–133. [Google Scholar]
- Zeng, N.; Dumortier, G.; Maury, M.; Mignet, N.; Boudy, V. Influence of additives on a thermosensitive hydrogel for buccal delivery of salbutamol: Relation between micellization, gelation, mechanic and release properties. Int. J. Pharm. 2014, 467, 70–83. [Google Scholar] [CrossRef]
- Djekic, L.; Krajisnik, D.; Martinovic, M.; Djordjevic, D.; Primorac, M. Characterization of gelation process and drug release profile of thermosensitive liquid lecithin/poloxamer 407 based gels as carriers for percutaneous delivery of ibuprofen. Int. J. Pharm. 2015, 490, 180–189. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagpal, M.; Singh, M.; Singh, T.G.; Aggarwal, G.; Dhingra, G.A. Improved antibacterial activity of topical gel-based on nanosponge carrier of cinnamon oil. BioImpacts 2021, 11, 23–31. [Google Scholar] [CrossRef]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef]
- De Seta, F.; Larsen, B. Antimicrobial Activity of a Vaginal Gel Formulation: Considerations Related to Vaginal Infection and Dysbiosis. Pathogens 2021, 10, 1576. [Google Scholar] [CrossRef]
- Borse, V.A.; Gangude, A.B.; Deore, A.B. Formulation and Evaluation of Antibacterial Topical Gel of Doxycycline Hyclate, Neem Oil and Tea Tree Oil. Indian J. Pharm. Educ. Res. 2020, 54, 206–212. [Google Scholar] [CrossRef]
- Figueiredo de Almeida Gomes, B.P.; Vianna, M.E.; Sena, N.T.; Zaia, A.A.; Ferraz, C.C.R.; de Souza Filho, F.J. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Awad, G.E.A.; Makhlouf, A.I.A. Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: Formulation development and in vivo therapeutic efficacy in rats. Eur. J. Pharm. Sci. 2018, 114, 255–266. [Google Scholar] [CrossRef]
- Alam, A.; Jawaid, T.; Alsanad, S.M.; Kamal, M.; Rawat, P.; Singh, V.; Alam, P.; Alam, P. Solubility Enhancement, Formulation Development, and Antibacterial Activity of Xanthan-Gum-Stabilized Colloidal Gold Nanogel of Hesperidin against Proteus vulgaris. Gels 2022, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Hosny, K.M.; Rizg, W.Y.; Eshmawi, B.A.; Badr, M.Y.; Safhi, A.Y.; Murshid, S.S.A. Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection. Gels 2022, 8, 241. [Google Scholar] [CrossRef]
- Simm, C.; May, R.C. Zinc and Iron Homeostasis: Target-Based Drug Screening as New Route for Antifungal Drug Development. Front. Cell. Infect. Microbiol. 2019, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiofur. J. Control. Release 2002, 85, 73–81. [Google Scholar] [CrossRef] [PubMed]



| Gels | Poloxamer 407 | Xanthan Gum | Lactate Buffer * | pH |
|---|---|---|---|---|
| G15/0.1 | 15 | 0.10 | 84.85 | 4.4 |
| G15/0.2 | 15 | 0.20 | 84.80 | 4.5 |
| G15/0.4 | 15 | 0.40 | 84.60 | 4.5 |
| G18/0.1 | 18 | 0.10 | 81.90 | 4.4 |
| G18/0.2 | 18 | 0.20 | 81.70 | 4.4 |
| G18/0.4 | 18 | 0.40 | 81.60 | 4.6 |
| Gels | Tsol-gel § (°C) | Determination of Tsol-gel | Leakage (cm) * |
|---|---|---|---|
| G15/0.1 | 22.7 ± 2.3 | G′-G″ crossover | 5.00 ± 0.00 |
| G15/0.2 | 23.7 ± 5.1 | onset of sharp G′ increase | 5.00 ± 0.00 |
| G15/0.4 | 25.4 ± 1.0 | onset of sharp G′ increase | 4.23 ± 0.25 |
| G18/0.1 | 21.4 ± 2.7 | G′-G″ crossover | 5.00 ± 0.00 |
| G18/0.2 | 20.5 ± 2.4 | onset of sharp G′ increase | 3.73 ± 0.30 |
| G18/0.4 | 18.6 ± 4.9 | onset of sharp G′ increase | 0.63 ± 0.32 |
| Formulation | Flux ± s.d. (μg/cm2/h) | Cal (mg/mL) | Release Rate (cm/h) × 10−3 | Reduction Ratio 1 |
|---|---|---|---|---|
| Sol-Cal | 13.31 ± 1.50 | 0.25 | 53.24 ± 6.00 | - |
| G18/0.4-Cal | 49.51 ± 0.71 | 0.25 | 198.04 ± 2.84 | 3.72 |
| Incubation Time | ||
|---|---|---|
| 3 h | 24 h | |
| Condition | CFU n°/sample (*) | CFU n°/sample (*) |
| AB (CTR) | 100 ± 24 | 1.255 ± 150 |
| AB Cal (0.031 mg/mL) | 94 ± 15 (−5%) | 880 ± 35 (−30%) |
| AB Cal (0.062 mg/mL) | 87 ± 18 (−13%) | 807 ± 41 (−35%) |
| AB Cal (0.125 mg/mL) | 82 ± 26 (−18%) | 616 ± 27 (−51%) |
| Incubation Time | ||
|---|---|---|
| 3 h | 24 h | |
| Condition | CFU n°/sample (*) | CFU n°/sample (*) |
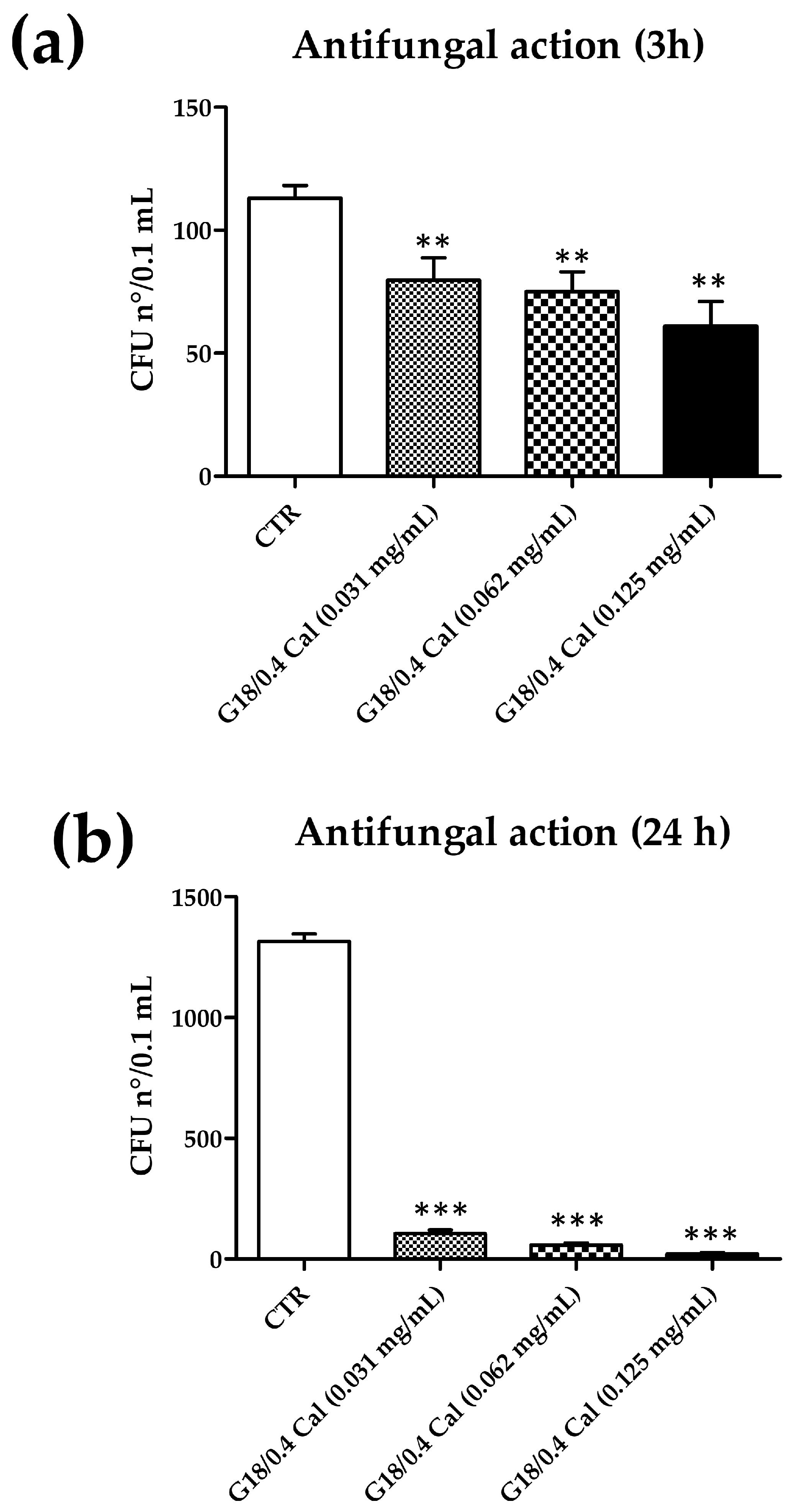
| G18/0.4 (CTR) | 113.0 ± 5.2 | 1.316 ± 31.0 |
| G18/0.4 Cal (0.031 mg/mL) | 79.7 ± 9.1 (−29%) | 105.1 ± 15.0 (−92%) |
| G18/0.4 Cal (0.062 mg/mL) | 75.0 ± 8.0 (−32%) | 57.7 ± 9.0 (−96%) |
| G18/0.4 Cal (0.125 mg/mL) | 61.0 ± 10.0 (−46%) | 22.0 ± 4.5 (−98%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellotti, D.; D’Accolti, M.; Pula, W.; Huang, N.; Simeliere, F.; Caselli, E.; Esposito, E.; Remelli, M. Calcitermin-Loaded Smart Gels Activity against Candida albicans: A Preliminary In Vitro Study. Gels 2023, 9, 165. https://doi.org/10.3390/gels9020165
Bellotti D, D’Accolti M, Pula W, Huang N, Simeliere F, Caselli E, Esposito E, Remelli M. Calcitermin-Loaded Smart Gels Activity against Candida albicans: A Preliminary In Vitro Study. Gels. 2023; 9(2):165. https://doi.org/10.3390/gels9020165
Chicago/Turabian StyleBellotti, Denise, Maria D’Accolti, Walter Pula, Nicolas Huang, Fanny Simeliere, Elisabetta Caselli, Elisabetta Esposito, and Maurizio Remelli. 2023. "Calcitermin-Loaded Smart Gels Activity against Candida albicans: A Preliminary In Vitro Study" Gels 9, no. 2: 165. https://doi.org/10.3390/gels9020165
APA StyleBellotti, D., D’Accolti, M., Pula, W., Huang, N., Simeliere, F., Caselli, E., Esposito, E., & Remelli, M. (2023). Calcitermin-Loaded Smart Gels Activity against Candida albicans: A Preliminary In Vitro Study. Gels, 9(2), 165. https://doi.org/10.3390/gels9020165